Abstract
Objectives:
This study aimed to: 1) examine changes in pain, psychosocial functioning, and healthcare utilization among children and adolescents with sickle cell disease (SCD) over a 2-year period; and 2) identify baseline biopsychosocial variables associated with the development and maintenance of chronic SCD pain at follow-up.
Method:
Forty-two youth (8–18 years old) with SCD completed a battery of self-report measures at baseline and 2-year follow-up. Analgesic, Anesthetic, and Addiction Clinical Trial Translational Innovations Opportunities and Networks and American Pain Society Pain Taxonomy (AAPT) diagnostic criteria were used to categorize patients into pain frequency groups at both time points: chronic (pain on most [≥ 15] days/month for the past 6 months, per AAPT diagnostic criteria), episodic (pain on 1–14 days/month), or asymptomatic (0 days/month).
Results:
At baseline, 31% (n=13) had chronic pain, 50% (n=21) episodic pain, and 19% (n=8) asymptomatic. At follow-up, 40.5% (n=17) had chronic pain, 52.4% (n=22) episodic pain, and 7.1% (n=3) asymptomatic. Between baseline and 2-year follow-up, 12% (n=5) developed chronic SCD pain. Depressive symptoms and admissions for pain significantly increased over time for youth with chronic pain (p’s<.05). An interaction effect revealed that baseline pain groups differed in their change in pain intensity over time (p<.01). Baseline psychosocial factors (i.e., higher functional disability, greater depressive symptoms, higher pain catastrophizing, and lower quality of life) were significantly associated with chronic pain at follow-up.
Discussion:
Biopsychosocial factors may be associated with the development and maintenance of chronic SCD pain and their relative contributions warrant further study.
Keywords: sickle cell disease, chronic pain, pediatric, biopsychosocial, longitudinal
Introduction
Recurrent acute pain episodes are a central feature of sickle cell disease (SCD) and a primary contributor of poor health-related quality of life and frequent healthcare utilization1–3. Daily pain diary studies among children and adults with SCD suggest a likely overlap of acute vaso-occlusive pain occurring within the context of ongoing chronic pain for some patients4,5. According to recently published diagnostic criteria developed by the Analgesic, Anesthetic, and Addiction Clinical Trial Translational Innovations Opportunities and Networks and American Pain Society Pain Taxonomy (AAPT), chronic SCD pain includes “reports of ongoing pain present on most days (≥ 15 days per month) over the past 6 months”6. Chronic pain has been estimated to occur in about 23% of children and adolescents and up to 29% in adults5,7 with SCD. Comparable to other pediatric chronic pain conditions, common biopsychosocial sequelae of chronic SCD pain in children and adolescents include functional impairment, frequent school absences, elevated depressive symptoms, high anxiety or catastrophic thinking, reduced quality of life, and frequent healthcare use7–11. Although there is a dearth of large-scale prospective longitudinal studies assessing the onset and maintenance of chronic SCD pain, it is likely that chronic SCD pain can begin in childhood and persist through adulthood4,5,12. Among youth with SCD, pain frequency, healthcare use, and activity reduction tend to increase with age13. However, not all youth with SCD experience enduring physical and psychosocial sequelae as they transition to adulthood14,15. Thus, early identification of youth at risk of developing chronic SCD pain is critical to advance treatments and disrupt the progression of functional disability and reduced quality of life into adulthood.
The natural course of chronic SCD pain and factors that contribute to the transition from acute to chronic pain in SCD remain largely unknown. However, sensitization of the central and peripheral nervous system, along with sickling related inflammation, and biopsychosocial risk factors are possible indicators of chronic SCD pain development16–18. Although research in chronic pain in pediatric SCD is sparse, longitudinal studies of other pediatric pain conditions (e.g., headache, abdominal pain, back pain) suggest that female gender, older age, lower socioeconomic status, higher pain frequency, elevated anxiety, and higher depression increase the risk of long-term persistence of pain19–23. In adults with SCD, evidence suggests that pain, somatic symptoms, depression, and anxiety may be positively associated with poorer biopsychosocial functioning and pain outcomes24,25. Given the nascent state of chronic SCD pain research, it remains unclear what biopsychosocial factors may contribute to the development or maintenance of chronic SCD pain in youth.
As a first step towards addressing this gap, the overall objective of this study was to evaluate longitudinal changes in episodic and chronic pain and related psychosocial sequelae in pediatric SCD across two time points over an approximate 2-year period. This observational cohort study aimed to: 1) examine changes in pain, psychosocial functioning, and healthcare utilization among youth with SCD from baseline to 2-year follow-up assessments; and 2) identify baseline psychosocial variables associated with the development and maintenance of chronic SCD pain at follow-up. A small proportion of youth were expected to transition from acute to chronic SCD pain over the course of 2 years. Pain, psychosocial functioning, and healthcare utilization were expected to worsen over time, particularly for youth with chronic SCD pain. Lastly, it was hypothesized that baseline psychosocial factors (i.e., older age, higher pain intensity, greater functional disability, elevated depressive symptoms, higher pain catastrophizing, reduced quality of life, and greater healthcare utilization) would be significantly associated with chronic pain at 2-year follow-up.
Materials and Methods
Setting
Participants included children and adolescents receiving care at outpatient comprehensive SCD clinics at three tertiary care locations at a large southeastern children’s hospital. The SCD program serves over 2000 children with SCD annually and includes approximately 15 pediatric hematologists, 8 advance practice providers, and a multi-disciplinary team of family support service providers (psychologists, social workers, school advocates, child life specialists, music therapists, and chaplains).
Participant Eligibility
Children and adolescents aged 8 to 18 years at time of initial enrollment and their parents were eligible to participate if patients were diagnosed with SCD and both patient and parent were English-speaking. Exclusion criteria included patients with significant documented cognitive or developmental disabilities, receiving chronic transfusions indicated for central nervous system (CNS) complications (e.g., stroke), or with comorbid medical condition in which pain is a common symptom (e.g., rheumatological or gastrointestinal conditions).
Study Procedures
Institutional Review Board approval was obtained prior to initiation of the study. Study coordinators reviewed clinic appointment lists and collaborated with the hematologists and/or nurse practitioners to identify potentially eligible patients. Patients and their parents were introduced to the study by a trained research coordinator who assessed their eligibility. If patients were eligible and expressed interest, the coordinator explained the study in greater detail. Parents and adolescents (18 years or older) provided written informed consent, children (8–10 years) provided verbal assent, and children and adolescents (11–17 years) provided written assent. For patient-parent dyads recruited over the phone, verbal consent and assent were obtained. Patients and parents were provided the option to complete web-based or pencil-and-paper measures during a routine outpatient clinic visit while waiting for their provider or at home. Patients and parents received a monetary incentive for the time dedicated to participation for both assessments.
At baseline assessment (T1) from 2014–2015, 180 patients were screened for study eligibility of which 61% (n=110) were deemed potentially eligible and approached for further screening and enrollment. A total sample of 100 children and adolescents with SCD and their parents participated in the baseline assessment with an intentional stratified sampling of patients with episodic pain (n=45), chronic pain (n=35), and a concurrent subset of patients without pain (n=20) to encompass a full range of pain frequency (range 0–31 days/month). Because this cohort study was not designed prospectively, parents and patients were contacted approximately 2 years later to consent for a follow-up assessment (T2) from 2016–2017 (M = 2.60 years, SD = 0.50, range 1.75–3.81 years from baseline). At follow-up, 41 participants declined participation or were lost to follow-up. Figure 1 illustrates primary reasons for attrition between baseline and follow-up, including participants who were lost to follow-up, transitioned to adult care without updated contact information, or declined future research participation. Patients were classified as lost to follow-up if they had no active contact information in the electronic medical record (e.g., no working phone number, returned mail) or had no active medical care in our children’s system (i.e., no clinic or hospital encounters in at least 1 year, no future scheduled appointments). Of the 59 participants eligible for follow-up, 49 patients and their parents consented to the follow-up assessment (83% consent rate) and 43 patient-parent dyads completed study procedures (73% participation rate). Routine monitoring of data quality revealed that 1 participant’s survey responses were invalid and were removed from analyses. The final sample size consisted of 42 children and adolescents and their parents who completed both baseline and follow-up assessments.
