Abstract
Opioid overdoses, many of which are attributed to use of illicit fentanyl, are currently one of the leading causes of death in the U.S. Although fentanyl has been used safely for decades in clinical settings, the widespread use of illicit fentanyl is a recent phenomenon. Starting in 2013, illicitly manufactured fentanyl and its analogs began to appear on the streets. These substances were added to or sold as heroin, often unbeknownst to the user. Because fentanyl is so potent, only small amounts are needed to produce pharmacological effects, but the margin between safe and toxic doses is narrow. Surprisingly little is known about the exact signaling mechanisms underlying fentanyl-related respiratory depression or the effectiveness of naloxone in reversing this effect. Similarly, little is known about the ability of treatment medications such as buprenorphine, methadone, or naltrexone to reduce illicit fentanyl use. The present article reviews the receptor, preclinical and clinical pharmacology of fentanyl, and how its pharmacology may predict the effectiveness of currently approved medications for treating illicit fentanyl use.
Keywords: Fentanyl, Heroin, Illicit, Pharmacology, Beta-arrestin, Abuse, Efficacy, Subjective effects, Self-administration, Pain, Methadone, Naltrexone, Buprenorphine, Treatment
1. Introduction: development of fentanyl
Paul Janssen synthesized fentanyl in 1960 with the rationale that synthesis of a highly potent drug with increased receptor specificity would exhibit a greater safety profile compared to morphine (Stanley, 1992; 2008). It was approved initially in the United States only as a combination medication with droperidol because of concerns about its extreme potency and greater propensity to produce muscle rigidity compared to other opioids. Despite these early concerns, the ability of fentanyl to provide cardiovascular stability and to block the stress response to surgical stimuli at high doses made it the mainstay of cardiac anesthesia. The clinical use of fentanyl was restricted to anesthesia until the 1990s when the development of non-injectable formulations was pursued. Today, numerous fentanyl-alone products are approved for use in the U.S. including oral transmucosal lozenges, effervescent buccal tablets, sublingual tablets, sublingual sprays, nasal sprays, transdermal patches, and injectable formulations. These products are used as anesthetic agents in surgical settings, treatments for chronic pain, and supplemental medications for breakthrough pain in patients with cancer (DEA, 2016). A number of other medications with chemical structures similar to fentanyl have been synthesized (e.g., sufentanil, alfentanil, and carfentanil), which are restricted for use in clinical anesthesia or more uncommonly used as nerve blocks, or, in the case of carfentanil, as a radiotracer in research studies using positron emission tomography (PET). These medications are substantially more potent than morphine: for example, carfentanil is 10,000 times more potent than morphine as an analgesic.
2. Epidemiology of illicit fentanyl use
Despite the current widespread use of fentanyl in clinical settings, an additional concern that delayed its initial approval in the U.S. was its potential for abuse (Stanley et al., 2008). The U.S. Drug Enforcement Agency eventually placed fentanyl, as well as the other fentanyl-like medications including sufentanil, alfentanil, and carfentanil into Schedule II of the Controlled Substances Act because it was believed that they had high potential for abuse. For decades after approval of fentanyl, however, reports of its abuse were low compared to other prescription opioid products, such as oxycodone and hydrocodone (Cicero et al., 2005; Katz et al., 2008). Most of the early reports suggested that fentanyl was being abused by healthcare professionals, such as anesthesiologists, who had easy access to it (e.g., Knisely et al., 2002; Silsby et al., 1984; Ward et al., 1983). Although later reports described non-medical use of the fentanyl transdermal patch by patients and/or individuals with substance use disorders (Jumbelic, 2010), overall prevalence rates of non-medical use of FDA-approved fentanyl products remained low. But in 2006, a surge in fentanyl-related overdose deaths and Drug Enforcement Agency (DEA) seizures of illicitly manufactured fentanyl occurred in the U.S. This “crisis” was attributed to fentanyl being mixed into heroin (Drug Enforcement Administration, 2016). The origin of this crisis was traced to a single clandestine laboratory that was manufacturing fentanyl illicitly, and when the laboratory was shut down, fentanyl overdose deaths and DEA seizures of fentanyl rapidly declined.
In the current fentanyl crisis, multiple clandestine laboratories around the world are manufacturing illicit fentanyl as well as a number of other compounds with similar chemical structures that until very recently would have eluded DEA scheduling but now is covered under a derivative law to prevent evasion of prosecution (Pichini et al., 2018). Beginning in 2013, a dramatic increase in fentanyl seizures occurred in the U.S.A. and by 2015, the number of fentanyl seizures was approximately 8 times higher than in 2006 (DEA Intelligence Brief, 2006). Synthesis of fentanyl is relatively straightforward compared to heroin, and because it is so potent, fentanyl is easy to conceal and transport for sale, so the risks to drug dealers of detection and arrest are reduced. It is purchased by dealers at low cost and added to heroin without the user’s knowledge, which results in enormous profits for the dealer. In addition to being used as an adulterant to heroin, fentanyl is being sold in pill form as counterfeit Norco®, a prescription pain medication containing hydrocodone and acetaminophen (DEA Intelligence Brief DEA-DCT-DIB-021-16, 2016), or CDN 80, which is meant to mimic a prescription pain medication containing oxycodone that is sold in Canada (European Monitoring Centre for Drugs and Drug Addiction, 2017). Of equal or greater concern is that fentanyl is being added to cocaine and sold as counterfeit Xanax® pills (a short-acting benzodiazepine anxiolytic used to treat anxiety disorders; DEA Intelligence Brief DEA-DCT-DIB-021-16, 2016). Because users of these substances typically have little or no tolerance to opioids, the risk of overdose may be higher. The tremendous rise in availability of illicit fentanyl has been associated with a rise in overdose deaths. More than 63,000 Americans died of drug overdoses in 2016, over 19,000 of which were related to synthetic opioids such as fentanyl and its analogs (https://www.cdc.gov/drugoverdose/data/statedeaths.html and https://www.cdc.gov/drugoverdose/data/fentanyl.html; accessed October 15, 2018). The concern is that the numbers of overdoses and deaths due to fentanyl will continue to increase in the coming years. Despite these alarming trends, relatively little is known about the exact signaling mechanisms that contribute to fentanyl-related overdose and death, and how effective current FDA-approved treatment medications for opioid use disorder may be against fentanyl. Subsequent sections of this review will describe the receptor pharmacology of fentanyl, the preclinical data on its abuse liability, the clinical pharmacology of fentanyl as it relates to abuse liability, and their implications for treatment of fentanyl abuse.
