Abstract
Background—
Cardiopulmonary exercise test (CPX) responses are strong predictors of outcomes in patients with heart failure. We recently developed a CPX score that integrated the additive prognostic information from CPX. The purpose of this study was to validate the score in a larger, independent sample of patients.
Methods and Results—
A total of 2625 patients with heart failure underwent CPX and were followed for cardiovascular (CV) mortality and major CV events (death, transplantation, left ventricular assist device implantation). Net reclassification improvement (NRI) for the score and each of its components were determined at 3 years. The VE/VCO2 slope was the strongest predictor of risk and was attributed a relative weight of 7, with weighted scores for abnormal heart rate recovery, oxygen uptake efficiency slope, end-tidal CO2 pressure, and peak VO2 having scores of 5, 3, 3, and 2, respectively. A summed score of >15 was associated with an annual mortality rate of 12.2% and a relative risk >9 for total events, whereas a score of <5 was associated with an annual mortality rate of 1.2%. The composite score was the most accurate predictor of CV events among all CPX responses considered (C indexes, 0.70 for CV mortality and 0.72 for the composite outcome). Each component of the score provided significant NRI compared with peak VO2 (category-free NRI, 0.61– 0.77), and the score provided significant NRI above clinical risk factors for both CV events and mortality (NRI, 0.63 and 0.65 for CPX score compared with clinical variables alone).
Conclusions—
These results validate the application of a simple, integrated multivariable score based on readily available CPX responses.
Keywords: epidemiology, exercise physiology, exercise testing, heart failure, oxygen consumption
Recent advances in therapy have resulted in a reduction in mortality for most forms of cardiovascular disease. However, success in treating other forms of cardiovascular disease along with aging of the population has resulted in an increase in the prevalence of chronic heart failure (HF).1,2 HF is now the leading cause of hospitalization among those aged >65 years, accounting for ≈20% of hospital admissions in this group.3 Therefore, a great deal of effort in recent years has been directed toward evaluation techniques designed to optimally stratify risk in these patients. A hallmark symptom of HF is exercise intolerance, typically evidenced by excessive shortness of breath, fatigue, or both. During the past 2 decades, the cardiopulmonary exercise test (CPX) has become an important procedure for quantifying the degree of exercise intolerance. Numerous studies have demonstrated that CPX responses powerfully stratify risk in patients with HF.4,5 Once generally limited to the assessment of peak VO2, indices of ventilatory inefficiency, heart rate recovery (HRR), and other responses have more recently been demonstrated to provide clinically significant and independent information for estimating prognosis in patients with HF.4–6
Clinical Perspective on p 218
There remains debate regarding the optimal application of CPX variables for estimating risk for mortality, hospitalization, or other outcomes in patients with HF. Similar to many other clinical tools, researchers have tended to take a binary approach when applying the CPX for this purpose. For example, a peak VO2 achieved ≤14 mL·kg−1·min−1 has been widely applied to define patients with HF at high risk for adverse events.4,7 More recently, there has been a growing awareness of the additional benefit of applying more complex statistical techniques and multivariate scores to predict risk in patients with cardiovascular disease,8–10 and HF specifically.6,11 The advantage of these approaches is that they permit the quantification of risk across the spectrum of abnormal responses10 and have been demonstrated to predict risk more accurately.6,10–12 These approaches have long been recommended for the standard exercise test to assist with the diagnosis of coronary artery disease8,9,13,14 and have recently been applied to CPX for estimating prognosis in patients with HF.6,11
We recently developed a CPX score using a summation of readily available responses that improved the prognostic utility of the test.6 CPX responses recently shown to be strong and independent predictors of outcomes in patients with HF pro-vided incremental, progressive, and independent information to the prediction of adverse outcomes. However, the sample used was relatively small, and a validation cohort for the score was not available. In addition, the association between a given risk marker and outcomes, despite generating a significant hazard, does not necessarily result in a higher reclassification of risk.15,16 Recently, statistical tests such as the net reclassifi-cation improvement (NRI) have been recommended to better quantify the ability of a measure to discriminate risk. The NRI improves on more standard indices of predictive modeling, such as the area under the receiver operating characteristic curve in that it more directly and incrementally evaluates the ability of new risk markers to classify subjects into higher or lower categories of risk.15–17 In the current study, we sought to: (1) validate a CPX score developed previously6 in a larger, independent sample of patients with HF; and (2) determine the contributions of individual and combined components of the CPX to enhance risk classification in patients with HF.