Figure 1.
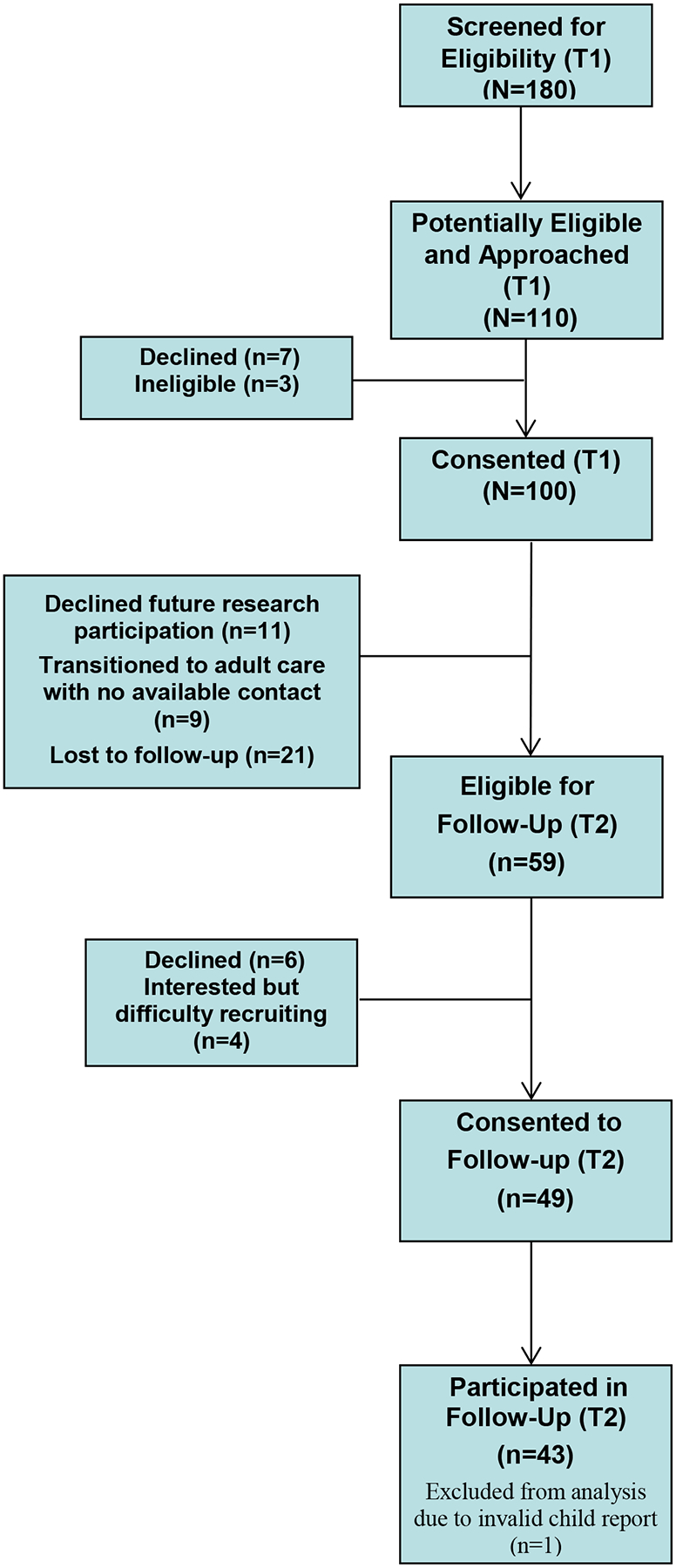
Study Flow Diagram for Initial (T1) and Follow-up (T2) Assessments
Measures
As part of a larger assessment protocol (see7,26 for detailed descriptions), the following measures were used to assess pain, psychosocial functioning, and healthcare utilization at baseline and follow-up.
Demographics.
Detailed demographic and background information, including patient and parent age, race, sex, highest education level, annual family income, and engagement in outpatient psychological therapy within the past 12 months (presence/absence at follow-up only) were reported by parents. Common SCD treatments (i.e., hydroxyurea, chronic transfusions) and disease-related complications, specifically avascular necrosis confirmed by MRI imaging, were determined based on medical chart review.
Pain characteristics.
Children and adolescents reported on their average pain intensity over the last two weeks using a numeric rating scale, with “0” indicating no pain, and “10” reflecting worst possible pain. Based on items adapted from prior research, patients reported on the number of days they had any pain in the past month (0–31 days) and on a 5-point Likert scale (0=none to 4=every day), and how long (i.e., duration) they have experienced the current level of pain frequency on a 5-point Likert scale ranging from 1 (only this month) to 5 (over 1 year)7,27,28. Following guidelines of the AAPT taxonomy, patients were classified into one of three pain groups: 1) chronic SCD pain, defined as ≥ 15 days of pain per month with duration of pain frequency ≥ 6 months6; 2) episodic pain, defined as 1–14 pain days in the past month29; or 3) asymptomatic, indicative of 0 pain days in the past month. A similar classification system was used in previous work to characterize pain chronicity of the baseline sample7 prior to publication of the AAPT taxonomy.
Functional Disability.
The Functional Disability Inventory (FDI) is a well-validated, 15-item, self-report instrument that assesses children’s and adolescents’ perceived difficulty to perform daily activities in home, school, recreational, and social settings30. Participants rated how much difficulty they have performing each of the activities on a 5-point Likert scale (0 = no trouble to 4 = impossible). Total scores range from 0 to 60, with the following established clinical reference points: 0–12 indicating no/minimal disability, 13–29 indicating moderate disability, and 30–60 indicating severe disability31. The FDI has been found to have high internal consistency, moderate to high test-retest reliability, and good predictive validity30,32. It has been used in prior research in pediatric SCD and chronic pain33–35. Internal reliabilities were 0.92 at baseline and 0.96 at follow-up.
Depressive Symptoms.
The Children’s Depression Inventory-2 (CDI-2) is a well-validated 24-item self-report measure used to assess depressive symptoms in children and adolescents in the past two weeks36. Total scores range from 0 to 54, and higher scores indicate greater severity of depressive symptoms. Clinical cutoffs for total CDI scores are: no/minimal depressive symptoms (<10), mild (10–19), moderate (20–28), and severe (> 28). Study coordinators immediately reviewed CDI results following patient completion and informed a licensed psychologist of reports of suicidality. A licensed psychologist conducted a risk assessment and devised a safety plan, if warranted. Patients also received referrals for mental health services, as appropriate. The CDI has strong psychometric properties and is frequently used in pediatric pain and SCD research37–42. Internal reliabilities were 0.85 at baseline and 0.90 at follow-up.
Pain Catastrophizing.
The Pain Catastrophizing Scale – child (PCS-C), is a 13-item questionnaire that assess thoughts and feelings about pain43,44. Items are rated on a 5-point Likert scale (0 = mildly to 4 = extremely). Total scores range from 0 to 52, with higher scores reflective of greater catastrophic thinking about pain. Total scores were used for analyses with the following clinical reference points: low (0–14), moderate (15–25), and high (≥ 26)45. The PCS-C has been used frequently in pediatric chronic pain research; although not yet used commonly in pediatric SCD8,9, it is well-validated in samples of youth with chronic pain and their parents44–46. Internal reliabilities were 0.92 at baseline and 0.96 at follow-up.
Quality of Life.
The Pediatric Quality of Life Sickle Cell Disease Module (PedsQL-SCD) is a well-validated 42-item measure that assesses 9 dimensions of health-related quality of life specific to SCD. Items are rated on a 5-point Likert scale (0 = never to 4 = almost always), and scores are transformed on a scale from 0 to 100. Total scores were used for analyses and higher scores are indicative of better quality of life. The PedsQL-SCD demonstrates excellent reliability and construct validity among patient self-report and has been widely used in pediatric SCD research28,47–49. Internal consistencies were 0.96 at baseline and 0.97 at follow-up.