3. Fentanyl pharmacology
Like most clinically used opioids, fentanyl produces its pharmacological effects via activation of the mu opioid receptor (MOR) with low affinity for delta and kappa opioid receptors. Fentanyl is a synthetic, lipophilic phenylpiperidine opioid agonist, unlike morphine, which is an alkaloid extracted from the opium poppy. Fentanyl is a highly efficacious agonist at the MOR with a 1.35 nM binding affinity (Ki) at recombinant human MORs (Volpe et al., 2011), an affinity similar to that reported using guinea pig membranes (1.2 nM Ki; Maguire et al., 1992). Albeit, a wide range of fentanyl binding affinities for the MOR have been reported (Ki = 0.007 (Chen et al., 1993) to > 200 nM (Traynor and Nahorski, 1995)), which most likely reflects differences in the radioligand, species, assay, or tissue used. This affinity is very similar to morphine binding at the MOR (Ki = 1.17 nM). Additionally, the elimination/clearance half-life is similar between fentanyl and morphine with t1/2 of 2–4 h for fentanyl and 2 h for morphine. This may be surprising, considering that fentanyl has a faster onset, much shorter duration of analgesic action, and higher analgesic potency compared to morphine. Human and preclinical studies show that fentanyl is 50 times (intramuscular), 150 times (subcutaneous), ~400 times (intravenous) or 10 times (epidural) more potent than morphine (Finch and DeKornfeld, 1967; Terenius, 1974; van den Hoogen and Colpaert, 1987), but most physicians accept and conversion charts report that fentanyl is approximately 100 times more potent than morphine. Additionally, fentanyl rapidly crosses the blood-brain barrier, resulting in greater analgesic potency, which is reflected in a half-life of ~5 min for equilibrium between plasma and cerebrospinal fluid. Thus, the greater analgesic potency and faster onset of fentanyl compared to morphine is not explained by binding affinity or half-life. Fentanyl levels rapidly decline due to redistribution to other tissues and fentanyl has rapid sequestration into body fat, contributing to its short duration of action. The difference in potency and onset and duration of action is, in part, attributed to the differential lipophilicity of these drugs. Of the clinically available MOR agonists, fentanyl and sufentanil are the most lipid soluble, whereas morphine is more hydrophilic. Using a classical octanol-water partition coefficient to measure lipid solubility, the co-efficient for morphine is 6 but > 700 for fentanyl (Lötsch et al., 2013). The difference in lipid solubility impacts not only the route of administration for clinical use but also the pharmacokinetics of metabolism and elimination. Additionally, the pharmacokinetic properties of fentanyl allowed for the development of unique clinical indications of non-injectable formulations ranging from treatment of cancer breakthrough pain using nasal formulations with direct access to the brain to transdermal release for treating chronic pain.
Fentanyl is poorly absorbed from the gastrointestinal tract but is exclusively metabolized where renal excretion accounts for less than 10% of the dose. Metabolism by piperidine N-dealkylation to norfentanyl, an inactive metabolite, is the predominant degradative pathway in humans, accounting for 99% of fentanyl metabolism (Labroo et al., 1997). Fentanyl metabolism is mediated almost exclusively by cytochrome P450 CYP3A4, together with CYP3A5 and CYP3A7 (Labroo et al., 1997). The involvement of CYP3A-dependent metabolism accounts for many adverse drug interactions, including the HIV protease inhibitor ritonavir (Olkkola et al., 1999). Ritonavir and the calcium channel blocker diltiazem have been reported to increase plasma levels and reduce elimination of fentanyl. Conversely, fentanyl can act as an enzyme inhibitor and reduce the clearance of sedative drugs such as midazolam. The short duration of action is in part due to the activity of P-glycoproteins within the blood-brain barrier that pumps fentanyl out of the central nervous system (CNS) (Wandel et al., 2002; Ziesenitz and van den Anker, 2013). The importance of these proteins is evident in that loperamide, an opioid used for treatment of diarrhea has negligible CNS effects, but this peripheral restriction is solely due to its high affinity for the P-glycoprotein substrate (Schinkel et al., 1996); loperamide produces CNS effects in P-glycoprotein knockout rodents (Tatke et al., 2018). Genetic polymorphisms in the ABCB1 gene that encodes for the P-glycoproteins (ABCB1 1236 TT (rs1128503), 2677 TT (rs2032582) and 3435TT (rs1045642)) causes CNS retention of fentanyl (Lötsch et al., 2013), resulting in adverse effects such as respiratory depression and sedation (Kesimci et al., 2012; Takashina et al., 2012). The ability of opioids to produce differential effects on nociception, respiratory depression, and constipation likely results from a combination of their chemistry, which will affect their distribution within the central nervous system, metabolism, receptor selectivity and receptor signaling.
4. Preclinical pharmacology of fentanyl
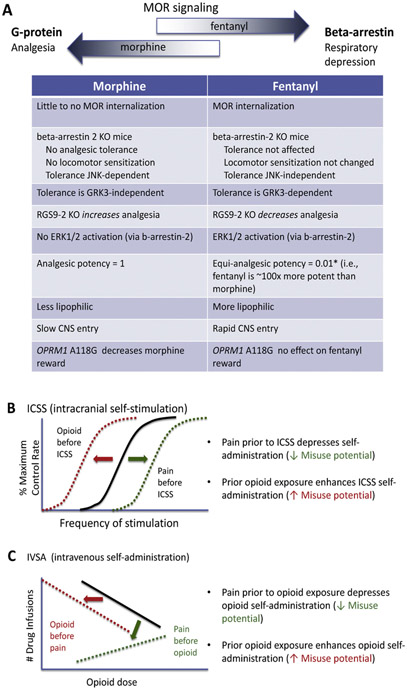
MOR belong to the superfamily of G-protein coupled receptors, a class of membrane-bound receptors that exhibit a seven transmembrane-spanning helical domain connected by intra- and extra-cellular loops. The MOR produces its effects via interactions with inhibitory heterotrimeric G-proteins (Gi/o), which are responsible for producing most opioid-related pharmacological effects, including analgesia and euphoria. However, MOR also produce G-protein-independent signaling through beta-arrestin complexes. A concerted effort is now underway to identify ligands with a bias towards G-protein signaling with less activation of beta-arrestins, as the arrestin signaling has been proposed to account for the life-threatening respiratory depressive effects of opioids (Fig. 1A, Groer et al., 2007; Manglik et al., 2016; Schmid et al., 2017; Schneider et al., 2016). A biased agonist is defined by the ability of agonist binding to the same receptor to differentially activate signaling cascades that results in the formation of different protein complexes that trigger different downstream cellular events. The beta-arrestin-2 knockout mouse is protected from morphine-induced respiratory depression and acute constipation (Raehal et al., 2005), although analgesic effects are enhanced by the absence of beta-arrestin 2 (Bohn et al., 1999). A spectrum of signaling bias for different opioid drugs was recently reviewed (Williams et al., 2013). Fentanyl exhibits signaling bias with greater arrestin relative to G-protein signaling, as measured by GTPγS binding in cells expressing mouse or human MORs (Schmid et al., 2017). This effect is evident in striatal neurons, where acute administration of fentanyl activates the mitogen-activated protein kinase (MAP kinase) ERK1/2 in a beta-arrestin-dependent manner, an effect that is absent following acute morphine (Macey et al., 2006), although ERK1/2 activation is produced following chronic morphine (Bilecki et al., 2005; Ligeza et al., 2008). In cell culture experiments, fentanyl promotes robust receptor phosphorylation, beta-arrestin-2 recruitment and receptor internalization but morphine has much weaker effects on these parameters. Perhaps surprising given the agonist bias of fentanyl for beta-arrestin signaling is that analgesic tolerance to fentanyl is not perturbed in beta-arrestin-2 knockout mice (Raehal and Bohn, 2011), whereas morphine tolerance is attenuated (Bohn et al., 2000). The above discussion specifically relates to differences between fentanyl and morphine, but comprehensive reviews of mechanisms underlying the development and maintenance of opioid tolerance have been published previously (Morgan and Christie, 2011; Williams et al., 2013).