Methods
This study was performed as part of an HF consortium; a multicenter, retrospective analysis including patients with HF from the exercise laboratories at the VA Palo Alto Health Care System and Stanford University, Palo Alto, CA; San Paolo Hospital, Milan, Italy; Virginia Commonwealth University, Richmond, VA; Brigham and Women’s Hospital, Boston, MA; and the LeBauer Cardiovascular Research Foundation, Greensboro, NC. A total of 2625 patients with chronic HF, tested between 1993 and 2010, were included. The sample in-cluded 1974 men and 651 women, with a mean age of 56±14 years. Eighty-nine percent of the subjects were independent from the original sample from which the score was developed. Inclusion criteria consisted of a diagnosis of HF18 and evidence of left ventricular sys-tolic dysfunction (ejection fraction [EF] <40%) or HF with preserved EF by 2-dimensional echocardiography obtained within 1 month of exercise testing. HF with preserved systolic function was considered to be present if the EF was normal (>45%) and the subject had a his-tory of decompensated HF. Subjects received routine follow-up care at the 5 institutions included in the study. All subjects were stable and receiving optimal medical therapy at the time of testing. The subjects completed a written informed consent, and institutional review board approval was obtained at each institution.
CPX Procedure and Data Collection
Symptom-limited CPX was performed on all patients using treadmill or cycle ergometer ramping protocols.19 A treadmill was used for testing in the American centers, whereas a cycle ergometer was used in the European center. We previously observed that optimal peak VO2 and VE/VCO2 slope threshold values for estimating prognosis were similar irrespective of mode of exercise in patients with HF.20 Ventilatory expired gas analysis was performed using a metabolic cart at all 5 centers (Medgraphics CPX-D or ULTIMA PFX, Minneapolis, MN; Orca Diagnostics, Santa Barbara, CA; Parvo Medics TrueOne 2400, Sandy, UT; or CareFusion Oxycon Pro, San Diego, CA). Before each test, the equipment was calibrated in standard fashion using reference gases. A standard 12-lead ECG was obtained at rest, each minute during exercise, and for at least 5 minutes during the recovery phase; blood pressure was measured using a standard cuff sphygmomanometer.
Minute ventilation (VE, body temperature and pressure, saturated [BTPS]), oxygen uptake (VO2, standard temperature and pressure, dry [STPD]), carbon dioxide production (VCO2, STPD), and other CPX variables were acquired breath-by-breath and averaged over 10-or 15-second intervals. VE and VCO2 responses throughout exercise were used to calculate the VE/VCO2 slope via least squares linear re-gression (y=mx+b, where m=slope). Previous work by our group and others has shown this method of calculating the VE/VCO2 slope to be optimal for estimating prognosis.21,22 The oxygen uptake efficiency slope (OUES) was calculated using [(VO2 (L/min) = m (log10VE)+b, where m=OUES)].23 HRR was defined as maximal heart rate minus heart rate at 1 minute in recovery.24 Resting end-tidal CO2 pressure (PetCO2) was derived from the average of a 2-minute sitting resting period before the test.25
End Points
The primary end point was cardiac-related mortality. A second com-posite end point including major cardiac events was also studied; this included cardiac transplantation, left ventricular assist device (LVAD) implantation, and cardiac-related death. Subjects were followed for ma-jor cardiac-related events for 3 years after their exercise test using the Social Security Death Index and hospital and outpatient medical chart review. Follow-up was performed by the HF program at each respective institution, providing a high likelihood that all major events were cap-tured. Individuals conducting the CPX were not involved in decisions regarding cause of death or heart transplant/LVAD implantation.