Healthcare Utilization.
Electronic medical record data were reviewed to gather the number of inpatient hospitalizations (admissions) and emergency department (ED) visits related to SCD pain within the last 12 months. ED visits for pain that subsequently required same-day inpatient hospitalization for further treatment were classified as admissions.
Statistical Analyses
On the basis of medium effect sizes determined from previous studies of differences in SCD pain chronicity7, a minimum of 35 patients across two repeated measures were needed to achieve power of at least .85 and detect a group by time interaction effect.
All data were entered into REDCap, a secure web application for online surveys and databases, and then exported into SPSS version 24 for analyses. First, baseline characteristics of all participants who completed both the baseline and follow-up assessment versus those who completed baseline assessment only were compared to ensure that there was no systematic source of bias due to selective attrition. Descriptive data on baseline and follow-up variables were computed to determine whether the data met the underlying assumptions of the proposed analytic procedures.
Using the criteria listed for pain classification described earlier, the sample was categorized into 3 groups: (1) chronic pain, (2) episodic pain, and (3) asymptomatic. Multivariate analysis of covariance analysis (MANCOVA) was conducted to compare patients in the chronic pain group to patients without chronic pain at follow-up on their functional outcomes (pain intensity, functional disability, quality of life), psychosocial characteristics (depressive symptoms, pain catastrophizing), and healthcare utilization (number of ED visits and hospital admission for pain). Due to multiple comparisons in this study, type-1 error inflation was controlled using the False Discovery Rate (FDR) technique, which has demonstrated superior type-1 error rates and statistical power balance in comparison to Bonferroni correction50.
Chi-square analyses were used to examine the distribution of patients in each pain group at baseline and follow-up to describe changes in pain frequency over time. Descriptive statistics were computed to compare patients who transitioned from episodic to chronic pain over time to patients with stable episodic pain from baseline to follow-up.
Linear mixed effect models with random effect for person-specific intercepts and slopes were used to assess change over time in pain, psychosocial functioning, and healthcare use. Random effects were retained based on their model contribution using Bayesian Information Criterion (BIC)51. At least two observations are required to evaluate change from baseline for each participant. Participants with only one observation (i.e., those who completed baseline but not follow-up assessments) contributed to the estimation of the mean level for their group at the nonmissing time point52. Missing data were assumed missing at random, indicating that the missingness was not associated with the unobserved value. Separate models were conducted for pain intensity, functional disability, depressive symptoms, pain catastrophizing, quality of life, inpatient admissions, and ED visits. Baseline patient characteristics (i.e., child age, SCD genotype, pain frequency group) and disease-modifying treatments (i.e., hydroxyurea, chronic transfusion therapy) were controlled for by including these variables as potential covariates. Additionally, time and the interaction effect for time and baseline pain group were initially included in the models. The most parsimonious model was selected using backward selection procedure. Specifically, all candidate variables in the model were initially included; variables with the largest p-value were iteratively removed until the p-values for all remaining variables were <.05 significant level. Main effects for variables that were part of an interaction term were not removed until the nonsignificant interaction term was removed from the model. Lastly, bivariate and spearman correlations were conducted to examine relations between pain characteristics, psychosocial, and healthcare use variables at baseline and follow-up.
Results
Sample Characteristics
Of the 100 patient-parent dyads from the baseline assessment, 57 patients were missing all patient-reported measures at follow-up and 57 parents were missing all parent-reported demographics at follow-up. For patients who completed both baseline and follow-up assessments, there were no missing data on primary variables of interest (i.e., pain intensity, functional disability, depression, catastrophizing, quality of life, and healthcare utilization). Attrition was related to child age such that patients who completed follow-up were significantly younger in baseline age (M = 12.76, SD = 2.25) than those who did not complete follow-up (M = 14.09, SD = 2.92; t [98] = 2.46, p<.05). Therefore, child age was included as a covariate in subsequent analyses, as appropriate. Participants who completed versus did not complete the follow-up assessment did not significantly differ on any other baseline demographics (i.e., child race, child sex, parent age, parent marital status, parent highest grade completed, annual family income) or baseline clinical or biopsychosocial factors (i.e., hemoglobin type, SCD treatments of hydroxyurea or chronic transfusion, pain characteristics, functional disability, depressive symptoms, quality of life, or healthcare use). There was no significant differential attrition based on baseline pain frequency group. Descriptive statistics on pain characteristics, psychosocial functioning, and healthcare use revealed skewness and kurtosis within the limits of normal distribution53.
Patient and parent baseline demographics for the sample at baseline and follow-up are presented in Table 1. At follow-up, patients were on average 14.95 years old (SD = 2.37, range 11–21). Most patients were female (59.5%), Black or African American (95.2%), and had hemoglobin type HbSS (78.6%). At follow-up, 69% (n=29) were prescribed hydroxyurea (adherence not verified) and 16% of patients (n=7) were receiving chronic transfusions. A total of 5 patients (11.9%) started hydroxyurea between baseline and follow-up assessments, of which 1 discontinued chronic transfusion and 1 was weaning off chronic transfusion. Two patients (4.8%) discontinued hydroxyurea by follow-up. Two patients (4.8%) started chronic transfusion by follow-up. At follow-up, parent reporters included mostly mothers (83.3%) and fathers (16.7%). Parent marital status, highest grade completed, and annual family income are detailed in Table 1.
Table 1.
Sample characteristics
| Baseline Characteristics | Baseline sample (n=100) | Follow-up Sample (n=42) |
|---|---|---|
| Child | ||
| Mean Age (SD) | 13.54 (2.74) | 14.95 (2.37) |
| Sex (female) | 61 (61.0) | 25 (59.5) |
| Race (African American) | 94 (94.0) | 40 (95.2) |
| Hemoglobin type | ||
| HbSS | 77 (77.0) | 33 (78.6) |
| HbSC | 15 (15.0) | 6 (14.3) |
| HbS β+Thalassemia | 5 (5.0) | 2 (4.8) |
| HbS β0Thalassemia | 3 (3.0) | 1 (2.4) |
| SCD Treatments or Complications | ||
| Hydroxyurea | 60 (60.0) | 29 (69.0) |
| Chronic Transfusion Therapy | 16 (16.0) | 7 (16.7) |
| Presence of Avascular Necrosis┼ | 3 (7.1) | 6 (14.3) |
| Parent | ||
| Reporter (mother/stepmother) | 85 (85.0) | 35 (83.3) |
| Marital Status | ||
| Married | 42 (42.0) | 13 (31.0) |
| Single | 37 (37.0) | 15 (35.7) |
| Divorced/Separated | 17 (17.0) | 11 (26.2) |
| Widowed | 4 (4.0) | 3 (7.1) |
| Highest grade completed | ||
| High School or less | 28 (28.0) | 10 (23.8) |
| Some college | 29 (29.0) | 12 (28.6) |
| College degree | 26 (26.0) | 14 (33.3) |
| Graduate or Professional degree | 16 (16.0) | 6 (14.3) |
| Missing | 1 (1.0) | |
| Annual family income | ||
| ≤$10,000 | 22 (22.0) | 10 (23.8) |
| $10,001–20,000 | 16 (16.0) | 8 (19.0) |
| $20,001–30,000 | 8 (8.0) | 3 (7.1) |
| $30,001–50,000 | 22 (22.0) | 9 (21.4) |
| $50,001–75,000 | 12 (12.0) | 4 (9.5) |
| ≥$75,001 | 12 (12.0) | 4 (9.5) |
| Prefer not to answer | 8 (8.0) | 4 (9.5) |
Note:
presence of avascular necrosis is based on the sample of n=42 that completed both baseline and follow-up assessments.