Fig. 1.
A. Pharmacological differences between fentanyl and prototypical opioid agonist morphine. Morphine binds to mu opioid receptors (MOR) and primarily produces signaling through activation of G-proteins, whereas fentanyl also activates beta-arrestin pathways that leads to respiratory depression. The enhanced respiratory depression of fentanyl compared to morphine may be due to their differences in intracellular signaling cascades. *Please note that equianalgesic conversion is dependent on route of administration and species. Opioid exposure prior to intracranial self-stimulation (ICSS) (B) or intravenous self-administration (IVSA) (C) produces activity predictive of increasing abuse potential. In contrast, pain prior to ICSS (B) or IVSA (C) has the opposite effect, where abuse potential is reduced. Note, the reduced abuse potential with prior pain to opioid exposure may not apply to individuals with comorbidities of post-traumatic stress disorder, depression and anxiety or the presence of catastrophizing (Evans & Cahill, 2016).
Fentanyl is a highly efficacious MOR agonist that results in less analgesic tolerance than lower efficacy MOR agonists such as morphine (Williams et al., 2013), although greater analgesic tolerance to fentanyl was reported following chronic administration in models of chronic pain (Imai et al., 2006; Narita et al., 2013). Fentanyl produces short-term tolerance (measured using a phasic thermal tail flick test) through a G-protein receptor kinase-dependent (GRK3) mechanism, whereas morphine produces tolerance through a c-Jun N-terminal kinase-dependent mechanism and not GRK3 (Terman et al., 2004; Kuhar et al., 2015). Similarly, beta-arrestin-2-dependent JNK cascade signaling was responsible for morphine analgesic tolerance and locomotor sensitization but not that of fentanyl (Mittal et al., 2012). Many studies have demonstrated that fentanyl and morphine differ with regard to mechanisms of opioid tolerance and reinforcing effects. For example, RGS9-2, a regulator of G-protein signaling, binds to the activated Gα subunit of G-proteins, thereby controlling MOR signal transduction, desensitization, analgesic tolerance and physical dependence. Knockout of RGS9-2 protein decreases fentanyl- but increases morphine-induced acute analgesia (Psifogeorgou et al., 2011). Additionally, a MOR polymorphism OPRM1 A118 G (using a humanized mouse model) reduces the ability of morphine to potentiate intracranial self-administration (ICSS, a positively reinforced operant behavior in which lever-press responding is maintained by delivery of electrical brain stimulation and is a hallmark of abuse potential) of the medial forebrain bundle and depresses morphine-induced dopamine release (implicated in reward). However, this polymorphism has no effect on fentanyl-induced ICSS or dopamine release (Robinson et al., 2015). Another point mutation T394 A at the MOR T394 phosphorylation site decreased opioid analgesic tolerance but increased intravenous heroin self-administration and dopamine release in the nucleus accumbens, suggesting that this mutation may increase susceptibility to opioid abuse (Wang et al., 2016).
There still exists a great debate over the influence of pain on the abuse potential of opioid analgesics. In pain models, a depression of ICSS is thought to capture the affective dimension of pain (Negus, 2013). In contrast to a chronic neuropathic pain model, acute visceral pain induced by intraperitoneal injection of lactic acid depressed ICSS (Ewan and Martin, 2011b; Altarifi et al., 2015). Systemic injection of a high-efficacy agonist such as fentanyl was more potent at blocking the depression of ICSS caused by an acute pain stimulus (Altarifi et al., 2015). In a model of chronic neuropathic pain, fentanyl, methadone and hydromorphone were less potent in facilitating ICSS (when electrical stimulation was in the ventral tegmental area) compared to pain-naïve controls, which was interpreted to reflect diminished abuse potential of opioids in chronic pain states (Fig. 1B, Ewan and Martin, 2011b). A previous study reported similar findings for heroin (Ewan and Martin, 2011a). Interestingly, morphine failed to facilitate ICSS in the presence of chronic neuropathic pain (Ewan and Martin, 2011b) or in an acute model of acute visceral pain (Altarifi et al., 2015).
Drug self-administration is more commonly used to measure reinforcing effects of drugs. Seminal studies examining the influence of chronic pain on opioid self-administration identified that the acquisition of heroin, morphine, fentanyl, hydromorphone and methadone self-administration was significantly reduced in the presence of chronic pain compared to sham control surgery, but there was no effect on responding for food (Fig. 1C, Martin et al., 2007). Importantly, the rate of drug intake correlated with reversal of mechanical allodynia (Martin et al., 2007). These data are consistent with reports that chronic inflammatory pain reduces acquisition of intravenous morphine self-administration (Lyness et al., 1989). Similarly, oral fentanyl self-administration was reduced or absent in three mouse models of chronic pain induced by complete Freund’s adjuvant inflammatory pain, spinal nerve ligation for neuropathic pain or a vincristine-induced neuropathy (Wade et al., 2013). Supporting the hypothesis that pain negatively influences the abuse potential of opioid analgesics are reports that non-contingent delivery of analgesics such as indomethacin (Lyness et al., 1989) or dexamethasone (Colpaert et al., 2001) decreased intravenous morphine or oral fentanyl self-administration, respectively. However, others have reported an increase in oral fentanyl consumption in a model of polyarthritis compared to pain-free or chronic neuropathic pain in rats (Kupers and Gybels, 1995). In contrast, intravenous heroin intake was increased if pain was induced after rodents were already dependent on opioids (Hipólito et al., 2015), which is consistent with clinical studies showing that patients with chronic pain had an increased risk of opioid analgesic misuse if they had a history of substance abuse, current high alcohol intake, long-term benzodiazepine use, or aberrant drug-related behavior (Fishbain et al., 2008; Højsted et al., 2013; Cragg et al., 2017; Hah et al., 2017). In non-pain conditions, rodents and non-human primates with extended access to intravenous heroin self-administration rapidly escalate their drug intake that is concomitant with development of analgesic tolerance and continued drug intake despite adverse consequences such as foot shock (Bozarth and Wise, 1985; Ahmed et al., 2000; Chen et al., 2006; Negus, 2006; Wade et al., 2015).
5. Clinical pharmacology of fentanyl: focus on abuse potential
5.1. Healthy volunteers
Some of the early studies of the abuse liability of fentanyl were conducted in normal, healthy volunteers who did not use drugs recreationally, although most of the participants used alcohol occasionally. In this population, fentanyl did not reliably increase positive subjective responses. At intravenous (i.v.) doses up to approximately 250 μg/70 kg, fentanyl increased positive subjective effects in 4 studies (e.g., it increased ratings of euphoria, feelings of well-being, or pleasantness of drug effects in Hoehe, 1988; Hoehe et al., 1988; Manner et al., 1987; and Matussek and Hoehe, 1989). In 3 other studies assessing i.v. fentanyl doses ranging between 50 μg/70 kg and approximately 200 μg/70 kg, fentanyl did not increase positive subjective responses (Ghoneim et al., 1975; Scamman et al., 1984; Zacny et al., 1996a), and in 2 additional studies, the positive subjective effects produced by fentanyl were equivocal (Zacny et al., 1992a, b). The negative findings reported by Ghoneim et al. (1975) and Scamman et al. (1984) were possibly due to the fact that the peak effects of fentanyl were missed because measurements of subjective responses did not begin until 30 min after drug administration. Zacny et al. (1996a) reported that participants did report feeling “high” and “coasting (spaced out)” after receiving 100 μg/70 kg i.v. fentanyl, but ratings of drug liking did not significantly differ from i.v. saline. In one of the 2 studies reporting equivocal results (Zacny et al., 1992a), ratings of drug liking were transient and did not coincide with increased scores on the Morphine-Benzedrine Group (MBG) scale of the Addiction Research Center Inventory (ARCI), a measure widely used at the time to assess drug-induced euphoria. In the other study reporting equivocal results (Zacny et al., 1992b), only a subset of participants (4 out of 6) reported liking the effects of fentanyl, but a relatively low dose was tested (50 μg/70 kg i.v.), so this outcome may not be entirely surprising.