Statistical Analysis
NCSS (Kayesville, UT) software and the Design and Hmisc libraries in S-Plus 7.0 and R (Seattle, WA) were used for all statistical analyses. Unpaired t tests were used for comparisons of continuous variables, and χ2 tests were used to compare categorical variables between those who experienced a cardiac event and those who did not. Receiver operating characteristic curve analysis was used to define optimal threshold values for each CPX response. Z tests were used to com-pare the areas under the receiver operating characteristic curves for CPX responses. Cox proportional hazards analysis was used to deter-mine age-adjusted hazard ratios for the 5 CPX variables included in the model, each expressed dichotomously using the threshold value. Optimal thresholds for each of the CPX variables were as follows: VE/VCO2 slope (≥34) abnormal HRR (≤6 beats at 1 minute), OUES (≤1.4), PetCO2 (<33 mm Hg), and peak VO2 (≤14 mL·kg−1·min−1). Each variable was assigned a weight according to the hazard ratios and summed to calculate the composite score. Proportional hazard assumptions were confirmed for each variable using the log [−log (survival function)] plot.
Kaplan–Meier analysis was used to determine overall and car-diovascular event-free survival characteristics for the summed score classifications 0 to 5, 6 to 10, 11 to 15, and >15. This analysis was repeated in 2 prespecified subgroups, which comprised subjects with left ventricular EF (LVEF) <30% and subjects with LVEF ≥30%. The log-rank test was used to determine statistical significance of the Kaplan–Meier analyses. Multivariable Cox proportional hazards analysis adjusted for age, sex, body mass index, EF, and HF patho-genesis was then used to calculate hazard ratios for each summed score classification group.
The predictive accuracies of each of the CPX responses were de-termined using both the right-censored concordance index (C index) validated with 200 bootstrap samples and the Akaike Information Criterion method.26 The predictive accuracy of the summed score was then evaluated via similar analyses in 4 prespecified subgroups: subjects with ischemic cardiomyopathy and nonischemic cardiomyopathy, and subjects with LVEF <30% and LVEF ≥30%. To further evaluate the reclassification characteristics of individual components of the CPX score, as well as the composite CPX score in compari-son with standard clinical risk factors (age, systolic blood pressure, HF pathogenesis, body mass índex, diuretic use, and LVEF), we calculated the category-free NRI index, modified for right-censored survival data according to the methods proposed by Pencina et al.27 The NRI was calculated for both cardiovascular-related mortality and major cardiovascular events. We corrected for overoptimism using 1000 bootstrap replicates and reported the median results and boot-strap estimated 95% confidence intervals (CIs).
Results
Baseline Characteristics of the Study Population and Development of the Summed Score
The study sample comprised 1974 men and 651 women with HF; 35% had an ischemic pathogenesis. The mean age of the cohort was 56±14 years, and the mean body mass index was 28.7±6.0 kg/m2. Subjects who died from cardiac causes were older and had a lower EF compared with subjects with no events (Table 1). Among CPX variables, peak VO2 (18.6±8.5 vs 14.1±5.4 mL·kg−1·min−1), peak heart rate, HRR, OUES, and PetCO2 were higher among those with no events. Conversely, resting heart rate, the VE/VCO2 slope and the CPX weighted summed score were lower among in the no event group versus those who died from cardiac causes. Peak VO2 was lower, whereas the VE/VCO2 slope and the weighted summed score were higher in patients who had a secondary outcome (LVAD or transplantation) versus both the no event and cardiac mortality groups.
Table 1.