Pain Group Classification
Patient-reported pain frequency and duration were used to determine pain group classification. Patient report of pain frequency in the past month (total number of pain days in past month, 0–31 days) and on a 5-point Likert scale (4=every day; 3=many; 2=some; 1=a few; 0=none) were highly intercorrelated (r = 0.85, p<.001). Total number of pain days significantly differed based on Likert-scale item responses (F=18.84, p<.001), such that patients who reported having pain in the past month on “many” or “every day” reported significantly higher number of total pain days in the past month (M = 19.18, SD = 8.41; M = 25.25, SD = 10.84, respectively) than those who reported having pain in the past month on “none” (M = 0.50, SD = 1.0), “a few” (M = 3.92, SD = 3.75), or “some” (M = 5.13, SD = 3.94) days. Given the high consistency between patient report of pain frequency regardless of question type (i.e., Likert scale versus open-ended), total pain days in the past month in addition to pain duration were used to classify patients into pain groups. At baseline, 8 (19%) patients were classified as asymptomatic, 21 (50%) patients were classified as having episodic pain, and 13 (31%) met the primary diagnostic criteria for chronic SCD pain. At 2-year follow-up, 3 (7.1%) patients were classified as asymptomatic, 22 (52.4%) had episodic pain, and 17 (40.5%) met diagnostic criteria for chronic SCD pain.
Patients identified as having chronic SCD pain at follow-up reported an average of 20.8 days (SD=5.14, range 15–31) of pain per month of which 29.4% (n=5) reported their pain frequency persisted for the past 6–12 months and 70.6% (n=12) reported pain duration persisted for over 1 year. Patients with episodic pain at follow-up (n=22) reported an average 4.27 days (SD=3.72, range 1–13) of pain per month; 45.4% (n=10) of these patients reported their pain frequency persisted for the past 3–5 months, 27.3% (n=6) persisted for the past 6–12 months, and 27.3% (n=6) persisted for over 1 year. Due to the low frequency of patients in the asymptomatic group, a MANCOVA analysis controlling for child age compared patients with chronic pain relative to those without chronic pain (i.e., asymptomatic and episodic groups combined). Results revealed a significant multivariate pain group effect at follow-up, Wilks’ λ = 0.37, F(7, 34) = 8.34, p<.001. Patients with chronic SCD pain had significantly higher pain intensity, functional disability, depressive symptoms, pain catastrophizing, reduced quality of life, and more frequent inpatient admissions and ED visits for pain than youth without chronic pain (Table 2).
Table 2.
Comparison of youth with SCD with chronic pain (n=17) and without chronic pain (n=25) on pain, functional outcomes, psychosocial characteristics, and healthcare utilization at 2-year follow-up
| Asymptomatic and Episodic
M (SD) |
Chronic M (SD) |
Univariate Tests df = (1, 40) |
Partial Eta Squared | ||
|---|---|---|---|---|---|
| F-value | p-value | ||||
| Pain intensity, NRS (0–10) | 2.31 (2.49) | 6.24 (1.95) | 28.26 | .01 | .42 |
| Functional disability (0–60) | 10.15 (12.69) | 24.53 (12.87) | 12.00 | .01 | .24 |
| Quality of life (0–100) | 64.35 (24.25) | 44.94 (13.09) | 8.91 | .01 | .18 |
| Depressive symptoms (0–56) | 8.35 (7.26) | 14.59 (9.11) | 5.43 | .04 | .13 |
| Pain Catastrophizing (0–52) | 17.88 (13.73) | 30.00 (11.80) | 9.16 | .01 | .18 |
| ED visits for pain (past 12 months) | 0.65 (1.26) | 2.29 (2.31) | 11.76 | .01 | .36 |
| Admissions for pain (past 12 months) | 0.77 (1.53) | 5.65 (4.89) | 20.94 | .01 | .18 |
Note: All statistics adjusted for child age at follow-up. False Discovery Rate (FDR) Type-1 error control was used for all pairwise comparisons.
Changes in Pain Frequency Over Time
Chi-square analysis revealed significant changes in the distribution of patients per pain group over time, X2 =0.89, p<.001 (see Figure 2). Specifically, 1 (2.4%) patient reported improvement in pain frequency moving from chronic pain at baseline to episodic pain at follow-up; 32 (76.2%) patients reported stable pain frequency (e.g., episodic at baseline and follow-up, chronic pain at baseline and follow-up); and 9 (21.4%) patients reported worsened pain frequency over time (i.e., changed from asymptomatic to episodic, or episodic to chronic pain from baseline to follow-up). Of the patients whose pain frequency worsened over time, 5 (11.9%) patients transitioned from asymptomatic or acute, episodic pain at baseline to chronic pain at 2-year follow-up. More detailed information regarding individual changes in patient treatment approaches and psychosocial functioning can be found in Supplementary Tables 1 and 2.
Figure 2.
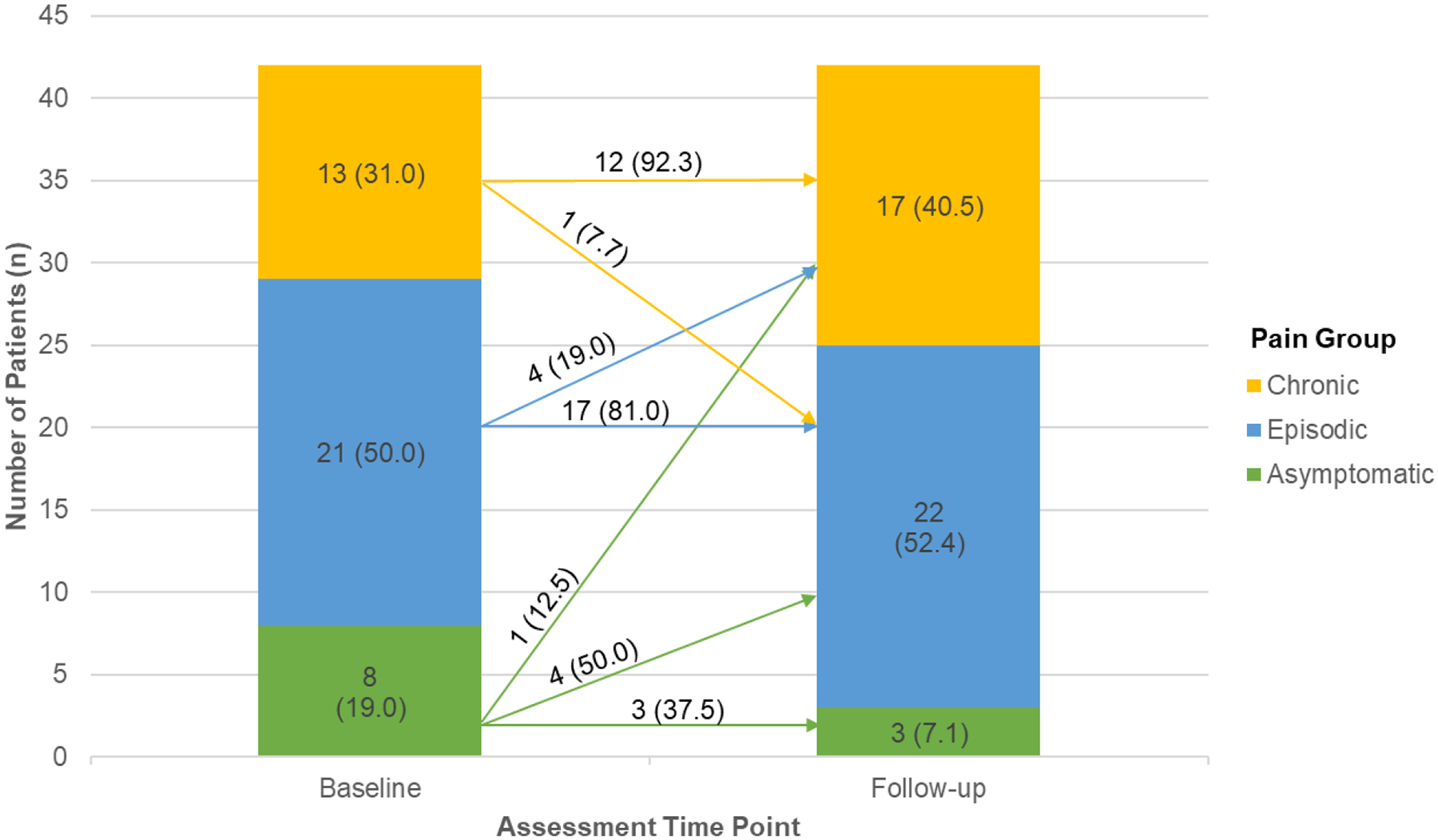
Frequency (n) and Percentage (%) of Patients by Pain Group Over Time. Arrows indicate number and percentage of patients who changed pain groups between assessment time points. Values in parentheses above arrows reflect the percentage of patients within Baseline Pain Group.