5.2. Illicit opioid users – subjective effects
By current standards, most assessments of the abuse liability of drugs are conducted in individuals who use them recreationally (Balster and Bigelow, 2003; Comer et al., 2012; Griffiths et al., 2003). It is generally assumed that recreational drug users are the most appropriate population for testing the abuse liability of drugs because by their behavior, these individuals have demonstrated that they can recognize drug effects and they like them, typically at doses that are higher than those used therapeutically. In 2017, the U.S. Food and Drug Administration (FDA) issued a guidance document for industry that recommended that recreational drug users who have a recent history of using substances in the same drug class as the test compound be enrolled to assess the abuse liability of drugs. The FDA specifically stated in their guidance document that “It is not recommended that drug-naïve subjects be used in HAP [human abuse potential] studies because this population has not been validated scientifically as being able to provide accurate information on the abuse potential of a drug.”
Supporting this recommendation is the fact that all of the studies that examined the subjective effects of fentanyl in experienced drug users have shown that fentanyl produces clear and dose-related increases in ratings of drug liking, good drug effects, and high (Baylon et al., 2000; Comer et al., 2008; Greenwald et al., 1996, 2005). Participants who were maintained on morphine (30 mg orally, given 4 times per day) reported that they would pay $8.50 for a fentanyl dose of 250 μg/70 kg i.v. compared to $2.50 for saline, and ratings of “bad drug effects” and “nauseated” were not significantly different from saline (Comer et al., 2008). Consistent with these results, doses up to 4.5 μg/kg (~315 μg/70 kg) did not significantly increase ratings of “bad effects” or “sick” in non-dependent recreational opioid users (Baylon et al., 2000). In contrast, 7 out of 8 healthy volunteers who received 3 μg/kg (~210 μg/70 kg) experienced nausea and 4 of them vomited; 3 of the 4 who vomited did so for up to 6 h after fentanyl administration (Scamman et al., 1984). Dizziness was reported by one additional subject, who remained prone for 8 h after drug administration (Scamman et al., 1984). It is not surprising that drug-inexperienced individuals would not report liking the effects of fentanyl.
5.3. Illicit opioid users – reinforcing effects
In addition to evaluating subjective responses following drug administration, the abuse potential of drugs in humans can be assessed by self-administration procedures (Comer et al., 2008, 2012; Haney and Spealman, 2008; Jones and Comer, 2013). Typically, participants are asked to make a response (such as finger presses on a computer mouse) in order to obtain drug, and a drug that is self-administered more than placebo is considered to be a reinforcer. One procedure for assessing the reinforcing effects of a drug uses a modified drug versus money progressive ratio schedule to assess reinforcing effects. Participants first receive a sample dose of drug and money and then during a later session, they have 10 opportunities to choose between 1/10th of the dose or money that was sampled previously. Each time drug or money is chosen, the number of responses (finger presses) progressively increases and the point at which responding stops is termed the “break point” (it is the highest ratio completed for drug and/or money). In morphine-dependent individuals, 250 μg/70 kg i.v. fentanyl produced a progressive-ratio break point value for drug that was significantly greater than placebo and similar to 12.5 mg/70 kg i.v. heroin, 25 mg/70 kg i.v. oxycodone, and 25 mg/70 kg i.v. morphine (Comer et al., 2008). Fentanyl also served as a reinforcer in methadone-maintained individuals (Greenwald and Roehrs, 2004).
Another way of assessing drug self-administration is through “behavioral economic” procedures (Bickel et al., 1993, 1995; Hursh, 1993). A re-analysis of data was performed from studies using a multiple-choice procedure (in which subjects made a series of choices between receiving a given drug dose and a range of money amounts; Greenwald, 2008; Griffiths et al., 1993). In this behavioral economic analysis of multiple-choice procedure data, “demand curves” were constructed by plotting drug choices as a function of unit price (response requirement divided by dose) for fentanyl, hydromorphone, and methadone (Greenwald, 2008). The demand curve for fentanyl was the most “inelastic” of the opioids that were tested, suggesting that fentanyl self-administration was the most resistant to change when unit price increases. However, several procedural differences across the studies from which the analysis was derived might have accounted for this finding, such as differences in route and method of drug administration (i.v. fentanyl cumulative dosing versus intramuscular hydromorphone acute dosing). Therefore, interpretation of the elasticity of fentanyl relative to the other opioids should be made with caution.
Another interesting study that examined the reinforcing effects of fentanyl was one in which recreational drug users (only one of whom reported recreational use of opioids) were asked to immerse their forearm in water maintained at different temperatures (37 °C, 10 °C, and 2 °C; Zacny et al., 1996b). For each of the temperatures, participants completed two consecutive sampling trials and three consecutive choice trials. They were instructed to choose one of the two infusion pumps (containing either saline or 50 μg i.v. fentanyl) five minutes before immersion of the forearm into the water. Under these conditions, fentanyl was self-administered significantly more than placebo under the two cold water conditions (77% of the time under both the 10 °C and 2 °C conditions) but not when the water was maintained at 37 °C (fentanyl was chosen 60% of the time, which did not differ from chance). The presence of pain also altered the subjective effects of fentanyl: participants reported feeling more elated after fentanyl administration compared to saline in the 37 °C condition, but not when they were asked to immerse their forearm in cold water (the 10 °C and 2 °C conditions). Some of these results were replicated in a subsequent study: oxycodone was self-administered only in the presence of a painful stimulus (hand immersions in water maintained at 2 °C), compared to a non-painful stimulus (hand immersions in water maintained at 37 °C; Comer et al., 2010). However, this outcome only occurred in participants who had used prescription opioids medically but had never used them recreationally. The participants who used prescription opioids recreationally self-administered oxycodone regardless of the presence or absence of pain (the 4 °C and 37 °C conditions). And unlike the results reported by Zacny et al. (1996b), the positive subjective responses produced by oxycodone did not differ in the presence and absence of pain in either group. Thus, the lack of reinforcing effects of fentanyl in the absence of pain in the study conducted by Zacny et al. (1996b) may have been due to the fact that the participants were not recreational users of opioids.