Demographic and Cardiopulmonary Exercise Test Comparisons Between Survivors and Nonsurvivors
| Variables | No Events (n=2290) | Cardiac Mortality (n=224) | Secondary Outcomes (Transplant/LVAD) (n=121) | P Value* |
|---|---|---|---|---|
| Age, y | 55.6±14 | 61.0±13† | 50.3±13‡ | <0.0001 |
| BMI, kg/m2 | 28.7±6.1 | 27.8±5.6 | 27.7±5.8† | 0.022 |
| Ejection fraction, % | 36.7±15.8 | 31.1±13.4† | 22.6±11.7‡ | <0.0001 |
| NYHA Class | 2.32±0.83 | 2.69±0.77† | 3.06±0.69‡ | <0.0001 |
| Medications (n%) | ||||
| β-Blocker | 1410 (66.1) | 112 (55.2)† | 88 (73.9)§ | 0.001 |
| ACE inhibitor | 1261 (55.1) | 135 (60.3) | 72 (59.5) | 0.229 |
| Diuretic | 1056 (54.5) | 93 (60.8) | 98 (82.4)‡ | <0.0001 |
| Exercise test responses‖ | ||||
| Resting heart rate, beats/min | 75±14 | 80±16† | 75±15§ | <0.0001 |
| Maximal heart rate, beats/min | 130±26 | 122±22† | 110±27‡ | <0.0001 |
| Peak VO2 , ml·kg−1·min−1 | 18.8±8.5 | 14.9±5.6† | 12.1±3.8‡ | <0.0001 |
| Peak RER | 1.10±0.14 | 1.10±0.17 | 1.14±0.16‡ | 0.008 |
| HRR, beats | 20.1±13.4 | 13.7±10.3† | 15.9±11.8† | <0.0001 |
| VE/VCO2 slope | 32.5±8.6 | 37.6±11.7† | 42.3±11.7‡ | <0.0001 |
| OUES | 2.09±0.89 | 1.60±0.65† | 1.31±0.61† | <0.0001 |
| PetCO2, mm Hg | 33.9±4.6 | 32.6±4.4† | 31.5±3.9† | <0.0001 |
| Weighted summed score | 5.26±4.3 | 7.4±4.6† | 8.6±4.2‡ | <0.0001 |
ACE indicates angiotensin receptor blocker; BMI, body mass index; HRR, heart rate recovery at 1 min; LVAD, left ventricular assist device; NYHA, New York Heart Association; OUES, oxygen uptake efficiency slope; PetCO2, end-tidal carbon dioxide pressure; and RER, respiratory exchange ratio.
P value represents main effect.
P<0.05 vs no events.
P<0.05 vs no events and cardiac mortality.
P<0.05 vs cardiac mortality.
Using standard clinical indications, reasons for stopping exercise included 22% overall fatigue/exhaustion, 31% shortness of breath/dyspnea, 22% leg fatigue, 6% chest discomfort, 5% claudication, 0.4% knee pain, and 13.6% for other reasons.
There were 412 total adverse events (290 deaths, 79 trans-plantations, and 43 LVAD implantations) during the mean 2.4±2.5 year follow-up. The weighted scores for abnormal CPX responses were derived from proportional hazards analysis and replicated from the previous score6; weighted scores of 7, 5, 3, 3, and 2 were applied for the VE/VCO2 slope, HRR, OUES, PetCO2, and peak VO2, respectively. When only those patients taking β-blockers were studied, the relative weights were similar, with the exception that there was a lower weight for HRR (weight=2).
Predictors of Adverse Events
Age-adjusted univariate predictors of cardiovascular mortality, secondary events, and total events are presented in Table 2. Each of the CPX responses in the score was significantly associated with each of the outcomes, with an abnormal VE/VCO2 slope generating the highest risk (hazard ratios, 3.2 [95% CI, 2.5–4.3]; 8.3 [95% CI, 5.4–12.9]; and 4.3 [95% CI, 3.5–5.3]; all P<0.001) for cardiovascular mortality, secondary events, and total events, respectively). With a weighted summed score of 0 to 5 as the reference group, risks for all event categories were significantly higher as the weighted summed score categories increased from 6 to 10, 11 to 15, and >15. For total events, a score of >15 was associated with a hazard ratio of >9. These results were similar among patients with preserved and reduced EF. Each CPX response and the composite score also significantly predicted risk when expressed as continuous variables.