Changes in Psychosocial and Functional Outcomes Over Time
Pain intensity.
The selected model for patient-reported average pain intensity included baseline pain group, F(2, 136) = 19.52, p < .001, and the pain group by time interaction effect, F(2, 136)= 5.00, p < .01) with random intercept; time was retained in the model but not significant. Additional covariates were removed from the model as they did not improve model fit. Patients with chronic SCD pain reported 3.89-points (95% CI [6.01, 1.76], p<.001) and 3.04 points (95% CI [4.70, 1.38], p < .001) higher pain intensity ratings than patients in the asymptomatic and episodic groups, respectively. It is important to note there was no significant change over time across groups; however, the rate of change over time in the episodic group (1.92-point decrease, 95% CI [0.11, 3.72], p<.05) was significantly different than the rate of change over time in the chronic pain group (0.78-point increase, p=.27; see Figure 3).
Figure 3.
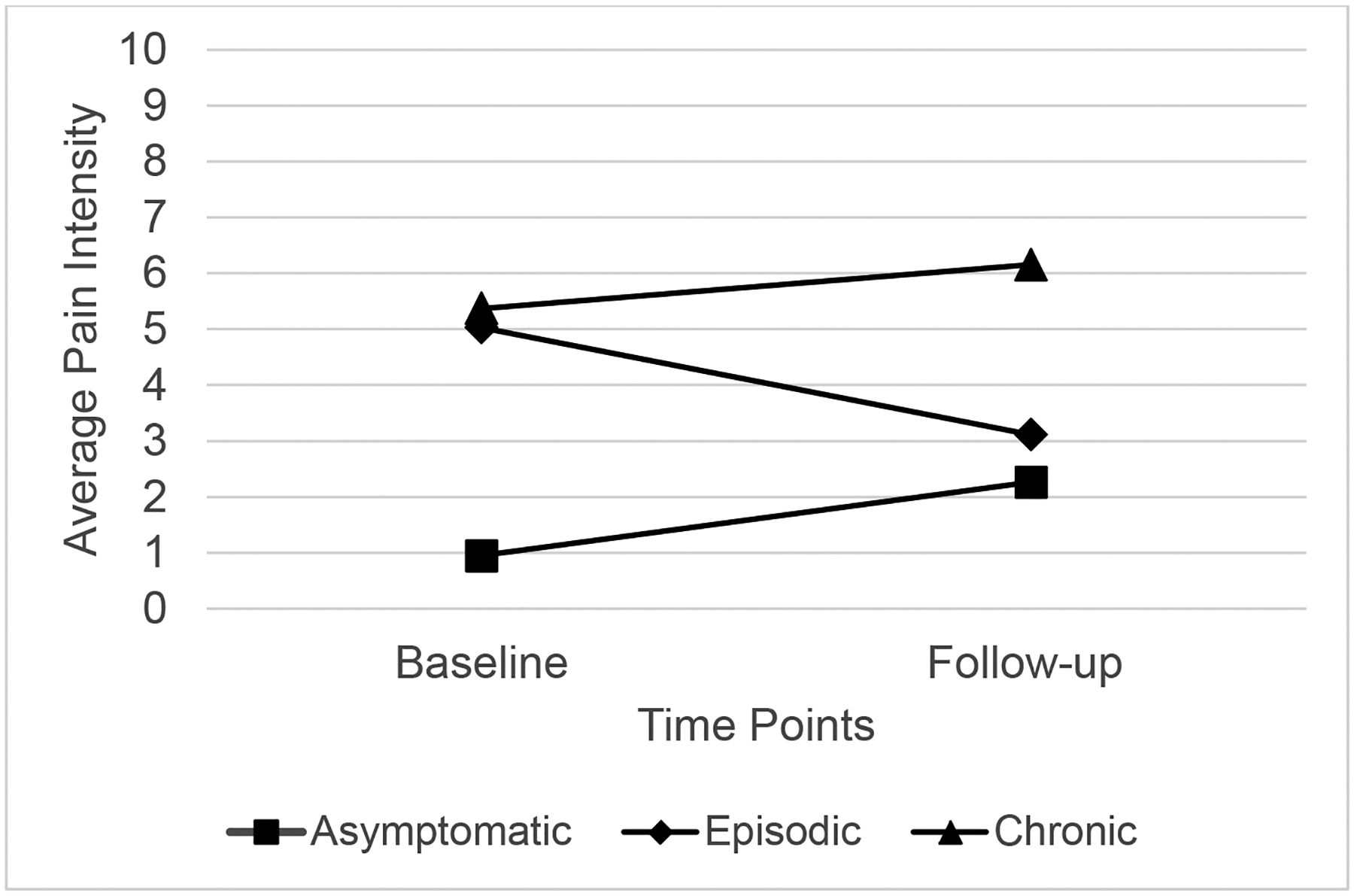
Change over time in Average Pain Intensity Scores at Baseline and 2-year follow-up by Baseline Pain Group
Depressive symptoms.
The most parsimonious model for patient-reported depressive symptoms included baseline pain frequency group and time with random intercept; the baseline pain group by time interaction effect was retained to improve fit although nonsignificant. Patients with chronic SCD pain reported 11.23-point (95% CI [16.78, 5.68], p<.001) and 6.69-point (95% CI [11.61, 3.10] p<.01) increase in depressive symptoms compared to youth in the asymptomatic and episodic groups, respectively, F(2, 136) = 14.79, p<.001. There was an average 3.68-point increase in depressive symptoms from baseline to follow-up for youth with chronic pain, F(1, 130) = 4.75, p<.05.
Inpatient Admissions.
The most parsimonious model for inpatient admissions included baseline pain frequency group and time with random intercept. The baseline pain group by time interaction effect was retained in the model although nonsignificant. Covariates (child age, SCD genotype, hydroxyurea, chronic transfusions) were not retained as they not improve model fit. Patients with chronic SCD pain had 4.95 (95% CI [7.26, 2.65], p<.001) more admissions compared to patients in the asymptomatic group and 2.74 (95% CI [4.55, 0.93], p<.01) more admissions than the episodic group, F(2, 136) =15.83, p<.001. There was an average 2.39 (95% CI [4.06, 0.72] increase in admissions from baseline to follow-up for youth with chronic pain, F(1, 136) =6.14, p<.01. The rate of change over time between baseline pain groups was not significant (p=.25).
There were no significant changes over time or pain group by time interaction effects for functional disability, quality of life, pain catastrophizing, or ED visits.
Baseline Predictors of Sickle Cell Chronic Pain at 2-Year Follow-up
Bivariate pearson product moment correlations were evaluated among child age, pain intensity, disability, depressive symptoms, pain catastrophizing, quality of life, and healthcare utilization at baseline and follow-up and displayed in Table 3. Although no baseline psychosocial variables were related to ED visits at follow-up, baseline disability, quality of life, and inpatient admissions were moderately related to inpatient admissions at follow-up (r’s=.30–.52, p’s<.05).
Table 3.
