Abstract
The majority of pancreatic cancers are diagnosed at an advanced stage, when surgical options are limited and treatment relies on systemic chemotherapy. In the NAPOLI-1 trial, liposomal irinotecan in combination with fluorouracil (nal-iri/5FU) was shown to improve overall survival when compared to fluorouracil alone for metastatic pancreatic cancer. Other retrospective studies have shown the combination of fluorouracil and conventional irinotecan (FOLFIRI) to be a viable option, though no randomized trials have compared nal-iri/5FU to FOLFIRI. The purpose of this single-center, retrospective, cohort study was to determine if nal-iri/5FU and FOLFIRI are similarly effective for the treatment of advanced pancreatic cancer. Due to the potential for treatment bias, inverse probability of treatment weighting was utilized to correct for baseline differences between the groups. The primary outcome of progression-free survival was similar at 4.1 months for nal-iri/5FU and 3.1 months for FOLFIRI. Overall survival and adverse effect frequency were also similar. Pegfilgrastim was used in 16% and 15% of patients, respectively, and nal-iri/5FU patients required significantly less atropine during treatment (36 vs. 70%). A cost analysis was conducted and concluded that the treatment with nal-iri/5FU was nearly 30 times more expensive than FOLFIRI treatment. Together, these data suggest a potential role for FOLFIRI for the treatment of advanced pancreatic cancer in the absence of clear benefits in effectiveness, toxicity, or cost for nal-iri/5FU.
Keywords: Metastatic pancreatic adenocarcinoma, Nanoliposomal irinotecan, FOLFIRI, Cost-effective
Background
Pancreatic cancer is estimated to be the fourth leading cause of cancer-related death in the United States in 2018, despite being only the 11th most common cancer in terms of new diagnoses. Pancreatic cancer also carries one of the poorest prognoses of any cancer type, with a 5-year relative survival rate of just 8% [1]. Approximately 80–85% of pancreatic cancer patients are diagnosed at an advanced stage, when surgical options are limited and treatment relies primarily on systemic chemotherapy [2]. This underscores the poor prognosis and the need for effective therapies in this setting.
Several advances have been made in the systemic treatment of advanced pancreatic cancers within the past decade. Phase III clinical trials support the use of FOLFIRINOX (leucovorin, 5-fluorouracil, irinotecan, and oxaliplatin) or gemcitabine with nab-paclitaxel (gem/nabP) as first-line treatment for patients with metastatic pancreatic cancer due to improved survival compared to a single-agent gemcitabine [3, 4]. Nanoliposomal irinotecan (nal-iri) is a novel formulation of irinotecan that gained regulatory approval in the United States in 2015 for the treatment of metastatic pancreatic cancer in combination with 5-fluorouracil (5FU) and leucovorin after progression on gemcitabine-based therapy [5]. This approval was based on the results of the NAPOLI-1 trial, a multinational phase III study that demonstrated improved survival for patients treated with nal-iri/5FU as compared to those treated with 5FU alone [6].
Prior to nal-iri/5FU, there was no systemic therapy specifically approved beyond first-line treatment for advanced pancreatic cancer. Treatment recommendations were extrapolated from the activity of FOLFIRINOX and gem/nabP in the first line, and fluoropyrimidine doublets were commonly recommended after the failure of gemcitabine-based treatment. Different combinations of 5FU and oxaliplatin were explored in phase III studies, though with conflicting results as to whether the addition of oxaliplatin conferred a survival advantage [7, 8]. The combination of conventional irinotecan and 5FU (FOLFIRI) has also been evaluated as a second-line treatment for advanced pancreatic cancer. Two retrospective studies have shown FOLFIRI to be a viable option following gemcitabine-based treatment [9, 10]. A phase II study compared FOLFIRI to oxaliplatin and 5FU (FOLFOX) for second-line treatment and found similar overall survival between both regimens of about 4 months [11]. In another multicenter phase II study, treatment with second-line FOLFIRI achieved an overall response rate of 36% [12].