The studies reviewed above highlight several important factors that must be considered when evaluating and interpreting results of abuse potential studies in humans, including the population selected for study (recreational opioid users should be examined), the assessment time points used (they should capture the expected pharmacokinetic profile of the drug, especially at early time points after drug administration), and the use of behavioral endpoints such as drug self-administration to provide greater clarity on the abuse liability of a drug. When all of these factors are considered, the pharmacological profile of fentanyl suggests that it has high potential for abuse in humans. However, the abuse liability of fentanyl relative to other mu opioid agonists remains somewhat unclear. The analysis by Greenwald (2008) suggests that fentanyl might have greater abuse liability than hydromorphone and methadone, but procedural inconsistencies in the studies that were examined make definitive conclusions difficult. The study by Comer et al. (2008) showed that fentanyl is more potent than heroin, morphine, and oxycodone, but it has similar abuse liability as the other drugs. In that study, testing higher doses of fentanyl and using higher progressive ratio values to avoid ceiling effects would have been helpful. Future studies using potentially more sensitive measures, such as a drug versus drug choice procedure or prospective assessments of demand curves for fentanyl compared to other mu opioids would be informative. Another way of approaching this issue is by asking opioid users directly how they perceive the effects of fentanyl. Cicero et al., 2017 asked 10,900 individuals who were entering treatment for opioid use disorder about fentanyl. This analysis was hampered by several variables, however, including the fact that both commercial and illicit fentanyl products are available to users and it is impossible to distinguish among them based on urine drug screens, illicit fentanyl is most often added to heroin and other drugs unbeknownst to the user, and the extent to which illicit fentanyl alone is available to users and sought out by them is unclear. Given the current patterns of illicit manufacturing, modern marketing techniques, and enormous profits to be made, however, it is likely that illicit fentanyl use will become even more widespread in the years to come (DEA Intelligence Brief DEA-DCT-DIB-021-16, 2016; Gilbert and Dasgupta, 2017).
6. Implications for treatment of illicit fentanyl use
The preclinical data reviewed above support the view that the pharmacology of fentanyl differs from other mu opioid agonists such as morphine. In contrast, it is unclear whether the pharmacology of fentanyl in humans as it relates to abuse liability differs significantly from other mu opioids, in part because the research procedures that could potentially make this differentiation (e.g., a drug versus drug choice paradigm or prospective behavioral economics procedures) have not been applied to this question. Whether the pharmacology of fentanyl in humans as it relates to toxicity differs from other opioids has also been understudied, even though the toxicity of fentanyl in clinical settings has been well characterized. While it is well known that fentanyl, like other opioid agonists, produces respiratory depression primarily via activation of opioid receptors in the pre-Bötzinger complex as well as actions in the Kolliker-Fuse and parabrachial nuclei of the pons (Lalley, 2006), recent clinical studies have also demonstrated that fentanyl induces chest wall rigidity that may contribute to fatalities (Burns et al., 2016). Further, the combination of fentanyl with other drugs of abuse or CNS depressants such as alcohol likely engages additional mechanisms, including cardiac arrhythmias, that lead to mortality. The knowledge gap in how fentanyl may differ from other opioid agonists is mainly due to the fact that fentanyl is used in a very different manner by a clinician administering the drug to a patient compared to a drug user self-administering fentanyl for its euphoric effects (i.e., a large bolus dose injected very rapidly, often in combination with alcohol or other drugs of abuse such as cocaine or benzodiazepines).
In addition to the research gaps regarding the relative abuse liability and toxicity of fentanyl compared to other opioid agonists, little information from controlled clinical trials is available about the effectiveness of treatment medications (methadone, buprenorphine, naltrexone) in reducing illicit fentanyl use, or naloxone for treating fentanyl-related overdose. Preclinical studies have clearly established that fentanyl interacts in a competitive manner with opioid antagonists such as naltrexone (e.g., Comer et al., 1992; Cornelissen et al., 2018). As such, simply increasing the antagonist dose should be effective if the euphoric effects of fentanyl are not completely suppressed (naltrexone) or the respiratory depressant effects of fentanyl are not completely reversed (naloxone). An important caveat to the latter statement is that the effectiveness of naloxone in reversing fentanyl-related overdoses is not clear when alcohol or other drugs have been co-ingested with fentanyl or if a synthetic fentanyl-like drug has been used.
Naloxone has been used for decades to reverse opioid-induced respiratory depression in both hospital (e.g., during surgery) and non-hospital settings (e.g., overdose by an illicit drug user). It has a rapid onset (within 2 min following intravenous administration) and short serum half-life (~1 h). In opioid-dependent individuals, naloxone can precipitate withdrawal, the severity of which may depend on multiple factors, such as the individual’s level of physical dependence, the amount and type of opioid agonist used during the overdose event, the time between the overdose event and administration of naloxone, and the amount of naloxone used to reverse the overdose. In order to avoid precipitating severe withdrawal, the American Heart Association recommends starting with a small dose of naloxone (0.4 mg intramuscularly or 2 mg intranasally). However, recent reports suggest that higher doses or repeated dosing of naloxone (due to recurrence of respiratory depression) may be required to reverse fentanyl-induced respiratory depression (Fairbairn et al., 2017; Lynn and Galinkin, 2018; Somerville et al., 2017). The reason that higher doses of naloxone may be required is not entirely clear. Possibilities are that a large dose of naloxone is needed simply because a large dose of fentanyl was used, a fentanyl analog was used that is not sensitive to naloxone, or, because the onset of fentanyl-induced respiration is so rapid, the naloxone was administered after the individual was already deceased. Another possibility is that fentanyl and naloxone may share an influx transporter into the brain and that when high doses of fentanyl are used, the transporter becomes saturated, so naloxone is not able to cross the blood-brain barrier (Lynn and Galinkin, 2018; Suzuki et al., 2010). Clearly, there is a dire need for more clinical studies to assess the effectiveness of naloxone in reversing respiratory depression induced by fentanyl and synthetic fentanyl-like drugs following various routes of administration (Fairbairn et al., 2017; Somerville et al., 2017).
The effectiveness of buprenorphine or methadone in reducing abuse of fentanyl by humans is also largely unknown. Studies conducted in rats have demonstrated that maintenance on buprenorphine was less effective in reducing the analgesic effects of opioid agonists with lower efficacy (morphine) compared to higher efficacy (etonitazene; Walker and Young, 2001). A study also was conducted in rhesus monkeys comparing the reinforcing effects of different opioid agonists in the presence and absence of morphine physical dependence (e.g., Winger and Woods, 2001). Through the mechanism of cross-tolerance, one would expect a rightward shift in the dose-effect curves for opioids when animals are physically dependent on morphine compared to no dependence. Although this outcome was demonstrated for most of the agonists tested, the rightward shift in the dose-effect curve for the higher efficacy agonist alfentanil was smaller than for the intermediate efficacy agonists, morphine and heroin. And the dose-effect curves for the lower efficacy agonists were shifted either downward (buprenorphine) or rightward to a much greater extent (nalbuphine) than the higher efficacy agonists (Winger and Woods, 2001). This pattern of effects has been demonstrated in several different species (rats, mice, monkeys, pigeons) across several different experimental assays (analgesia, drug discrimination, schedule-controlled responding for food, self-administration; Barrett et al., 2001, 2003; Duttaroy and Yoburn, 1995; Negus et al., 2003; Paronis and Holtzman, 1992, 1994; Pitts et al., 1998; Smith and Picker, 1998; Walker and Young, 2001, 2002; Walker et al., 1995, 1998; Winger and Woods, 2001; Young et al., 1991). Therefore, for both buprenorphine and opioid agonist maintenance, the general finding is that the effects of higher efficacy agonists are more difficult to block than lower efficacy agonists. To the extent that these findings can be extrapolated to humans, the data suggest that methadone and buprenorphine may be less effective in treating fentanyl abuse than it is in treating heroin abuse.
