Pulmonary arterial hypertension (PAH) is a progressive and fatal disease with no cure. Pulmonary arterial pressure (PAP) is a function of cardiac output and pulmonary vascular resistance (PVR). In patients with PAH, the increased PAP is mainly due to increased PVR. The major causes for the elevated PVR and PAP are sustained pulmonary vasoconstriction, concentric pulmonary vascular remodeling, in situ thrombosis, and increased pulmonary vascular wall stiffness. Current therapies, however, target mainly vasoconstriction, and despite the advent of 14 medical therapies across three vasodilation pathways (prostacyclin, nitric oxide, and endothelin-1), the disease remains severe and life threatening. Pulmonary vascular remodeling is caused, at least in part, by increased proliferation and/or survival of resident pulmonary vascular cells, and antiproliferative/proapoptotic strategies are now under development as disease-modifying therapies for PAH.
Yet the mechanisms for vasoconstriction (and/or vasodilation) and vascular remodeling are not entirely separate. Drugs that are effective for treatment of PAH may attenuate pulmonary vasoconstriction and also inhibit pulmonary vascular remodeling. One of the therapeutic examples is prostacyclin or its chemically stable analog, treprostinil, a potent vasodilator that is currently in use for the treatment of PAH. It has been noted that, in addition to its vasodilatory effect, prostacyclin or treprostinil, when given in high doses, attenuates experimental pulmonary hypertension (PH) by inhibiting proliferation of pulmonary artery smooth muscle cells (PASMCs). Interestingly, in addition to canonical activation of the EP2 (prostaglandin E2 receptor 2) and the PPAR-γ (peroxisome proliferator–activated receptor-γ), prostacyclin inhibits cell proliferation induced by PDGF (platelet-derived growth factor) and transforming growth factor β, suggestive of EP2-independent mechanisms of action (1–3).
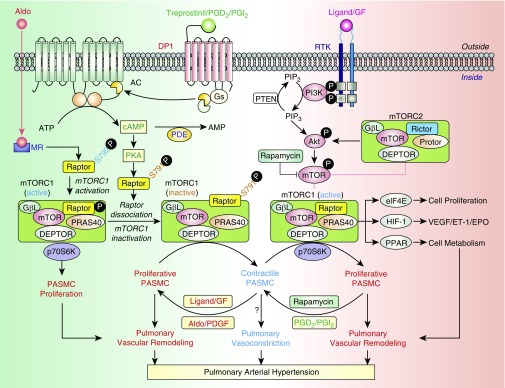
In this issue of the Journal, He and colleagues (pp. 1263–1276) uncover another enticing link between the vasodilatory prostacyclin axis and antiproliferative signaling in the pulmonary vasculature (4). The authors demonstrate that DP1 (D prostanoid receptor subtype 1), for which treprostinil acts as an agonist, is downregulated in PASMCs from patients with PAH and animals with experimental PH. Intriguingly, DP1 deficiency exacerbated pulmonary artery (PA) remodeling in mice with hypoxia-induced PH via activation of the mTOR (mechanistic target of rapamycin) complex 1 (mTORC1) (Figure 1). The mTOR, a fundamental regulator of cellular homeostasis, acts through two functionally distinct complexes, mTORC1 (mTOR-Raptor) and mTORC2 (mTOR-Rictor), to modulate multiple cellular processes, including cell growth, proliferation, and survival (5). Smooth muscle mTORC1 is activated in PAH/PH by multiple events, including hypoxia, the growth factor–mediated activation of receptor tyrosine kinase cascade, and the mTORC2–Akt axis (6–9) and, in turn, promotes PASMC proliferation and induces pulmonary vascular remodeling and PH (6–8, 10). The study by He and colleagues further expands our understanding of the important role of mTORC1 in PAH by providing compelling evidence that vasodilators (e.g., prostacyclin or treprostinil) target mTORC1 activity to inhibit PASMC proliferation and attenuate pulmonary vascular remodeling (4).
Figure 1.