To date, no randomized clinical trials have compared nal-iri/5FU to FOLFIRI, so it is unknown whether one regimen is clinically superior to the other for advanced pancreatic cancer patients. Preclinical data demonstrate that nal-iri administration results in a more than fivefold higher intratumoral concentration of the active metabolite of irinotecan (SN-38) compared to serum levels [13]. However, the collective data suggest that FOLFIRI may achieve survival outcomes similar to the more expensive nal-iri/5FU regimen. It is also possible that the increased cost of nal-iri may be justified if proven to be unequivocally more effective than conventional irinotecan in this setting, or if nal-iri/5FU is comparatively less toxic. This retrospective, single-institution analysis was conducted to begin to address this gap in clinical knowledge.
Methods
Patients
This was a single-center, retrospective, cohort study of all consecutive patients aged 18–89 years, with locally advanced or metastatic pancreatic cancer treated with either nal-iri/5FU or FOLFIRI at The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute from October 2015 to August 2018. Patients were required to have previously received a gemcitabine-based therapy, either as the first-line treatment of unresectable or metastatic disease, or as neoadjuvant or adjuvant treatment if within the preceding 6 months. Incarcerated or pregnant patients were excluded from this study. The electronic medical record was utilized to collect data on patient demographics, Eastern Cooperative Oncology Group (ECOG) performance status, baseline CA 19–9 and albumin levels, M stage, prior cancer treatment, date of diagnosis, starting doses of nal-iri/5FU and FOLFIRI as well as any dose reductions or treatment delays, adverse events, date of disease progression, and survival. This study was approved by The Ohio State University Institutional Review Board with a waiver of consent documentation due to the retrospective nature of the study.
Treatment
Patients received either nal-iri/5FU or FOLFIRI on days 1 and 15 of a 28-day cycle. Nal-iri/5FU consisted of liposomal irinotecan 70 mg/m2 administered over 90 min, followed by leucovorin 200–400 mg/m2 given as a 30-min infusion, and 5FU 2400 mg/m2 infused over 46 h. The FOLFIRI regimen was given as conventional irinotecan 180 mg/m2 infusion given over 90 min, followed by 5FU 2400 mg/m2 given over 46 h. At our institution, it is a common clinical practice to omit leucovorin and 5FU bolus when FOLFIRI is used in the palliative setting. Granulocyte-colony stimulating factor (G-CSF) was not routinely administered for primary prophylaxis of febrile neutropenia.
Outcomes
The primary outcome of this study was progression-free survival (PFS). Secondary outcomes included time to treatment failure (TTF), overall survival (OS), frequency of dose reductions or treatment delays, and frequency of adverse effects. All adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.03).
PFS was defined as the time from the first dose of chemotherapy to progression or death, while TTF was the time from the first dose of chemotherapy to the day of treatment discontinuation. For the purposes of recording dose reductions, omission of leucovorin and/or 5FU bolus was not considered.
Cost analysis
The cost for a course of treatment was estimated based on the observed TTF for each regimen, and included drug costs, administration costs, and adverse event costs similar to the method previously described by Goldstein and colleagues [14]. Drug costs were calculated using the unit cost of each drug from the 2019 average sales price by the Centers for Medicare and Medicaid services (CMS) [15]. Doses were calculated using a body surface area of 1.86 m2 based on the U.S. mean values [14, 16]. The fees for chemotherapy administration were based on Current Procedure Terminology (CPT) codes using methods previously described by Tumeh et al. [17]. The cost of adverse events was based on pegfilgrastim use and estimated by CMS average sales price [15]. Pegfilgrastim costs were based on the proportion of patients observed to receive primary prophylaxis (beginning with the first dose of chemotherapy) or secondary prophylaxis (beginning with the second dose of chemotherapy) for the duration of treatment.