In sum, a great deal is known about the pharmacology of fentanyl using preclinical models and when it is used therapeutically in humans for anesthesia or analgesia. However, studies are desperately needed to elucidate the physiological mechanisms underlying fentanyl overdose so that effective treatments can be developed to reduce the risk of death. Similarly, studies to evaluate the most effective maintenance doses and dosing regimens of naltrexone, methadone, and buprenorphine for treating fentanyl abuse are urgently needed to address the public health crisis posed by use of illicit fentanyl.
Acknowledgements
The writing of the manuscript was supported by U54DA037842 (PI: Levin, Project PI: Comer), R01DA039169 (PI: Comer), R01DA035207 (PI: Comer), Shirley & Stefan Hatos Foundation (PI: Cahill), NIH NIDA R01DA041781 (Co-I: Cahill), and DOD W81XWH-15-1-0435 (PI: Cahill). The authors would also like to thank Lauren Noble for her assistance with the manuscript.
References
- Ahmed SH, Walker JR, Koob GF, 2000. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22 (4), 413. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS, 2015. Effects of μ-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of μ-agonist efficacy and noxious stimulus intensity. J. Pharmacol. Exp. Ther 352 (2), 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE, 2003. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 70 (3), S13–S40. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ, 2001. Importance of sex and relative efficacy at the μ opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology 158, 154–164. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, Picker MJ, 2003. Use of irreversible antagonists to determine the relative efficacy of μ-opioids in a pigeon drug discrimination procedure: Comparison of β-funaltrexamine and clocinnamox. J. Pharmacol. Exp. Ther 306 (3), 1061–1070. [DOI] [PubMed] [Google Scholar]
- Baylon GJ, Kaplan HL, Somer G, Busto UE, Sellers EM, 2000. Comparative abuse liability of intravenously administered remifentanil and fentanyl. J. Clin. Psychopharmacol 20 (6), 597–606. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, 1993. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend. 33 (2), 173–192. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, 1995. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology 118 (3), 250–259. [DOI] [PubMed] [Google Scholar]
- Bilecki W, Zapart G, Lięza A, Wawrzczak-Bargiela A, Urbański MJ, Przewłocki R, 2005. Regulation of the extracellular signal-regulated kinases following acute and chronic opioid treatment. Cell. Mol. Life Sci. CMLS 62 (19-20), 2369–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT, 1999. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 286 (5449), 2495–2498. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG, 2000. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408 (6813), 720. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA, 1985. Toxicity associated with long-term intravenous heroin and cocaine self administration in the rat. J. Am. Med. Assoc 254 (1), 81–83. [PubMed] [Google Scholar]
- Burns G, DeRienz RT, Baker DD, Casavant M, Spiller HA, 2016. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin. Toxicol 54 (5), 420–423. [DOI] [PubMed] [Google Scholar]
- Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB, 1993. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 52 (4), 389–396. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF, 2006. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31 (12), 2692. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Munoz A, 2005. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002-2004. J. Pain 6 (10), 662–672. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Kasper ZA, 2017. Increases in self-reported fentanyl use among a population entering drug treatment: the need for systematic surveillance of illicitly manufactured opioids. Drug Alcohol Depend. 177, 101–103. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Slot LB, Attal N, Koek W, 2001. Opiate self administration as a measure of chronic nociceptive pain in arthritic rats. Pain 91 (1-2), 33–45. [DOI] [PubMed] [Google Scholar]
- Comer SD, Burke TF, Lewis JW, Woods JH, 1992. Clocinnamox: a novel, systemically-active, irreversible opioid antagonist. J. Pharmacol. Exp. Ther 262 (3), 1051–1056. [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ, 2008. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology 33 (5), 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J, 2010. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 109 (1-3), 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Zacny JP, Dworkin RH, Turk DC, Bigelow GE, Foltin RW, et al. , 2012. Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. Pain 153 (12), 2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen JC, Obeng S, Rice KC, Zhang Y, Negus SS, Banks ML, 2018. Application of receptor theory to the design and use of fixed-proportion mu-opioid agonist and antagonist mixtures in rhesus monkeys. J. Pharmacol. Exp. Ther 365, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg A, Hau JP, Woo SA, Liu C, Doyle-Waters MM, Hohl CM, 2017. Risk factors for addiction among patients receiving prescribed opioids: a systematic review protocol. Syst. Rev 6 (1), 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2016. Counterfeit Prescription Pills Containing Fentanyl: a Global Threat. DEA Intelligence Brief, DEA-DCT-DIB-021-16, 1–10. [Google Scholar]
- Duttaroy A, Yoburn BC, 1995. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82, 1226–1236. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2017. European Drug Report 2017: Trends and Developments. European Monitoring Centre for Drugs and Drug Addiction. [Google Scholar]
- Ewan EE, Martin TJ, 2011a. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiol.: J. Am. Soc. Anesthesiol 114 (3), 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2011b. Rewarding Electrical Brain Stimulation in Rats after Peripheral Nerve InjuryDecreased Facilitation by Commonly Abused Prescription Opioids. Anesthesiol.: J. Am. Soc. Anesthesiol 115 (6), 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn N, Coffin PO, Walley AY, 2017. Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: challenges and innovations responding to a dynamic epidemic. Int. J. Drug Policy 46, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch JS, DeKornfeld TJ, 1967. Clinical investigation of the analgesic potency and respiratory depressant activity of fentanyl, a new narcotic analgesic. J. Clin. Pharmacol 7 (1), 46–51. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS, 2008. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 9 (4), 444–459. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP, Thatcher JW, 1975. The effect of diazepam and fentanyl on mental, psychomotor and electroencephalographic functions and their rate of recovery. Psychopharmacologia 44 (1), 61–66. 10.1007/bf00421185. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Dasgupta N, 2017. Silicon to syringe: cryptomarkets and disruptive innovation in opioid supply chains. Int. J. Drug Policy 46, 160–167. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, 2008. Behavioral economic analysis of drug preference using multiple choice procedure data. Drug Alcohol Depend. 93 (1-2), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Roehrs TA, 2005. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology 30 (1), 212–221. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, June HL, Stitzer ML, Marco AP, 1996. Comparative clinical pharmacology of short-acting mu opioids in drug abusers. J. Pharmacol. Exp. Ther 277 (3), 1228–1236. [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA, 2003. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 70 (3), S41–S54. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM, 2007. An opioid agonist that does not induce μ-opioid receptor—arrestin interactions or receptor internalization. Mol. Pharmacol 71 (2), 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah JM, Sturgeon JA, Zocca J, Sharifzadeh Y, Mackey SC, 2017. Factors associated with prescription opioid misuse in a cross-sectional cohort of patients with chronic non-cancer pain. J. Pain Res 10, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R, 2008. Controversies in translational research: drug self-administration. Psychopharmacology (Berl.) 199 (3), 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Moron JA, 2015. Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J. Neurosci 35 (35), 12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehe M, 1988. Influence of the menstrual cycle on neuroendocrine and behavioral responses to an opiate agonist in humans: preliminary results. Psychoneuroendocrinology 13 (4), 339–344. [DOI] [PubMed] [Google Scholar]