Correlations of pain, psychosocial, and healthcare use variables at baseline and 2-year follow-up
| Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1. Child Age | 0.97** | 0.09 | 0.25 | 0.11 | 0.19 | 0.01 | −0.06 | 0.14 | −0.17 |
| 2. Pain Intensity | 0.39 | 0.37* | 0.22 | 0.41** | 0.26 | 0.47** | −0.49** | 0.29 | 0.12 |
| 3. Pain Frequency Group+ | 0.47** | 0.50** | 0.70** | 0.53** | 0.44** | 0.46** | −0.52** | 0.52** | 0.22 |
| 4. Functional Disability | 0.14 | 0.35* | 0.39* | 0.58** | 0.49** | 0.52** | −0.50** | 0.30* | 0.12 |
| 5. Depressive Symptoms | 0.17 | 0.40** | 0.36* | 0.65** | 0.62** | 0.51** | −0.49** | 0.12 | 0.15 |
| 6. Pain Catastrophizing | 0.20 | 0.48* | 0.38** | 0.54** | 0.39* | 0.55** | −0.60** | 0.20 | 0.28 |
| 7. Quality of Life | −0.17 | −0.54** | −0.47* | −0.62** | −0.38* | −0.62** | 0.75** | −0.37** | −0.22 |
| 8. Inpatient Admissions | 0.34* | 0.39* | 0.52** | 0.35* | 0.14 | 0.32* | −0.39** | 0.52** | −0.37 |
| 9. ED Visits | −0.08 | 0.18 | 0.27 | 0.11 | 0.31* | 0.10 | −0.27 | 0.19 | 0.53** |
Note: All values represent Pearson correlations except
Pain Frequency Group values represent Spearman’s rho
p<.05;
p<.01
Spearman correlations evaluated associations between baseline psychosocial factors and pain frequency group at follow-up. Baseline pain group (ρ=.70, p<.01) and inpatient admissions (ρ=.52, p<.01) demonstrated large, positive associations with pain group at follow-up. Additionally, higher levels of baseline functional disability, depressive symptoms, and pain catastrophizing, and lower quality of life had moderate associations with pain group at follow-up (ρ’s=.36–.47, all p’s <.05).
Discussion
Chronic pain has gained greater recognition as a distinct and serious complication in SCD6. Consistent assessment of chronic SCD pain is a first and essential step needed to improve management of this complex problem. By utilizing the AAPT evidence-based classification system that defined core diagnostic criteria for chronic pain associated with SCD6, this is one of the first studies to a) longitudinally examine changes in chronic pain and associated psychosocial functioning in a clinical cohort of youth with SCD and b) identify biopsychosocial risk factors that may be associated with development of chronic SCD pain. In line with our prior findings, patients who met the core diagnostic criteria for chronic SCD pain demonstrated higher pain intensity, greater functional disability, reduced health-related quality of life, greater depressive symptoms and pain catastrophizing, and more healthcare utilization than patients without chronic SCD pain. This pattern of increased difficulty with daily activities and psychosocial distress remains consistent with past studies of persistent and chronic SCD pain in youth7,11 as well as other pediatric chronic pain conditions54,55.
Over the course of approximately 2 years, youth with chronic SCD pain reported an increase in depressive symptoms and inpatient admissions after adjusting for patient characteristics, such as child age, which can impact disease severity13. In addition, patient report of average pain intensity changed variably over time based on pain frequency group at baseline. Contrary to expectations, there was no meaningful change over time in pain intensity for youth with chronic pain; however, those in the episodic pain group at baseline reported reductions in pain intensity by 2-year follow-up. Possible multimodal therapies, such as changes in pain medication regimen, additional disease-modifying treatments, and participation in physical or outpatient psychological therapy may have contributed to reductions in pain intensity for the episodic pain group. For example, 33% of patients in the episodic group at baseline engaged in psychological therapy between baseline and follow-up assessments, which may have contributed to improvements. Specific changes in pain medications, engagement in physical therapy, and content or dosage of outpatient psychological therapy were not easily assessed in this study as some of these services were completed outside our institution; thus, their relative contributions to reductions in pain intensity or possible prevention of progression to chronic pain remain unclear for this sample and warrant future prospective and controlled studies.
Contrary to expectations, functional disability, health-related quality of life, pain catastrophizing, and ED visits for pain remained relatively stable over time across pain groups. The overall pattern of stability in psychosocial functioning is consistent with most of the sample also exhibiting relatively stable pain frequency over time. Changes to SCD treatments that occurred between assessments may have partially served as mitigating factors. That is, about 24% of the sample experienced changes to SCD treatments, such as initiating hydroxyurea (7%) or chronic transfusion therapy (7%), discontinuing hydroxyurea (4.8%), or switching off chronic transfusion therapy to hydroxyurea (4.8%). For the subset of patients who experienced changes in their SCD treatments, a majority had stable pain frequency from baseline to follow-up and it is possible that treatment changes may have diminished worsening symptoms or functioning. Notably, only 2 (4.8%) patients who had changes to their SCD treatments (either discontinued chronic transfusion or started hydroxyurea) demonstrated a change in their pain frequency group from asymptomatic to episodic over time. Further study is required to clarify what additional multimodal treatments are pursued (e.g., physical therapy, integrative therapies) as well as adherence to medications and treatments that may contribute to patterns of change and stability within the context of disease progression over time.
Our second objective was to better understand the possible individual and psychosocial factors associated with the transition from episodic to chronic pain as well as maintenance of chronic pain at follow-up. Although identification of risk factors of the acute to chronic pain transition in SCD remains limited, results from this study found that n=5 (12%) children and adolescents from this clinical cohort developed chronic pain over the course of 2 years. Although it is difficult to draw conclusions from a select few cases, it is important to note that patients who developed chronic pain ranged in age (12–17), offering some preliminary indication of the development of chronic pain across the developmental spectrum rather than only focused on older adolescents and adults. Additionally, changes in SCD treatments and disease progression may be possible contributors to the development of chronic SCD pain. For example, the development or progression of AVN, greater utilization of disease-modifying treatments and/or adjuvant medications may be plausible factors that could modify the development of chronic pain but have not yet been studied. A combination of biopsychosocial factors and disease progression could be considered as possible contributors to the onset of chronic SCD pain over time.
Several baseline psychosocial factors were associated with the maintenance of chronic pain at follow-up. Specifically, youth with higher functional disability, greater depressive symptoms and pain catastrophizing, reduced quality of life, and more inpatient admissions at baseline were more likely to report chronic pain at follow-up. Routine screening and monitoring of psychosocial functioning and patterns of healthcare utilization may help support early identification of patients who are at risk for developing chronic pain. Emerging research suggests that brief pain screening may help identify youth at increased vulnerability for developing chronic pain11,56,57, allowing opportunity to intervene earlier to improve pain management and disrupt the progression to chronic SCD pain.
Study findings should be considered within the context of some limitations. Given the study aims, the stratified sampling with focus on recurrent and chronic pain in combination with the modest sample size that completed both baseline and follow-up assessments limits the generalizability of findings to the general population of youth with SCD. Related, the number of baseline psychosocial constructs examined in predicting chronic pain at follow-up was restricted due to the sample size. A larger sample and greater statistical power would allow inclusion of additional variables to consider as potential risk factors for chronic pain or the acute to chronic pain transition. Patients who completed baseline and follow-up were significantly younger than those who only completed baseline, which likely reflects the subset of patients who transitioned to adult care between assessments. Future prospective longitudinal studies should make intentional effort to assess and follow participants into young adulthood to optimize long-term monitoring. These limitations may potentially underestimate the incidence rate of acute to chronic pain transition as chronic pain most commonly emerges in middle to late adolescence. Comparable to other longitudinal cohort studies with multiple assessment time points58, there was variability in the time period between assessments that occasionally ranged beyond 2 years that may have diminished potential time effects. Last, in the absence of standardized, validated measures to assess chronic SCD pain, chronic pain frequency was determined based on patient self-report and is subject to bias and random measurement error. Nonetheless, results provide valuable insights about the development and maintenance of chronic SCD pain that can be used to inform the development of larger, prospective longitudinal studies that improve the standardized assessment of chronic SCD pain and follow patients through young adulthood. The findings offer new and important clinical implications for the assessment and treatment of chronic pain in pediatric SCD. Strategies to support research engagement likely contributed to higher retention rates compared to other behavioral studies in SCD in which participation and retention rates range from 60–80%13,59. As the first observational longitudinal study on chronic SCD pain in youth, the use of the AAPT diagnostic criteria offers additional consensus towards identifying and characterizing chronic pain within the context of SCD.