Statistical analysis
Inverse probability of treatment weighting (IPTW) using propensity scores was used to account for potential differences in patient characteristics between the FOLFIRI group and the nal-iri/5FU group. Propensity scores were calculated by a logistic regression model with treatment regimen as the outcome, and patient characteristics and potential confounding variables (age, sex, BMI, insurance, prior treatment, number of prior systemic therapies, metastatic disease, site of metastatic disease, baseline CA, baseline albumin, and baseline ECOG) as the independent variables. Patient characteristics are presented for each group both with and without application of IPTW, along with standardized differences between groups for both methods to assess the balance achieved by IPTW. IPTW was applied to all analyses of outcomes. Kaplan–Meier curves were generated for each group for PFS, OS, and TTF outcomes. Median time to event and/or event estimates at select follow-up times are presented for each outcome. Frequency of discontinuation, dose reduction/delay, and toxicity are reported for each group. No formal statistical hypothesis tests were performed, since the study hypothesis is non-inferiority of FOLFIRI and the limited sample size did not provide adequate power for formal tests of non-inferiority.
Results
Patient characteristics
A total of 82 patients were screened for inclusion. Of these, 5 patients were excluded for not having received prior gemcitabine therapy and 2 patients did not receive study treatment. Of the remaining 75 patients, 35 received nal-iri/5FU and 40 received FOLFIRI.
Baseline characteristics between the groups prior to IPTW adjustment were well balanced with the exception of gender, site of metastases, prior treatment, and baseline ECOG, each having a standardized difference greater than 0.2 (Table 1). After IPTW weighting of the data, both treatment groups were balanced with regard to all baseline characteristics, and time to event analyses and safety data are reported using the IPTW-adjusted groups. Nearly all patients (88%) had metastatic disease, with 71% of those patients having hepatic involvement. More than half of patients in each group received at least two prior systemic therapies. Prior chemotherapy exposure included gemcitabine, fluoropyrimidines, and oxaliplatin. Approximately one-third of patients had previous exposure to irinotecan, which was nearly always in the neoadjuvant setting (7 of 10 nal-iri/5FU patients and 11 of 13 FOLFIRI patients).
Table 1.
Patient characteristics by treatment
| Variable | Unadjusted data |
Adjusted by IPTWa |
||||
|---|---|---|---|---|---|---|
| Nal-iri (n = 35) |
FOLFIRI (n = 40) |
Std. Diff.b |
Nal-iri (n = 35.4) |
FOLFIRI (n = 39.3) |
Std. Diff.b |
|
| Age, median (IQR) | 63 (60–71) | 64 (60–70) | 0.01 | 63 (58–71) | 64 (59–69) | 0.02 |
