Extremely premature neonates born at the canalicular to saccular stage of lung development (22–28 wk of gestation) are at very high risk of developing bronchopulmonary dysplasia (BPD). The premature lung, now having to complete lung development in the extrauterine environment, is subjected to many adverse exposures, including hyperoxia, that promote the development of BPD. Many developmental pathways are precisely orchestrated for optimal lung maturation. Decreased or sustained activation of these pathways may contribute to the pathogenesis of this disease or may impair recovery of the lung from injury. Identification of these novel pathways and their mediators is crucial for the establishment of mechanisms leading to BPD and the development of novel therapeutic strategies.
The Wnt signaling pathway is critical both during embryonic development and in lung diseases throughout the lifespan (1). The Wnt family of proteins includes a large number of members that control a variety of developmental processes, including cell fate, proliferation, polarity, and migration. Wnt signaling consists of canonical, β-catenin–dependent signaling and two noncanonical pathways, including planar cell polarity and calcium-calmodulin–dependent protein kinase II/protein kinase C signaling. The canonical signaling pathway involves a number of proteins, including the transmembrane receptor Frizzled, coreceptors, and a variety of proteins that make up a “destruction complex” that control degradation versus nuclear translocation of β-catenin. On translocation to the nucleus, β-catenin activates several Wnt target genes (1). In distal lung development, Wnts provide spatiotemporal cues to coordinate an intricate crosstalk between the lung epithelium and mesenchymal cells (2). Frank and colleagues showed that Wnt signaling is reactivated during alveologenesis and leads to proliferation of type 2 alveolar epithelial cells (AECs), whereas inhibition of Wnt signaling decreased proliferation and promoted transdifferentiation of type 2 AECs to type 1 AECs (3). Increased Wnt/β-catenin activity occurs in patients with BPD, whereas inhibition of WNT/β-catenin signaling attenuates hyperoxia-induced lung injury in neonatal rodent models (4–6).
In this issue of the Journal, studies by Sucre and colleagues (pp. 1249–1262) focus on the role of Wnt5a, a noncanonical Wnt that is required for normal distal lung morphogenesis (7). The authors chose to study Wnt5a because of previously published reports of its role in lung diseases such as idiopathic pulmonary fibrosis (8) and chronic obstructive pulmonary disease (9). It is known that Wnt5a−/− mice die immediately after birth and show abnormal functional coupling of capillaries and the developing alveoli and thickening of the intersaccular interstitium (10). On the other hand, Wnt5a overexpression in distal lung epithelium using the SPC promoter reduced epithelial branching and dilated distal airways (11).
In the current studies, the critical involvement of NFκB (nuclear factor-κB) in the expression of Wnt5a and the mechanistic role of Wnt5a in the changes noted because of hyperoxia exposure during lung development was studied using organotypic cocultures, ex vivo precision-cut lung slices (PCLS), and in vivo mouse models. Hyperoxia exposure of organotypic cocultures resulted in increased expression of the fibrotic genes ACTA2, COL1A1, and ELN and decreased expression of the alveogenesis genes FOXM1, MYB, and MCM2. In examining the Wnt signaling pathway, hyperoxia was associated with increased nuclear accumulation of phosphorylated β-catenin and expression of AXIN2. Hyperoxia increased the expression of Wnt2b, Wnt5a, Wnt9a, and Wnt16 and decreased the expression of Wnt4, Wnt10a, and Wnt11. The increased Wnt5a expression was in mesenchymal cells. Addition of Wnt5a to cultures in normoxia demonstrated the same gene expression changes as observed with hyperoxia, and blockade of Wnt5a using a neutralizing antibody reversed the changes in gene expression observed in hyperoxia-exposed cultures. Alveolarization was decreased in PCLS exposed to hyperoxia, and this was abrogated in the presence of anti-Wnt5a antibody. In the in vivo mouse model of BPD (85% oxygen exposure from PN2 to PN14), increased expression of Wnt5a was noted in hyperoxia-exposed mouse lungs. Human samples from patients with BPD confirmed the increase in Wnt5a expression as compared with samples from babies who had succumbed to nonrespiratory causes. Next, pharmacologic or genetic inhibition of NFκB in PCLS exposed to hyperoxia showed decreased expression of Wnt5a and normal alveolarization. Thus, these exciting studies open the possibility of using Wnt5a as a potential therapeutic target to prevent or limit the severity of BPD.