Our findings may inform future directions to strengthen the state of chronic SCD pain research. Additional work is needed focused on prospective, longitudinal studies in pediatric SCD that integrate the biopsychosocial model of pain, including disease-related biological factors, disease severity including the development and progression of AVN, and psychosocial functioning to identify risk and protective factors associated with the transition from acute to chronic pain as well as characterize the natural history of chronic SCD pain, particularly from adolescence through young adulthood. The 12% preliminary incidence rate of the acute to chronic pain transition may help inform the sample sizes required to plan well-designed rigorous longitudinal studies. Studies focused on developing a standardized, validated assessment of chronic SCD pain may consider employing electronic daily diaries60 or ecological momentary assessments61 to examine patterns of variation between and within individuals over time. Routine screening focused on physical functioning, depressive symptoms, quality of life, and admission frequency may enhance early identification of patients who are at risk of developing chronic pain, which could theoretically help facilitate engagement and referrals for more robust multimodal pain interventions. Engagement in multidisciplinary treatment, including adjunctive medications, physical therapy, psychological therapies, and other non-pharmacological interventions is essential for chronic SCD pain management warranting further attention and support62.
Supplementary Material
Acknowledgements
The authors extend sincere thanks and appreciation to the children and families who participated in this research by sharing their time and experience. Special thanks are also given to the clinical research coordinators and assistants for their time and effort in supporting the study: Leann Schilling, Shelley Mays, Natasha Morris, Mitchell Turner, Bailey Sturdivant, and Anne Felder.
Disclosures: Funding for this work was supported by the National Institute of Health, National Center for Advancing Translational Sciences (NCATS) [grant number UL1TR000454] and the Emory and Children’s Pediatric Seed Grant Program. Preparation of this paper was supported by the National Heart, Lung, and Blood Institute (NHLBI) [grant number 1K23Hl133457-01A1] to Soumitri Sil, PhD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SS, LLC, AW, MB, FA have no conflicts of interest to report. NB has research funding from NHLBI. CD has research funding from Pfizer, Micelle BioPharma, Novartis, Merck, Katz Foundation, and NIH/NICHD/NCATS; he is a consultant for Pfizer, Novartis, Global Blood Therapeutics, Epizyme, Micelle BioPharma, Modus Therapeutics, Hilton Publishing Company, and Ironwood Pharmaceutics; and he is on the advisory board of Pfizer, Novartis, and Micelle BioPharma.
References
- 1.Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, Smith-Whitley K, Wang W. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatric blood & cancer. 2010;55(3):485–94. Epub 2010/07/27. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. Jama. 2010;303(13):1288–94. Epub 2010/04/08. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 3.Kauf TL, Coates TD, Huazhi L, Mody‐Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. American journal of hematology. 2009;84(6):323–7. [DOI] [PubMed] [Google Scholar]
- 4.Dampier C, Ely B, Brodecki D, O’Neal P. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002;3(6):461–70. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 5.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Annals of internal medicine. 2008;148(2):94–101. [DOI] [PubMed] [Google Scholar]
- 6.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT Diagnostic Criteria for Chronic Sickle Cell Disease Pain. J Pain. 2017. Epub 2017/01/10. doi: 10.1016/j.jpain.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Sil S, Cohen LL, Dampier C. Psychosocial and Functional Outcomes in Youth With Chronic Sickle Cell Pain. Clin J Pain. 2016;32(6):527–33. Epub 2015/09/18. doi: 10.1097/ajp.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 8.Sil S, Dampier C, Cohen LL. Pediatric Sickle Cell Disease and Parent and Child Catastrophizing. J Pain. 2016;17(9):963–71. Epub 2016/06/07. doi: 10.1016/j.jpain.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein-Leever A, Cohen LL, Dampier C, Sil S. Parent pain catastrophizing predicts child depressive symptoms in youth with sickle cell disease. Pediatric blood & cancer. 2018;65(7):e27027 Epub 2018/03/08. doi: 10.1002/pbc.27027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig NN, Sil S, Khowaja MK, Cohen LL, Dampier C. Executive Functioning Mediates the Relationship Between Pain Coping and Quality of Life in Youth With Sickle Cell Disease. J Pediatr Psychol. 2018. Epub 2018/07/28. doi: 10.1093/jpepsy/jsy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sil S, Cohen LL, Dampier C. Pediatric pain screening identifies youth at risk of chronic pain in sickle cell disease. Pediatric blood & cancer. 2019;66(3):e27538. doi: doi: 10.1002/pbc.27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro BS, Dinges DF, Orne EC, Bauer N, Reilly LB, Whitehouse WG, Ohene-Frempong K, Orne MT. Home management of sickle cell-related pain in children and adolescents: natural history and impact on school attendance. Pain. 1995;61(1):139–44. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 13.Gil KM, Thompson RJ, Keith BR, Tota-Faucette M, Noll S, Kinney TR. Sickle Cell Disease Pain in Children and Adolescents: Change in Pain Frequency and Coping Strategies Over Time. Journal of Pediatric Psychology. 1993;18(5):621–37. doi: 10.1093/jpepsy/18.5.621. [DOI] [PubMed] [Google Scholar]
- 14.Gil KM, Porter L, Ready J, Workman E, Sedway J, Anthony KK. Pain in Children and Adolescents With Sickle Cell Disease: An Analysis of Daily Pain Diaries. Children’s Health Care. 2000;29(4):225–41. [Google Scholar]
- 15.Noll RB, Vannatta K, Koontz K, Kalinyak K, Bukowski WM, Davies WH. Peer Relationships and Emotional Well-Being of Youngesters with Sickle Cell Disease. Child Development. 1996;67(2):423. doi: 10.2307/1131824. [DOI] [PubMed] [Google Scholar]
- 16.Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. European journal of haematology. 2014;93(2):89–95. Epub 2014/04/17. doi: 10.1111/ejh.12340. [DOI] [PubMed] [Google Scholar]
- 17.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain. 2015;156(4):722–30. Epub 2015/01/30. doi: 10.1097/j.pain.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–83. Epub 2011/06/29. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Incledon E, O’Connor M, Giallo R, Chalkiadis GA, Palermo TM. Child and Family Antecedents of Pain During the Transition to Adolescence: A Longitudinal Population-based Study. The Journal of Pain. doi: 10.1016/j.jpain.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Huguet A, Tougas ME, Hayden J, McGrath PJ, Chambers CT, Stinson JN, Wozney L. Systematic Review of Childhood and Adolescent Risk and Prognostic Factors for Recurrent Headaches. The Journal of Pain. 2016;17(8):855–73.e8. doi: 10.1016/j.jpain.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Dunn KM, Jordan KP, Mancl L, Drangsholt MT, Le Resche L. Trajectories of pain in adolescents: A prospective cohort study. PAIN®. 2011;152(1):66–73. doi: 10.1016/j.pain.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain. 2008;138(1):11–21. Epub 2007/12/21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 23.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. PAIN. 2011;152(12):2729–38. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Levenson JL, McClish DK, Dahman BA, Bovbjerg VE, de ACV, Penberthy LT, Aisiku IP, Roberts JD, Roseff SD, Smith WR. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosomatic medicine. 2008;70(2):192–6. Epub 2007/12/26. doi: 10.1097/PSY.0b013e31815ff5c5. [DOI] [PubMed] [Google Scholar]