| Male | 21 (60) | 19 (48) | 0.25 | 18 (50) | 20 (51) | 0.01 |
| BMI | 0.04 | 0.03 | ||||
| > 25 kg/m2 | 20 (57) | 22 (55) | 18 (50) | 20 (51) | ||
| ≤ 25 kg/m2 | 15 (43) | 15 (38) | 15 (44) | 15 (39) | ||
| Insurance | 0.11 | 0.09 | ||||
| Government | 20 (57) | 25 (63) | 20 (46) | 24 (61) | ||
| Private | 15 (43) | 15 (38) | 15 (44) | 15 (39) | ||
| Prior treatment | ||||||
| Surgery | 11 (31) | 12 (30) | 0.03 | 12 (33) | 12 (32) | 0.04 |
| Radiation | 7 (20) | 12 (30) | 0.23 | 9 (24) | 9 (24) | 0.01 |
| Gemcitabine alone | 12 (34) | 16 (40) | 0.12 | 13 (36) | 15 (38) | 0.03 |
| Gemcitabine combo | 33 (94) | 33 (83) | 0.37 | 31 (88) | 35 (88) | 0.01 |
| Fluoropyrimidine | 13 (37) | 17 (43) | 0.11 | 14 (39) | 16 (41) | 0.04 |
| Irinotecan | 10 (29) | 13 (33) | 0.09 | 11 (31) | 13 (33) | 0.03 |
| Oxaliplatin | 10 (29) | 16 (40) | 0.24 | 12 (33) | 14 (35) | 0.04 |
| Other chemo | 4 (11) | 3 (8) | 0.13 | 4 (9) | 4 (10) | 0.04 |
| Number of prior systemic therapies | 0.06 | 0.06 | ||||
| 1 | 14 (40) | 17 (43) | 16 (44) | 17 (42) | ||
| 2 | 14 (40) | 15 (38) | 13 (37) | 14 (36) | ||
| ≥ 3 | 7 (20) | 8 (20) | 7 (19) | 8 (21) | ||
| Metastatic disease | 30 (86) | 33 (83) | 0.09 | 32 (89) | 34 (86) | 0.09 |
| Site of metastases | 0.3 | 0.13 | ||||
| Hepatic only | 11 (31) | 17 (43) | 14 (38) | 16 (40) | ||
| Extrahepatic | 8 (23) | 6 (15) | 6 (17) | 7 (18) | ||
| Both | 11 (31) | 10 (25) | 12 (34) | 11 (29) | ||
| Baseline CA 19–9 | 0.04 | 0.01 | ||||
| ≥ 1500 U/mL | 19 (54) | 21 (53) | 18 (50) | 20 (50) | ||
| < 1500 U/mL | 16 (46) | 19 (48) | 18 (50) | 20 (50) | ||
| Baseline albumin | 0.09 | 0.03 | ||||
| ≥ 3.5 g/dL | 23 (66) | 28 (70) | 25 (71) | 27 (69) | ||
| < 3.5 g/dL | 12 (34) | 21 (30) | 10 (29) | 12 (31) | ||
| Baseline ECOG | 0.32 | 0.04 | ||||
| 0 | 7 (20) | 5 (13) | 5 (15) | 6 (15) | ||
| 1 | 22 (63) | 31 (78) | 26 (73) | 29 (74) | ||
| 2 | 6 (17) | 4 (10) | 4 (12) | 4 (11) | ||
Data are presented as n (%) unless otherwise indicated
IPTW inverse probability of treatment weighting, Std. Diff. standardized difference, IQR interquartile range, BMI body mass index, CA 19–9 cancer antigen 19–9, ECOG Eastern Cooperative Oncology Group performance status score
Reported weighted counts are rounded to the nearest integer and percentages are calculated based on the unrounded weighted counts
Standardized difference is used to compare balance in measured variables. A standardized difference > 0.2 may indicate imbalance between groups
Treatment outcomes
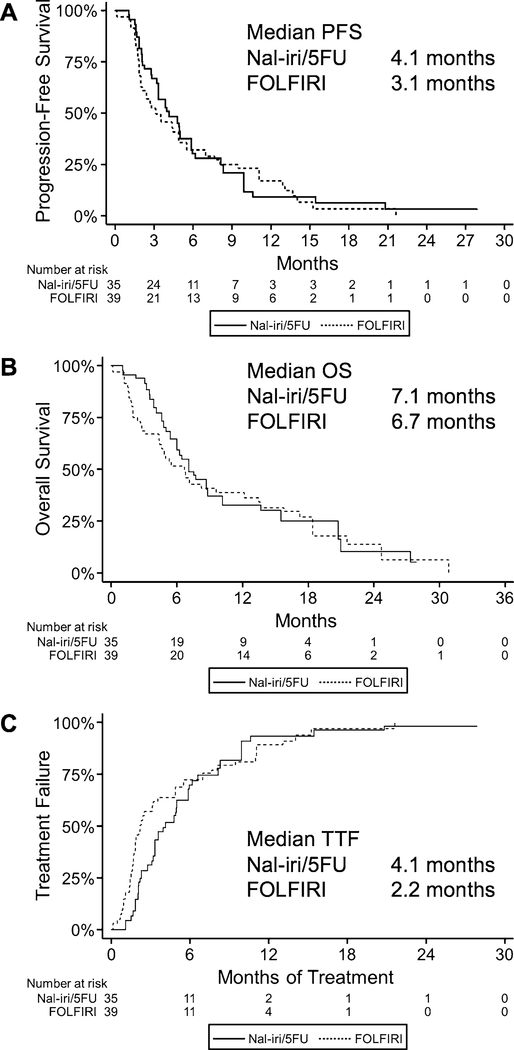
The primary outcome of PFS was similar between the two treatment groups (median 4.1 months for nal-iri/5FU vs. 3.1 months for FOLFIRI; Fig. 1a). OS was also comparable, with a median of 7.1 months and 6.7 months in the nal-iri/5FU and FOLFIRI groups, respectively (Fig. 1b). TTF was nearly 2 months longer in the nal-iri/5FU group (4.1 months vs. 2.2 months; Fig. 1c).