The interplay between the developing lung (exposed to various postnatal stressors including hyperoxia) and other physiological factors (circulation and the immune system) is critical in the pathogenesis of injury. The three-dimensional organotypic coculture model with type 2 AECs and mesenchymal cells (from canalicular stage of lung development) used in these studies was able to localize the expression of Wnt5a to the mesenchymal compartment. Although this model has several advantages over two-dimensional culture systems (12) and preserves the spatial context and the lung microenvironment, it is a static system that does not have the ability to incorporate the contribution of systemic immune cells that may be recruited to the injured lung (13). Wnt5a may also play a role in the developing endothelium (14), which was not studied by Sucre and colleagues (7). For example, in a recent study by Yuan and colleagues, loss of endothelial Wnt5a led to small vessel loss in pulmonary arterial hypertension (15).
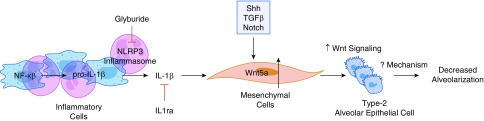
The authors show that the blockade of NFκB resulted in decreased Wnt5a and improved alveolarization. However, NFκB also drives the transcription of pro-IL1β, and the NLRP3 inflammasome controls the formation of mature IL1β, a system that is critical for the inflammatory process and the pathogenesis of BPD (16). Interestingly, IL1β increases the expression of Wnt5a in myofibroblasts (17), endothelial cells (18), and chondrocytes (19–21). Ge and colleagues showed induction of Wnt5a by IL1β in chondrocytes (22), and this was blocked by NFκB inhibition. They further showed recruitment of NFκB p65 to the Wnt5a promoter after IL1β treatment. Thus, the decrease in Wnt5a expression secondary to NFκB inhibition could have been secondary to decreased IL1β in vivo. Therefore, whether NFκB acts directly on Wnt5a in this model remains to be clarified. Use of NLRP3 inflammasome inhibitors such as glyburide and IL1β blockers such as IL1 receptor antagonist in these models would help define this pathway. In addition, multiple other signaling pathways such as Hedgehog, TGFβ, and Notch can also regulate Wnt5a expression (23). Figure 1 shows the potential pathways that could regulate the expression of mesenchymal Wnt5a in the development of BPD.
Figure 1.
NFκB (nuclear factor-κB) drives the transcription of pro-IL1β, and the NLRP3 inflammasome controls the formation of mature IL1β. IL1β increases the expression of Wnt5a in the mesenchyme, leading to increased Wnt signaling in type 2 alveolar epithelial cells. Wnt5a expression can also be increased by several other signaling pathways such as Hedgehog, TGFβ, and Notch. The mechanisms leading to impaired alveolar development secondary to increased Wnt signaling in the pulmonary mesenchyme and type 2 alveolar epithelial cells still need to be elucidated.
NFκB-mediated increase in Wnt5a expression impaired alveolarization in this study. However, other studies have reported beneficial effects of NFκB signaling in alveolarization (24), specifically protecting the saccular and alveolar lung from hyperoxia-induced injury (25). Even though the upstream regulation of Wnt5a may be through NFκB, therapeutic strategies focused on Wnt5a may alleviate hyperoxic lung injury while preserving the other beneficial aspects of NFκB signaling in the developing lung. The mechanisms leading to impaired alveolar development secondary to increased Wnt signaling in the pulmonary mesenchyme and type 2 AECs need to be elucidated. We look forward to future studies that will build on the ex vivo results in the in vivo model and delineate the potential mechanisms accounting for Wnt5a-mediated effects.
Supplementary Material
Footnotes
Supported by NIH grant K08-HL127103 and grants R03-HL141572 and R01-HL14775 (K.L.).
