Extended Data Figure 3. Plasma membrane localization and phosphorylation are required for BIK1 ubiquitination.
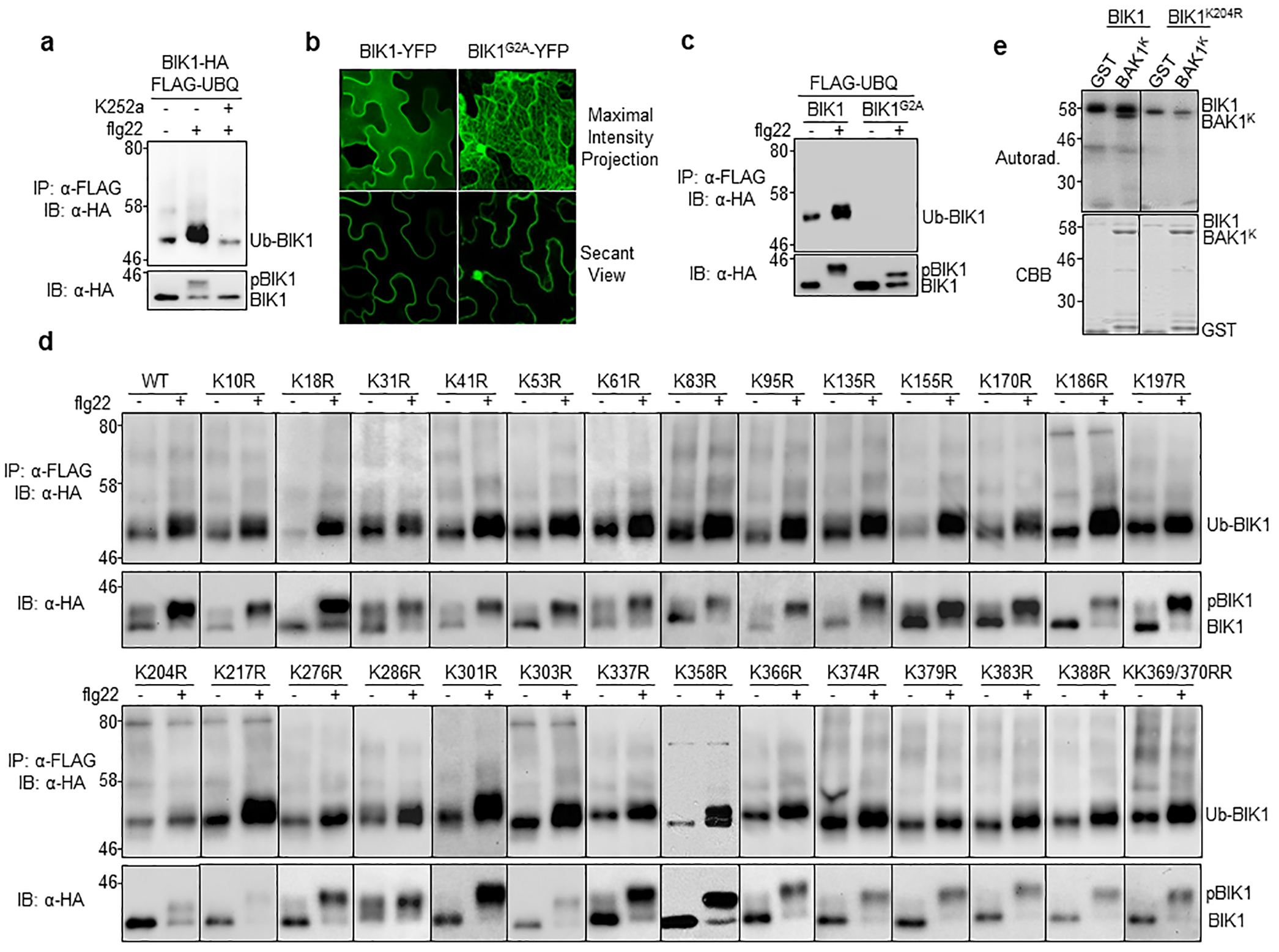
a. The kinase inhibitor K252a blocks flg22-induced BIK1 ubiquitination. Protoplasts transfected with FLAG-UBQ and BIK1-HA were treated with 1 μM K252a for 30 min prior to 100 nM flg22 treatment. b. BIK1G2A no longer localizes to PM. BIK1-YFP or BIK1G2A-YFP was expressed in N. benthamiana for imaging analysis. c. BIK1G2A compromises flg22-induced monoubiquitination. BIK1-HA or BIK1G2A-HA was co-expressed with FLAG-UBQ in protoplasts. d. Single K-to-R mutations of BIK1 fail to block flg22-induced ubiquitination without altering kinase activity. HA-tagged BIK1 WT or mutants was co-expressed with FLAG-UBQ in protoplasts. e. BIK1K204R exhibits reduced autophosphorylation and phosphorylation activity on BAK1. An in vitro kinase assay was performed using GST-BIK1 or GST-BIK1K204R as a kinase and GST or GST-BAK1K (BAK1 kinase domain without detectable autophosphorylation activity) as a substrate with [γ−32P] ATP. Proteins were separated with SDS-PAGE and analyzed by autoradiography (Autorad.) (top). Protein loading is shown by CBB staining (bottom). The experiments were repeated at least twice with similar results.