Fig. 1.
Survival outcomes of patients treated with nal-iri/5FU or FOLFIRI for advanced pancreatic cancer. Kaplan–Meier curves are shown with associated median estimates for progression-free survival (a), overall survival (b), and time to treatment failure (c)
Safety and tolerability
Patients discontinued treatment for a variety of reasons, including disease progression (63%), personal or physician preference (24%), and death (9%). More patients in the nal-iri/5FU group discontinued treatment for disease progression (75 vs. 53%), while FOLFIRI patients had more discontinuations for patient or physician preference (38 vs. 11%), which is consistent with the shorter TTF observed in that group (Table 2).
Table 2.
Safety outcomes
| Nal-iri (n = 35.4) |
FOLFIRI (n = 39.3) |
|
|---|---|---|
| Reason for discontinuationa | ||
| Disease progression | 26 (75) | 21 (53) |
| Toxicity | 0 (0) | 2 (4) |
| Patient preference | 2 (7) | 11(28) |
| Physician preference | 1 (4) | 4 (10) |
| Death | 5 (13) | 2 (5) |
| ≥ 1 treatment delay | 24 (66) | 14 (36) |
| Dose reductions | ||
| Initial dose reduction | 4 (12) | 14 (34) |
| ≥ 1 dose reduction | 14 (39) | 19 (48) |
| G-CSF use | 5 (16) | 6 (15) |
| Atropine | 13 (36) | 27 (70) |
IPTW-adjusted; reported weighted counts are rounded to the nearest integer, percentages are calculated based on the unrounded weighted counts
G-CSF granulocyte-colony stimulating factor
One patient in the nal-iri group continued on treatment at the time of data collection
The frequency of treatment delays and dose reductions was also different between the two groups. Two-thirds of patients in the nal-iri/5FU group had their treatment delayed as compared to only about one-third of FOLFIRI patients. In contrast, FOLFIRI patients were more frequently dosereduced (48 vs. 39%; Table 2). Initial dose reductions were more common in the FOLFIRI group (35 vs. 12%).
The use of G-CSF as prophylaxis for febrile neutropenia was similar between both groups (Table 2). No patients in this study received G-CSF de novo as primary prophylaxis for the evaluated treatments without having demonstrated a need for growth factor support during prior chemotherapy. All patients in the FOLFIRI group in whom G-CSF was used received pegfilgrastim as primary prophylaxis (15%) after demonstrating the need for G-CSF with prior chemotherapy regimens. In contrast, only 6% of patients in the nal-iri/5FU group received primary prophylaxis, while the remaining 10% of patients received pegfilgrastim as secondary prophylaxis after severe neutropenia. Atropine use for the management of acute diarrhea was different between groups with nearly twice as many patients in the FOLFIRI group requiring atropine as compared to the nal-iri/5FU group (70% vs. 36%; Table 2).