Originally Published in Press as DOI: 10.1164/rccm.202002-0277ED on February 26, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ota C, Baarsma HA, Wagner DE, Hilgendorff A, Königshoff M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol Cell Pediatr. 2016;3:34. doi: 10.1186/s40348-016-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Volckaert T, De Langhe SP. Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development. Dev Dyn. 2015;244:342–366. doi: 10.1002/dvdy.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Popova AP, Bentley JK, Anyanwu AC, Richardson MN, Linn MJ, Lei J, et al. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L439–L448. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hummler SC, Rong M, Chen S, Hehre D, Alapati D, Wu S. Targeting glycogen synthase kinase-3β to prevent hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol. 2013;48:578–588. doi: 10.1165/rcmb.2012-0383OC. [DOI] [PubMed] [Google Scholar]
- 6. Li J, Yu K-H, Oehlert J, Jeliffe-Pawlowski LL, Gould JB, Stevenson DK, et al. Exome sequencing of neonatal blood spots and the identification of genes implicated in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:589–596. doi: 10.1164/rccm.201501-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sucre JMS, Vickers KC, Benjamin JT, Plosa EJ, Jetter CS, Cutrone A, et al. Hyperoxia injury in the developing lung is mediated by mesenchymal expression of Wnt5A. Am J Respir Crit Care Med. 2020;201:1249–1262. doi: 10.1164/rccm.201908-1513OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rydell-Törmänen K, Zhou X-H, Hallgren O, Einarsson J, Eriksson L, Andersson-Sjöland A, et al. Aberrant nonfibrotic parenchyma in idiopathic pulmonary fibrosis is correlated with decreased β-catenin inhibition and increased Wnt5a/b interaction. Physiol Rep. 2016;4:e12727. doi: 10.14814/phy2.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baarsma HA, Skronska-Wasek W, Mutze K, Ciolek F, Wagner DE, John-Schuster G, et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med. 2017;214:143–163. doi: 10.1084/jem.20160675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- 11. Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 12. Sucre JMS, Jetter CS, Loomans H, Williams J, Plosa EJ, Benjamin JT, et al. Successful establishment of primary type II alveolar epithelium with 3D organotypic coculture. Am J Respir Cell Mol Biol. 2018;59:158–166. doi: 10.1165/rcmb.2017-0442MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu G, Betts C, Cunoosamy DM, Åberg PM, Hornberg JJ, Sivars KB, et al. Use of precision cut lung slices as a translational model for the study of lung biology. Respir Res. 2019;20:162. doi: 10.1186/s12931-019-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang D-H, Yoon J-Y, Lee S-H, Bryja V, Andersson ER, Arenas E, et al. Wnt5a is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving both Wnt/beta-catenin and protein kinase Calpha. Circ Res. 2009;104:372–379. doi: 10.1161/CIRCRESAHA.108.185405. [DOI] [PubMed] [Google Scholar]
- 15. Yuan K, Shamskhou EA, Orcholski ME, Nathan A, Reddy S, Honda H, et al. Loss of endothelium-derived Wnt5a is associated with reduced pericyte recruitment and small vessel loss in pulmonary arterial hypertension. Circulation. 2019;139:1710–1724. doi: 10.1161/CIRCULATIONAHA.118.037642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Savani RC. Modulators of inflammation in bronchopulmonary dysplasia. Semin Perinatol. 2018;42:459–470. doi: 10.1053/j.semperi.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raymond M, Marchbank T, Moyer MP, Playford RJ, Sanderson IR, Kruidenier L. IL-1β stimulation of CCD-18co myofibroblasts enhances repair of epithelial monolayers through Wnt-5a. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1270–G1278. doi: 10.1152/ajpgi.00458.2011. [DOI] [PubMed] [Google Scholar]
- 18. Lee JG, Heur M. Interleukin-1β-induced Wnt5a enhances human corneal endothelial cell migration through regulation of Cdc42 and RhoA. Mol Cell Biol. 2014;34:3535–3545. doi: 10.1128/MCB.01572-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ge X, Ma X, Meng J, Zhang C, Ma K, Zhou C. Role of Wnt-5A in interleukin-1beta-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheum. 2009;60:2714–2722. doi: 10.1002/art.24779. [DOI] [PubMed] [Google Scholar]
- 20. Shi S, Man Z, Li W, Sun S, Zhang W. Silencing of Wnt5a prevents interleukin-1β-induced collagen type II degradation in rat chondrocytes. Exp Ther Med. 2016;12:3161–3166. doi: 10.3892/etm.2016.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang S-G, Ryu J-H, Kim I-C, Jho E-H, Jung H-C, Kim K, et al. Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J Biol Chem. 2004;279:26597–26604. doi: 10.1074/jbc.M401401200. [DOI] [PubMed] [Google Scholar]
- 22. Ge X-P, Gan Y-H, Zhang C-G, Zhou C-Y, Ma K-T, Meng J-H, et al. Requirement of the NF-κB pathway for induction of Wnt-5A by interleukin-1β in condylar chondrocytes of the temporomandibular joint: functional crosstalk between the Wnt-5A and NF-κB signaling pathways. Osteoarthritis Cartilage. 2011;19:111–117. doi: 10.1016/j.joca.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 23. Katoh M, Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med. 2009;23:763–769. doi: 10.3892/ijmm_00000190. [DOI] [PubMed] [Google Scholar]
- 24. Iosef C, Alastalo T-P, Hou Y, Chen C, Adams ES, Lyu S-C, et al. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1023–L1036. doi: 10.1152/ajplung.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang G, Abate A, George AG, Weng Y-H, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest. 2004;114:669–678. doi: 10.1172/JCI19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.