- Hoehe M, Duka T, Doenicke A, 1988. Human studies on the μ opiate receptor agonist fentanyl: neuroendocrine and behavioral responses. Psychoneuroendocrinology 13 (5), 397–408. [DOI] [PubMed] [Google Scholar]
- Højsted J, Ekholm O, Kurita GP, Juel K, Sjøgren P, 2013. Addictive behaviors related to opioid use for chronic pain: a population-based study. Pain 154 (12), 2677–2683. [DOI] [PubMed] [Google Scholar]
- Hursh SR, 1993. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 33 (2), 165–172. [DOI] [PubMed] [Google Scholar]
- Imai S, Narita M, Hashimoto S, Nakamura A, Miyoshi K, Nozaki H, Hareyama N, Takagi T, Suzuki M, Narita M, Suzuki T, 2006. Differences in tolerance to anti-hyperalgesic effects between chronic treatment with morphine and fentanyl under a state of pain. Japan. J. Psychopharmacol 26 (5-6), 183–192. [PubMed] [Google Scholar]
- Jones JD, Comer SD, 2013. A review of human drug self-administration procedures. Behav. Pharmacol 24 (5-6), 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbelic MI, 2010. Deaths with transdermal fentanyl patches. Am. J. Forensic Med. Pathol 31 (1), 18–21. [DOI] [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A, Benoit C, Butler SF, 2008. Internet-based survey of nonmedical prescription opioid use in the United States. Clin. J. Pain 24 (6), 528–535. 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- Kesimci E, Engin AB, Kanbak O, Karahalil B, 2012. Association between ABCB1 gene polymorphisms and fentanyTs adverse effects in Turkish patients undergoing spinal anesthesia. Gene 493 (2), 273–277. [DOI] [PubMed] [Google Scholar]
- Knisely JS, Campbell ED, Dawson KS, Schnoll SH, 2002. Tramadol post-marketing surveillance in health care professionals. Drug Alcohol Depend. 68 (1), 15–22. [DOI] [PubMed] [Google Scholar]
- Kuhar JR, Bedini A, Melief EJ, Chiu YC, Striegel HN, Chavkin C, 2015. Mu opioid receptor stimulation activates c-Jun N-terminal kinase 2 by distinct arrestin-dependent and independent mechanisms. Cell. Signal 27 (9), 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Gybels J, 1995. The consumption of fentanyl is increased in rats with nociceptive but not with neuropathic pain. Pain 60 (2), 137–141. [DOI] [PubMed] [Google Scholar]
- Labroo RB, Paine MF, Thummel KE, Kharasch ED, 1997. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab. Dispos 25 (9), 1072–1080. [PubMed] [Google Scholar]
- Lalley PM, 2006. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol 290, R1387–R1396. [DOI] [PubMed] [Google Scholar]
- Ligeza A, Wawrzczak-Bargiela A, Kaminska D, Korostynski M, Przewlocki R, 2008. Regulation of ERK1/2 phosphorylation by acute and, 2018 of ERK1/2 phosphorylation by acute and chronic morphine-implications for the role of cAMP-responsive element binding factor (CREB)-dependent and Ets-like protein-1 (Elk-1)-dependent transcription; small interfering RNA-based strategy. FEBS J. 275 (15), 3836–3849. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Walter C, Parnham MJ, Oertel BG, Geisslinger G, 2013. Pharmacokinetics of non intravenous formulations of fentanyl. Clin. Pharmacokinet 52 (1), 23–36. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL, Heavner JE, Iacono CU, Garvin RD, 1989. Morphine self administration in the rat during adjuvant-induced arthritis. Life Sci. 45 (23), 2217–2224. [DOI] [PubMed] [Google Scholar]
- Lynn RR, Galinkin JL, 2018. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther. Adv. Drug Saf 9 (1), 63–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C, 2006. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J. Biol. Chem 281 (45), 34515–34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire P, Tsai N, Kamal J, Cometta-Morini C, Upton C, Loew G, 1992. Pharmacological profiles of fentanyl analogs at μ, δ and κ opiate receptors. Eur. J. Pharmacol 213 (2), 219–225. [DOI] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. , 2016. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manner T, Kanto J, Salonen M, 1987. Simple devices in differentiating the effects of buprenorphine and fentanyl in healthy volunteers. Eur. J. Clin. Pharmacol 31 (6), 673–676. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC, 2007. Opioid Self-administration in the Nerve-injured RatRelevance of Antiallodynic Effects to Drug Consumption and Effects of Intrathecal Analgesics. Anesthesiol.: J. Am. Soc. Anesthesiol 106 (2), 312–322. [DOI] [PubMed] [Google Scholar]
- Matussek N, Hoehe M, 1989. Investigations with the specific mu-opiate receptor agonist fentanyl in depressive patients: growth hormone, prolactin, cortisol, noradrenaline and euphoric responses. Neuropsychobiology 21 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- Mittal N, Tan M, Egbuta O, Desai N, Crawford C, Xie C-W, Evans CJ, Walwyn W, 2012. Evidence that behavioral phenotypes of morphine in beta-arr2−/− mice are due to the unmasking of JNK signaling. Neuropsychopharmacology 37, 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ, 2011. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br. J. Pharmacol 164 (4), 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Imai S, Nakamura A, Ozeki A, Asato M, Rahmadi M, Sudo Y, Hojo M, Uezono Y, Devi LA, Kuzumaki N, Suzuki T, 2013. Possible involvement of prolonging spinal μ-opioid receptor desensitization in the development of anti-hyperalgesic tolerance to μ-opioids under a neuropathic pain-like state. Addict. Biol 18 (4), 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, 2006. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J. Pharmacol. Exp. Ther 317 (2), 711–723. [DOI] [PubMed] [Google Scholar]
- Negus SS, 2013. Expression and treatment of pain-related behavioral depression. Lab Anim. (NY) 42 (8), 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Gatch MB, Mello NK, 2003. Effects of heroin and its metabolites on schedule-controlled responding and thermal nociception in rhesus monkeys: Sensitivity to antagonism by quadazocine, naltrindole and β-funaltrexamine. Drug Alcohol Depend. 70, 17–27. [DOI] [PubMed] [Google Scholar]
- Olkkola KT, Palkama VJ, Neuvonen PJ, 1999. Ritonavir’s role in reducing fentanyl clearance and prolonging its half-life. Anesthesiology 91 (3), 681–685. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG, 1992. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperdine or fentanyl in rats. J. Pharmacol. Exp. Ther 262 (1), 1–9. [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG, 1994. Sensitization and tolerance to the discriminative stimulus effects of mu-opioid agonists. Psychopharmacology 114, 601–610. [DOI] [PubMed] [Google Scholar]