The most frequently observed adverse events in either group included anemia, fatigue, and pain (Table 3). Several toxicities were more frequent in the nal-iri/5FU group, including anorexia, diarrhea, and mucositis, though neutropenia and thrombocytopenia were more common for FOLFIRI patients. There were no distinct differences in the frequency of grade 3 or 4 toxicity for patients treated with nal-iri/5FU or FOLFIRI, with the exception of numerically more patients having grade 3/4 fatigue in the FOLFIRI group (9% vs. 4%), while poor performance status (ECOG ≥ 3) was more common in the nal-iri/5FU group (12 vs. 5%; Table 3).
Table 3.
Adverse events
| Eventa | Nal-iri (n = 35.4) |
FOLFIRI (n = 39.3) |
||
|---|---|---|---|---|
| Any grade |
Grade 3/4 |
Any grade |
Grade 3/4 |
|
| Neutropenia | 8 (23) | 5 (13) | 15 (37) | 3 (9) |
| Thrombocytopenia | 12 (35) | 1 (2) | 20 (51) | 2 (6) |
| Anemia | 35 (100) | 4 (12) | 37 (95) | 5 (13) |
| Fatigue | 35 (100) | 1 (4) | 36 (93) | 4 (9) |
| Nausea/vomiting | 28 (80) | 0 (0) | 31 (78) | 1 (2) |
| Anorexia | 30 (84) | 0 (0) | 27 (69) | 1 (2) |
| Diarrhea | 27 (76) | 1 (3) | 24 (60) | 0 (0) |
| Mucositis | 18 (50) | 1 (3) | 12 (30) | 0 (0) |
| Pain | 30 (83) | 0 (0) | 32 (81) | 2 (6) |
| HFS | 7 (20) | 1 (2) | 9 (23) | 0 (0) |
| ECOG ≥ 3 | 4 (12) | 2 (5) | ||
IPTW-adjusted; reported weighted counts are rounded to the nearest integer, percentages are calculated based on the unrounded weighted counts
HFS hand-foot syndrome, ECOG Eastern Cooperative Oncology Group performance status score
Based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE, version 4.03)
Cost analysis
The monthly costs of treatment were estimated based on the calculated drug costs, administration costs, and the cost of management for key adverse effects. The monthly cost of drugs for nal-iri/5FU was $12,039 and $123 for FOLFIRI. Monthly administration costs were also higher for nal-iri/5FU as compared to FOLFIRI ($632 vs. $487). The cost of pegfilgrastim was similar between both groups ($215 vs. $212). The total cost of a course of treatment was estimated using the observed median time to treatment failure. Based on these estimates, nal-iri/5FU had a final cost of $52,834 for a time to treatment failure of 4.1 months, while FOLFIRI treatment totaled $1809 over a period of 2.2 months.
Discussion
This single-center retrospective study showed that both nal-iri/5FU and FOLFIRI treatment lead to similar survival outcomes in patients with advanced pancreatic cancer. Both median PFS and OS were similar between the two groups. Visual inspection of the time-to-event curves did not demonstrate a clear survival advantage of nal-iri/5FU compared to FOLFIRI. In addition, patients experienced adverse effects with a similar frequency and severity between the two treatment groups.
Survival outcomes in this study were similar to those published in the past trials. The phase 3 NAPOLI-1 trial showed PFS for patients treated with nal-iri/5FU to be 3.1 months and OS 6.2 months [18]. More recently, a retrospective real-world experience was published by Glassman and colleagues that reported PFS of 2.9 months [19]. FOLFIRI lacks phase 3 data in this setting, though retrospective and prospective studies can provide estimates of survival outcomes. Zaniboni and colleagues conducted a phase 2 trial of FOLFIRI in advanced pancreatic cancer with PFS of 3.3 months [12]. A retrospective study further supports this finding with a PFS of 3.7 months [9]. The observed PFS of 4.1 months and 3.1 months in this study for nal-iri/5FU and FOLFIRI, respectively, are in line with the outcomes reported in previous trials. Overall survival is likewise similar between this study and previously published results. Furthermore, the head-to-head comparison in this study of a similar patient population treated during the same period of time at the same institution supports the interpretation of similar survival outcomes between these two treatment regimens.