- 25.Sogutlu A, Levenson JL, McClish DK, Rosef SD, Smith WR. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: the PiSCES project. Psychosomatics. 2011;52(3):272–9. Epub 2011/05/14. doi: 10.1016/j.psym.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Sil S, Cohen LL, Dampier C. Pediatric pain screening identifies youth at risk of chronic pain in sickle cell disease. Pediatric blood & cancer. 2018:e27538 Epub 2018/11/06. doi: 10.1002/pbc.27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zempsky WT, O’Hara EA, Santanelli JP, Palermo TM, New T, Smith-Whitley K, Casella JF. Validation of the Sickle Cell Disease Pain Burden Interview–Youth. The Journal of Pain. 2013;14(9):975–82. doi: 10.1016/j.jpain.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panepinto JA, Torres S, Bendo CB, McCavit TL, Dinu B, Sherman-Bien S, Bemrich-Stolz C, Varni JW. PedsQL sickle cell disease module: feasibility, reliability, and validity. Pediatric blood & cancer. 2013;60(8):1338–44. Epub 2013/02/27. doi: 10.1002/pbc.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Field JJ, Ballas SK, Campbell CM, Crosby LE, Dampier C, Darbari DS, McClish DK, Smith WR, Zempsky WT. Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks-American Pain Society-American Academy of Pain Medicine Pain Taxonomy Diagnostic Criteria for Acute Sickle Cell Disease Pain. J Pain. 2018. Epub 2018/12/24. doi: 10.1016/j.jpain.2018.12.003. [DOI] [Google Scholar]
- 30.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16(1):39–58. [DOI] [PubMed] [Google Scholar]
- 31.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory (FDI) among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121(1–2):77–84. Epub 2006/02/17. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9(9):771–83. [DOI] [PubMed] [Google Scholar]
- 34.Robinson MR, Daniel LC, O’Hara EA, Szabo MM, Barakat LP. Insurance status as a sociodemographic risk factor for functional outcomes and health-related quality of life among youth with sickle cell disease. J Pediatr Hematol Oncol. 2014;36(1):51–6. Epub 2013/10/19. doi: 10.1097/mph.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palermo TM, Witherspoon D, Valenzuela D, Drotar DD. Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain. 2004;109(3):461–70. Epub 2004/05/26. doi: 10.1016/j.pain.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs M Children’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems Inc.; 1992. [Google Scholar]
- 37.Conte P, Walco G, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis and Rheumatism. 2003;48(10):2923–30. [DOI] [PubMed] [Google Scholar]
- 38.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108(3):221–9. Epub 2004/03/20. doi: 10.1016/j.pain.2003.11.008S0304395903004585 [pii]. [DOI] [PubMed] [Google Scholar]
- 39.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clinical Journal of Pain. 2001;17(4):341–9. [DOI] [PubMed] [Google Scholar]
- 40.Logan DE, Claar RL, Guite JW, Kashikar-Zuck S, Lynch-Jordan A, Palermo TM, Wilson AC, Zhou C. Factor Structure of the Children’s Depression Inventory in a Multisite Sample of Children and Adolescents With Chronic Pain. The Journal of Pain. 2013;14(7):689–98. doi: 10.1016/j.jpain.2013.01.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehlo MG, Kamfar HZ. Depression and quality of life in children with sickle cell disease: the effect of social support. BMC psychiatry. 2015;15:78 Epub 2015/04/17. doi: 10.1186/s12888-015-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gartstein MA, Short AD, Vannatta K, Noll RB. Psychosocial adjustment of children with chronic illness: an evaluation of three models. Journal of developmental and behavioral pediatrics : JDBP. 1999;20(3):157–63. Epub 1999/07/07. doi: 10.1097/00004703-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): A preliminary validation. PAIN. 2006;123(3):254–63. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 44.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104(3):639–46. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 45.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: Proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. PAIN®. 2014;155(11):2360–7. doi: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995;7(4):524. doi: Doi 10.1037//1040-3590.7.4.524. [DOI] [Google Scholar]
- 47.Connolly ME, Bills SE, Hardy SJ. Neurocognitive and psychological effects of persistent pain in pediatric sickle cell disease. Pediatric blood & cancer. 2019;66(9):e27823 Epub 2019/05/28. doi: 10.1002/pbc.27823. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell SL, Schlenz AM, Kanter J. Health-related Quality of Life in Children With Sickle Cell Disease Undergoing Chronic Red Cell Transfusion Therapy. J Pediatr Hematol Oncol. 2019;41(4):307–12. Epub 2019/04/27. doi: 10.1097/mph.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 49.Hardy SJ, Bills SE, Wise SM, Hardy KK. Cognitive Abilities Moderate the Effect of Disease Severity on Health-Related Quality of Life in Pediatric Sickle Cell Disease. J Pediatr Psychol. 2018;43(8):882–94. Epub 2018/04/17. doi: 10.1093/jpepsy/jsy019. [DOI] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 51.Seltman H Mixed Models Experimental Design and Analysis 2018. p. 357–78. [Google Scholar]
- 52.Bolger N, Laurenceau J-P. Intensive longitudinal methods: An introduction to diary and experience sampling research. Little TD, editor. New York: The Guilford Press; 2013. [Google Scholar]
- 53.Tabachnick BG, Fidell LS. Using Multivariate Statistics. Boston, MA: Pearson Education, Limited; 2012. [Google Scholar]
- 54.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and Functional Disability in Chronic Pediatric Pain. The Clinical Journal of Pain. 2001;17(4):341–9. [DOI] [PubMed] [Google Scholar]
- 55.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. Journal of Developmental & Behavioral Pediatrics. 2000;21(1):58–69. [DOI] [PubMed] [Google Scholar]
- 56.Heathcote LC, Rabner J, Lebel A, Hernandez JM, Simons LE. Rapid Screening of Risk in Pediatric Headache: Application of the Pediatric Pain Screening Tool. J Pediatr Psychol. 2018;43(3):243–51. Epub 2017/10/20. doi: 10.1093/jpepsy/jsx123. [DOI] [PubMed] [Google Scholar]
- 57.Simons LE, Smith A, Ibagon C, Coakley R, Logan DE, Schechter N, Borsook D, Hill JC. Pediatric Pain Screening Tool: rapid identification of risk in youth with pain complaints. Pain. 2015;156(8):1511–8. Epub 2015/04/24. doi: 10.1097/j.pain.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reeve BB, Edwards LJ, Jaeger BC, Hinds PS, Dampier C, Gipson DS, Selewski DT, Troost JP, Thissen D, Barry V, Gross HE, DeWalt DA. Assessing responsiveness over time of the PROMIS((R)) pediatric symptom and function measures in cancer, nephrotic syndrome, and sickle cell disease. Qual Life Res. 2018;27(1):249–57. Epub 2017/09/09. doi: 10.1007/s11136-017-1697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barakat LP, Patterson CA, Weinberger BS, Simon K, Gonzalez ER, Dampier C. A prospective study of the role of coping and family functioning in health outcomes for adolescents with sickle cell disease. J Pediatr Hematol Oncol. 2007;29(11):752–60. Epub 2007/11/07. doi: 10.1097/MPH.0b013e318157fdac. [DOI] [PubMed] [Google Scholar]
- 60.Bakshi N, Smith ME, Ross D, Krishnamurti L. Novel Metrics in the Longitudinal Evaluation of Pain Data in Sickle Cell Disease. Clin J Pain. 2017;33(6):517–27. Epub 2016/09/02. doi: 10.1097/ajp.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 61.May M, Junghaenel DU, Ono M, Stone AA, Schneider S. Ecological Momentary Assessment Methodology in Chronic Pain Research: A Systematic Review. The journal of pain : official journal of the American Pain Society. 2018;19(7):699–716. Epub 2018/01/31. doi: 10.1016/j.jpain.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tick H, Nielsen A, Pelletier KR, Bonakdar R, Simmons S, Glick R, Ratner E, Lemmon RL, Wayne P, Zador V. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. Explore (New York, NY). 2018;14(3):177–211. Epub 2018/05/08. doi: 10.1016/j.explore.2018.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