mTORC1, a potential therapeutic target for pulmonary arterial hypertension (PAH). Activation of DP1 (D prostanoid receptor subtype 1) by prostaglandin D2 (PGD2), prostacyclin (PGI2), or the PGI2 analog treprostinil leads to the cAMP/PKA (protein kinase A)-mediated phosphorylation of Raptor at ser791 (S791). The DP1-mediated Raptor phosphorylation at S791 results in dissociation of Raptor from the mTORC1 complex and, subsequently, loss of mTORC1 activity, inhibition of pulmonary artery smooth muscle cell (PASMC) proliferation, and phenotypical transition of PASMCs from the proliferative to contractile phenotype. Activation of RTK (receptor tyrosine kinase) by mitogenic agonist/ligands and GFs (growth factors) (e.g., PDGF [platelet-derived growth factor]) leads to activation of the PI3K/Akt/mTORC1/p70S6K signaling cascade and, subsequently, PASMC proliferation, pulmonary vascular remodeling, and pulmonary hypertension (PH). Aldosterone (Aldo), by binding to the MR (mineralocorticoid receptor) or aldosterone receptor, a nuclear receptor that functions as a transcription factor, activates Akt and induces phosphorylation of Raptor at ser792 (S792). The Aldo/MR-mediated Raptor phosphorylation at S792 increases mTORC1 activity and promotes PASMC proliferation by increasing p70S6K phosphorylation. The proposed mechanisms indicate that 1) mitogenic and vasoconstrictor agonists (e.g., Aldo) and GFs (e.g., PDGF) activate mTORC1 by phosphorylating its critical component, Raptor (at S792); enhance PASMC proliferation through p70S6K and other factors (e.g., elF4E, HIF-1α [hypoxia-inducible factor-1α], and PPAR-γ [peroxisome proliferator-activated receptor-γ]); and, ultimately, induce pulmonary vascular remodeling and PH; and 2) antiproliferative and vasodilator agonists (e.g., PGI2 or treprostinil) reduce mTORC1 activity by phosphorylation of Raptor (at S791), inhibit PASMC proliferation, and, potentially, attenuate pulmonary vascular remodeling and PH. The mTORC1-associated phenotypical switching of PASMCs from the contractile to proliferative phenotype induced, for example, by PDGF is probably an early pathogenic mechanism of pulmonary vascular remodeling in PAH. The rapamycin-induced inhibition of mTOR and mTORC1, along with PGI2/treprostinil-mediated mTOR dissociation from the mTORC1 complex, would synergistically exert a therapeutic effect on PAH/PH by attenuating progression (or inducing regression) of pulmonary vascular remodeling. AC = adenylyl cyclase; PDE = phosphodiesterase.
The prostaglandin and aldosterone axes reciprocally regulate vascular tone, and the balance of these vasoactive agents plays an important role in homeostatic regulation of the pulmonary vascular structure and function and, ultimately, PAP. Aghamohammadzadeh and colleagues recently reported that the vasoconstrictor aldosterone plays a pro-proliferative role by promoting Ser792 phosphorylation of raptor, the core protein in mTORC1 complex, leading to PASMC-specific mTORC1 activation, hypertrophic vascular remodeling, and PH (11). New data from He and colleagues (4) show that the vasodilator prostacyclin (or its analog, treprostinil) could, in turn, reduce mTORC1 activity via DP1-dependent protein kinase A–induced phosphorylation of raptor at Ser791, resulting in its dissociation from mTORC1, loss of mTORC1 function, and amelioration of PH (4). Further supporting the important role of raptor in mTORC1 activation, a recent study from Tang and colleagues showed that genetic deletion of raptor in smooth muscle cells (SMCs) hinders development of experimental PH in mice (10). Together, these findings demonstrate reciprocal regulation of mTORC1 activity by vasoconstrictors and vasodilators and suggest the role for raptor (phosphorylated at Ser791 or Ser792) as a growth factor/RTK (receptor tyrosine kinase)-independent rheostat modulating mTORC1 activity and PASMC proliferation, depending on the balance of vasoconstrictors and vasodilators (Figure 1).
SMCs in normal PAs exhibit a fully differentiated, quiescent, and contractile phenotype. Vascular and endothelial injury, as well as imbalanced release of various growth and fibrotic factors from circulating and inflammatory cells, results in transition from contractile phenotype to synthetic or proliferative phenotype. SMCs with synthetic or proliferative phenotypes are highly proliferative, migratory, and dedifferentiated with upregulated extracellular matrix protein synthesis. The mTORC1 activity also plays an important role in regulating phenotypical transitions of vascular SMCs (12). Activation of mTORC1 by, for example, PDGF-BB, promotes the transition of SMCs from a contractile (fully differentiated) phenotype to a synthetic (highly proliferative) phenotype, whereas inhibition of mTORC1 with rapamycin (or activation of cAMP/PKA [protein kinase A] signaling by prostacyclin) induces SMC differentiation and inhibits SMC proliferation (13, 14). The emerging role of vasoconstrictors (e.g., aldosterone) and vasodilators (e.g., prostacyclin) in regulating mTORC1 activity also suggests the role for the early-stage vasoconstriction in mTORC1-dependent switching of PASMCs from contractile to synthetic/proliferative phenotype and, consequently, development of pulmonary vascular remodeling as PAH progresses.
Although the study from He and colleagues sheds light on the novel role of the DP1-mTORC1 axis in SMC pathology in PAH, several important questions remain to be answered (4). Prostaglandin D2/DP1 signaling plays an important role in inflammatory response (15), a key player in PAH pathogenesis, and the link between DP1 and mTORC1 in the inflammation and immunity in PAH remains to be established. Furthermore, the mTORC1 is upregulated in right ventricle (RV) myocardium from rats with severe experimental PH and contributes to cardiomyocyte hypertrophy (16). Although prostanoid receptors and PKA play important roles in RV remodeling (17), the role of DP1 in the right heart is not well studied, and a potential link between DP1 and mTORC1 in the hypertrophic RV response to PAH remains to be determined.