The majority of nal-iri/5FU patients discontinued treatment for disease progression or death. While a majority of FOLFIRI patients also discontinued treatment for disease progression, a larger proportion discontinued for patient or physician preference than in the nal-iri/5FU group. This likely contributed to a shorter median time to treatment failure in the FOLFIRI group (2.2 vs. 4.1 months). Despite discontinuing treatment earlier, however, median PFS was only slightly shorter and OS about the same for the FOLFIRI as compared to nal-iri/5FU.
Dose modifications were approached differently depending on treatment regimen. Two-thirds of nal-iri/5FU patients had at least one treatment delay, whereas only about one-third of FOLFIRI patients were delayed. This contrasts with one-third of FOLFIRI patients who initiated treatment with a reduced dose, compared to 12% of nal-iri/5FU patients. This study was not designed to evaluate this difference in approach to toxicity management, but potential explanations are physician familiarity with each regimen and prior exposure to irinotecan for individual patients. FOLFIRI is commonly used in gastrointestinal malignancies and oncologists may be more comfortable making empiric dose adjustments based on patient performance status, ability to tolerate prior chemotherapy, or toxicities seen during treatment. In contrast, nal-iri/5FU is a newer regimen and oncologists may prefer to forego initial dose reductions, opting instead to delay treatment when toxicities arise.
Chemotherapy dose intensity is traditionally expected to correlate with successful outcomes. The pre-emptive dose reductions seen in this study represent real-world oncology practices, and compared to prior data this does not seem to have produced significantly poorer outcomes than those seen in clinical trials. Specifically, Glassman and colleagues noted a median starting dose of 55 mg/m2 for nal-iri in their real-world data study, which was not associated with significantly inferior outcomes [19]. Other studies have shown a similar maintenance of effectiveness for different regimens used in advanced pancreatic cancer despite reductions in dose intensity. For example, Ahn and colleagues reported promising results for gem/nabP administered every other week as opposed to weekly for 3 out of 4 weeks leading to a 33% decrease in dose intensity [20]. Collectively, these data support the continued exploration of dosing strategies that balance reduced intensity and toxicity without compromising outcomes.
The decision to select one treatment over another involves consideration of several factors, particularly efficacy and safety, and secondarily may include cost or convenience. The evidence presented by this study, and by past trials, suggests that nal-iri/5FU and FOLFIRI have little difference in survival outcomes. Additionally, our data neither support a meaningful difference in tolerability between regimens, nor a difference in utilization of G-CSF for supportive care. The one appreciable difference noted in this population was atropine use for the management or prevention of acute diarrhea, which was used in twice as many patients who received FOLFIRI. This difference is not unexpected, given the liposomal encapsulation of nal-iri prevents the parent irinotecan compound from inhibiting acetylcholinesterase.
The cost analysis in this study, which took into account drug costs, administration costs, and the costs of pegfilgrastim use, showed nal-iri/5FU treatment is considerably more expensive than FOLFIRI. The United Kingdom’s National Institute for Health and Care Excellence (NICE) recently concluded the incremental cost-effectiveness ratio per quality-adjusted life year gained for nal-iri/5FU when compared to FOLFOX far exceeded the £50,000 threshold for cost-effectiveness. NICE therefore did not recommend nal-iri/5FU for the treatment of metastatic pancreatic cancer [21]. The consideration of efficacy, toxicity, and cost clearly supports the consideration of FOLFIRI in place of nal-iri/5FU for the treatment of advanced pancreatic cancer, though further studies are needed to conclusively determine the role of each regimen.