- Pichini S, Solimini R, Berretta P, Pacifici R, Busardo FP, 2018. Acute intoxications and fatalities from illicit fentanyl and analogues: an update. Ther. Drug Monit 40 (1), 38–51. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Allen RM, Walker EA, Dykstra LA, 1998. Clocinnamox antagonism of the antinociceptive effects of mu opioids in squirrel monkeys. J. Pharmacol. Exp. Ther 285, 1197–1206. [PubMed] [Google Scholar]
- Psifogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, Gold SJ, et al. , 2011. A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia. J. Neurosci 31, 5617–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM, 2011. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60 (1), 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM, 2005. Morphine side effects in β-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther 314 (3), 1195–1201. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Vardy E, DiBerto JF, Chefer VI, White KL, Fish EW, Chen M, Gigante E, Krouse MC, Sun H, Thorsell A, Heilig M, Malanga CJ, 2015. Receptor reserve moderates mesolimbic responses to opioids in a humanized mouse model of the OPRM1 A118G polymorphism. Neuropsychopharmacology 40 (11), 2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamman FL, Ghoneim MM, Korttila K, 1984. Ventilatory and mental effects of Alfentanil and fentanyl. Acta Anaesthesiol. Scand 28 (1), 63–67. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L, 1996. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest 97 (11), 2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM, 2017. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171 (5), 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Provasi D, Filizola M, 2016. How oliceridine (TRV-130) binds and stabilizes a μ opioid receptor conformational state that selectively triggers G protein signaling pathways. Biochemistry 55 (46), 6456–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silsby HD, Kruzich DJ, Hawkins MR, 1984. Fentanyl citrate abuse among health care professionals. Mil. Med 149 (4), 227–228. [PubMed] [Google Scholar]
- Smith MA, Picker MJ, 1998. Tolerance and cross-tolerance to the rate-suppressing effects of opioids in butorphanol-treated rats: influence of maintenance dose and relative efficacy at the mu receptor. Psychopharmacology 140 (1), 57–68. [DOI] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnel J, Gladden RM, Zibbell JE, Green TC, Younkin M, Ruiz S, Babakhanlou-Chase H, Chan M, Callis BP, Kuramoto-Crawford J, Nields HM, Walley AY, 2017. Characteristics of fentanyl overdose – massachusetts, 2014-2016. MMWR Morb. Mortal. Wkly. Rep 66 (14), 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley TH, 1992. The history and development of the fentanyl series. J. Pain Symptom Manage 7 (3), S3–S7. [DOI] [PubMed] [Google Scholar]
- Stanley TH, Egan TD, Van Aken H, 2008. A tribute to Dr. Paul A. J. Janssen: entrepreneur extraordinaire, innovative scientist, and significant contributor to anesthesiology. Anesth. Analg 106 (2), 451–462 table of contents. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ohmuro A, Miyata M, Furuishi T, Hidaka S, Kugawa F, Fukami T, Tomono K, 2010. Involvement of an influx transporter in the blood-brain barrier transport of naloxone. Biopharm. Drug Dispos 31, 243–252. [DOI] [PubMed] [Google Scholar]
- Takashina Y, Naito T, Mino Y, Yagi T, Ohnishi K, Kawakami J, 2012. Impact of CYP3A5 andABCB1 gene polymorphisms on fentanyl pharmacokinetics and clinical responses in cancer patients undergoing conversion to a transdermal system. Drug Metab. Pharmacokinet 27 (4), 414–421. [DOI] [PubMed] [Google Scholar]
- Tatke A, Janga KY, Avula B, Wang X, Jablonski MM, Khan IA, Majumdar S, 2018. P-glycoprotein restricts ocular penetration of loperamide across the blood-ocular barriers: a comparative study on Mdr1a knock-out and wild type Sprague Dawley rats. AAPS PharmSciTech 19 (4), 1662–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L, 1974. Contribution of ‘receptor’affinity to analgesic potency. J. Pharm. Pharmacol 26 (2), 146–148. [DOI] [PubMed] [Google Scholar]
- Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C, 2004. G protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br. J. Pharmacol 141 (1), 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR, 1995. Modulation by mu-opioid agonists of guanosine-5’-O-(3-[35S] thio) triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol 47 (4), 848–854. [PubMed] [Google Scholar]
- van den Hoogen RHWM, Colpaert FC, 1987. Epidural and subcutaneous morphine, meperidine (pethidine), fentanyl and sufentanil in the rat: analgesia and other in vivo pharmacologic effects. Anesthesiology 66 (2), 186–194. [DOI] [PubMed] [Google Scholar]
- Volpe DA, Tobin GAM, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL, 2011. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol 59 (3), 385–390. [DOI] [PubMed] [Google Scholar]
- Wade CL, Krumenacher P, Kitto KF, Peterson CD, Wilcox GL, Fairbanks CA, 2013. Effect of chronic pain on fentanyl self-administration in mice. PLoS One 8 (11) e79239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, 2015. Compulsive like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40 (2), 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Young AM, 2001. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology 154 (2), 131–142. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM, 2002. Clocinnamox distinguishes opioid agonists according to relative efficacy in normal and morphine-treated rats trained to discriminate morphine. J. Pharmacol. Exp. Ther 302, 101–110. [DOI] [PubMed] [Google Scholar]
- Wandel C, Kim R, Wood M, Wood A, 2002. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology 96 (4), 913–920. [DOI] [PubMed] [Google Scholar]
- Wang X-F, Barbier E, Chiu YT, He Y, Zhan J, Bi GH, Zhang HY, Feng B, Liu-Chen LY, Wang JB, Xi ZX, 2016. T394A mutation at the μ opioid receptor blocks opioid tolerance and increases vulnerability to heroin self-administration in mice. J. Neurosci 36 (40), 10392–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CF, Ward GC, Saidman LJ, 1983. Drug abuse in anesthesia training programs: A survey: 1970 through 1980. J. Am. Med. Assoc. 1983;250 (7), 922–925. 10.1001/jama.1983.03340070028021. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ, 2013. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev 65 (1), 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Woods JH, 2001. The effects of chronic morphine on behavior reinforced by several opioids or by cocaine in rhesus monkeys. Drug Alcohol Depend. 62,181–189. [DOI] [PubMed] [Google Scholar]
- Young AM, Kapitsopoulos G, Makhay MM, 1991. Tolerance to morphine-like stimulus effects of mu opioid agonists. J. Pharmacol. Exp. Ther 257 (2), 795–805. [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Zaragoza JG, de Wit H, 1992a. Subjective and behavioral responses to intravenous fentanyl in healthy volunteers. Psychopharmacology 107 (2-3), 319–326. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Zaragoza JG, de Wit H, 1992b. Effects of fasting on responses to intravenous fentanyl in healthy volunteers. J. Subst. Abuse 4 (2), 197–207. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Coalson DW, Klafta JM, Klock PA, Alessi R, Rupani G, Young CJ, Patil PG, Apfelbaum JL, 1996a. Midazolam does not influence intravenous fentanyl-induced analgesia in healthy volunteers. Pharmacol. Biochem. Behav 55 (2), 275–280. [DOI] [PubMed] [Google Scholar]
- Zacny JP, McKay MA, Toledano AY, Marks S, Young CJ, Klock PA, Apfelbaum JL, 1996b. The effects of a cold-water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug Alcohol Depend. 42 (2), 133–142. [DOI] [PubMed] [Google Scholar]
- Ziesenitz VC, van den Anker JN, 2013. Impact of CYP3A and ABCB1 polymorphisms on the pharmacokinetics and pharmacodynamics of fentanyl. Int. J. Clin. Pharmacol. Ther 51 (12), 991–992. [DOI] [PubMed] [Google Scholar]