He and colleagues made an important observation that mTORC1 inhibitor rapamycin reduces PH in mice with SMC-specific DP1 deficiency (4), suggesting potential attractiveness of combining treprostinil and rapamycin as a therapeutic strategy to treat PAH. Of note, there has been significant interest in inhibition of mTORC1 to treat PAH, and a phase I/II clinical trial of an albumin-bound nanoparticle form of sirolimus (rapamycin), which increases accumulation of the drug in the lung, is ongoing (clinicaltrials.gov NCT02587325). Results presented to date have found good tolerability and potential clinical efficacy in 6-minute-walk distance, PVR, cardiac output, N-terminal pro–brain natriuretic peptide level, and the EmPHasis10 quality of life questionnaire in patients with PAH with functional class III disease on standard therapies, including prostacyclin and its analogs (18). Although not completed yet, this clinical study provides new insights into potential benefits of combining treprostinil with mTORC1 inhibition and paves the way for further studies of this combination as a therapeutic intervention in PAH.
Supplementary Material
Footnotes
Supported by NIH/NHLBI grants R01 HL113178 and HL130261 (E.A.G.), P01HL103455 and R01AG058659 (M.A.S.), and R35HL135870 (J.X.-J.Y.).
Originally Published in Press as DOI: 10.1164/rccm.202001-0087ED on January 22, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lambers C, Kornauth C, Oberndorfer F, Boehm PM, Tamm M, Klepetko W, et al. Mechanism of anti-remodelling action of treprostinil in human pulmonary arterial smooth muscle cells. PLoS One. 2018;13:e0205195. doi: 10.1371/journal.pone.0205195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel JA, Shen L, Hall SM, Benyahia C, Norel X, McAnulty RJ, et al. Prostanoid EP2 receptors are up-regulated in human pulmonary arterial hypertension: a key anti-proliferative target for treprostinil in smooth muscle cells. Int J Mol Sci. 2018;19:E2372. doi: 10.3390/ijms19082372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901. doi: 10.1183/09031936.00097107. [DOI] [PubMed] [Google Scholar]
- 4. He Y, Zuo C, Jia D, Bai P, Kong D, Chen D, et al. Loss of DP1 aggravates vascular remodeling in pulmonary arterial hypertension via mTORC1 signaling. Am J Respir Crit Care Med. 2020;201:1263–1276. doi: 10.1164/rccm.201911-2137OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, et al. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, et al. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;48:568–577. doi: 10.1165/rcmb.2012-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, et al. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 2011;25:1922–1933. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension: from cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang H, Wu K, Wang J, Vinjamuri S, Gu Y, Song S, et al. Pathogenic role of mTORC1 and mTORC2 in pulmonary hypertension. JACC Basic Transl Sci. 2018;3:744–762. doi: 10.1016/j.jacbts.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aghamohammadzadeh R, Zhang Y-Y, Stephens TE, Arons E, Zaman P, Polach KJ, et al. Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. FASEB J. 2016;30:2511–2527. doi: 10.1096/fj.201500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu M, Gomez D. Smooth muscle cell phenotypic diversity. Arterioscler Thromb Vasc Biol. 2019;39:1715–1723. doi: 10.1161/ATVBAHA.119.312131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45:A25–A32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14. Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. 2012;64:540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vijay R, Fehr AR, Janowski AM, Athmer J, Wheeler DL, Grunewald M, et al. Virus-induced inflammasome activation is suppressed by prostaglandin D2/DP1 signaling. Proc Natl Acad Sci USA. 2017;114:E5444–E5453. doi: 10.1073/pnas.1704099114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pena A, Kobir A, Goncharov D, Goda A, Kudryashova TV, Ray A, et al. Pharmacological inhibition of mTOR kinase reverses right ventricle remodeling and improves right ventricle structure and function in rats. Am J Respir Cell Mol Biol. 2017;57:615–625. doi: 10.1165/rcmb.2016-0364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viswanathan G, Mamazhakypov A, Schermuly RT, Rajagopal S. The role of G protein-coupled receptors in the right ventricle in pulmonary hypertension. Front Cardiovasc Med. 2018;5:179. doi: 10.3389/fcvm.2018.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon M, Gomberg-Maitland M, Oudiz RJ, Machado RF, Rischard FP, Elinoff JM, et al. ABI-009, nab-sirolimus, an mTOR inhibitor with high lung accumulation in preclinical models is active in patients with severe pulmonary arterial hypertension [abstract] Am J Respir Crit Care Med. 2019;199:A4409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.