Several limitations merit consideration with regard to this study. This study was a retrospective analysis and therefore includes limitations inherent to that design. Specifically, this study was reliant on the accurate documentation for the reporting of adverse effects, and there was a lack of consistency with regard to toxicity management and dose reductions or treatment delays. In addition, there is a possibility of treatment selection bias, though this was anticipated and IPTW adjustments were utilized to correct for baseline differences between groups. Finally, the small sample size prevented formal non-inferiority hypothesis testing due to inadequate power.
In conclusion, this study found similar survival and toxicity outcomes for advanced pancreatic cancer patients treated with either nal-iri/5FU or FOLFIRI. Future work is needed to confirm the results of this study with an adequately powered trial testing non-inferiority. These data, in combination with the cost analysis, support the consideration of FOLFIRI for the treatment of advanced pancreatic cancer following a gemcitabine-based therapy, especially when financial toxicity may be of particular concern.
Footnotes
Compliance with ethical standards
Conflict of interest Anne Noonan served as a paid member of the Data Safety Monitoring Board for Helsinn in 2015–2016 and a paid advisory board member for QED Therapeutics and Exelixis in 2019. No other authors have any potential conflicts to declare.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–1. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onivyde (irinotecan liposome) [package insert]. Basking Ridge: Ipsen Biopharmaceuticals, Inc.; 2017. [Google Scholar]
- 6.Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57. [DOI] [PubMed] [Google Scholar]
- 7.Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy-Thind S, Zulfiqar M, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol. 2016;34(32):3914–20. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32(23):2423–9. [DOI] [PubMed] [Google Scholar]
- 9.Gebbia V, Maiello E, Giuliani F, Borsellino N, Arcara C, Colucci G. Irinotecan plus bolus/infusional 5-Fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale. Am J Clin Oncol. 2010;33(5):461–4. [DOI] [PubMed] [Google Scholar]
- 10.Neuzillet C, Hentic O, Rousseau B, Rebours V, Bengrine-Lefevre L, Bonnetain F, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol. 2012;18(33):4533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo C, Hwang JY, Kim JE, Kim TW, Lee JS, Park DH, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101(10):1658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaniboni A, Aitini E, Barni S, Ferrari D, Cascinu S, Catalano V, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol. 2012;69(6):1641–5. [DOI] [PubMed] [Google Scholar]
- 13.Kalra AV, Kim J, Klinz SG, Paz N, Cain J, Drummond DC, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74(23):7003–13. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DA, Krishna K, Flowers CR, El-Rayes BF, Bekaii-Saab T, Noonan AM. Cost description of chemotherapy regimens for the treatment of metastatic pancreas cancer. Med Oncol. 2016;33(5):48. [DOI] [PubMed] [Google Scholar]
- 15.ASP drug pricing files. Centers for Medicare and Medicaid Services. https://www.cms.gov/Medicare/Medicare-Fee-for-ServicePart-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html Accessed 10 Apr 2019.
- 16.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. Natl Health Stat Rep. 2018;122:1–16. [PubMed] [Google Scholar]
- 17.Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2005;5(2):153–62. [DOI] [PubMed] [Google Scholar]
- 18.Wang-Gillam A, Hubner RA, Siveke JT, Von Hoff DD, Belanger B, de Jong FA, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur J Cancer. 2019;108:78–87. [DOI] [PubMed] [Google Scholar]
- 19.Glassman DC, Palmaira RL, Covington CM, Desai AM, Ku GY, Li J, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer. 2018;18(1):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn DH, Krishna K, Blazer M, Reardon J, Wei L, Wu C, et al. A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Ther Adv Med Oncol. 2017;9(2):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleeman N, Abdulla A, Bagust A, Beale S, Richardson M, Stainthorpe A, et al. Pegylated liposomal irinotecan hydrochloride trihydrate for treating pancreatic cancer after gemcitabine: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(3):289–99. [DOI] [PubMed] [Google Scholar]