Table 2.
Age-adjusted Univariate Proportional Hazards Analysis
| Cardiac Mortality (n=224) |
LVAD/Transplant (n=121) |
Total Events (n=412)* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Age, y | 1.37 | 1.20–1.57 | <0.0001 | 1.46 | 1.21–1.77 | <0.0001 | 1.10 | 0.98–1.21 | 0.11 |
| BMI, kg/m2 | 1.11 | 0.97–1.27 | 0.14 | 1.09 | 0.90–1.33 | 0.39 | 1.11 | 1.00–1.25 | 0.06 |
| Ejection fraction, % | 1.55 | 1.33–1.81 | <0.0001 | 3.33 | 2.56–4.33 | <0.0001 | 1.95 | 1.71–2.23 | <0.0001 |
| NYHA Class | 1.92 | 1.39–2.66 | <0.0001 | 3.90 | 2.65–5.75 | <0.0001 | 2.53 | 1.99–3.21 | <0.0001 |
| Pathogenesis (ischemic) | 1.01 | 1.36–2.34 | <0.0001 | 1.98 | 1.34–2.95 | 0.0007 | 1.72 | 1.40–2.10 | <0.0001 |
| Peak VO2 ≤14 mL·kg−1·min−1 | 1.98 | 1.52–2.57 | <0.0001 | 6.07 | 4.06–9.05 | <0.0001 | 3.10 | 2.54–3.77 | <0.0001 |
| HRR≤6 beats at 1 min | 1.75 | 1.09–2.79 | 0.02 | 2.04 | 0.99–4.22 | 0.054 | 2.14 | 1.55–2.96 | <0.0001 |
| VE/VCO2 slope ≥34 | 3.24 | 2.47–4.26 | <0.0001 | 8.32 | 5.36–12.92 | <0.0001 | 4.29 | 3.48–5.29 | <0.0001 |
| OUES≤1.4 | 2.33 | 1.56–3.44 | <0.0001 | 5.75 | 2.79–11.90 | <0.0001 | 2.87 | 2.08–3.94 | <0.0001 |
| PetCO2<33 mm Hg | 1.77 | 1.25–2.51 | 0.001 | 3.11 | 1.96–4.95 | <0.0001 | 2.38 | 1.86–3.04 | <0.0001 |
| Weighted summed score | |||||||||
| 0 to 5 (n=1398) | 1 | (Reference) | 1 | (Reference) | 1 | (Reference) | |||
| 6 to 10 (n=841) | 2.33 | 1.72–3.16 | <0.0001 | 5.21 | 3.26–8.33 | <0.0001 | 2.74 | 2.16–3.48 | <0.0001 |
| 10 to 15 (n=335) | 3.26 | 2.28–4.67 | <0.0001 | 7.16 | 4.20–12.21 | <0.0001 | 4.6 | 3.55–5.98 | <0.0001 |
| >15 (n=38) | 4.31 | 1.87–9.9 | 0.0005 | 12.4 | 4.72–32.57 | <0.0001 | 9.25 | 5.75–14.88 | <0.0001 |
Age, BMI, and ejection fraction are increments using SD. BMI indicates body mass index; CI, confidence interval; HR, hazard ratios; HRR, heart rate recovery at 1 min; LVAD, left ventricular assist device; NYHA, New York Heart Association; OUES, oxygen uptake efficiency slope; and PetCO2, end-tidal carbon dioxide pressure.
Includes LVAD, transplant, and all causes of death.
Relationships Between Summed Score and Outcomes
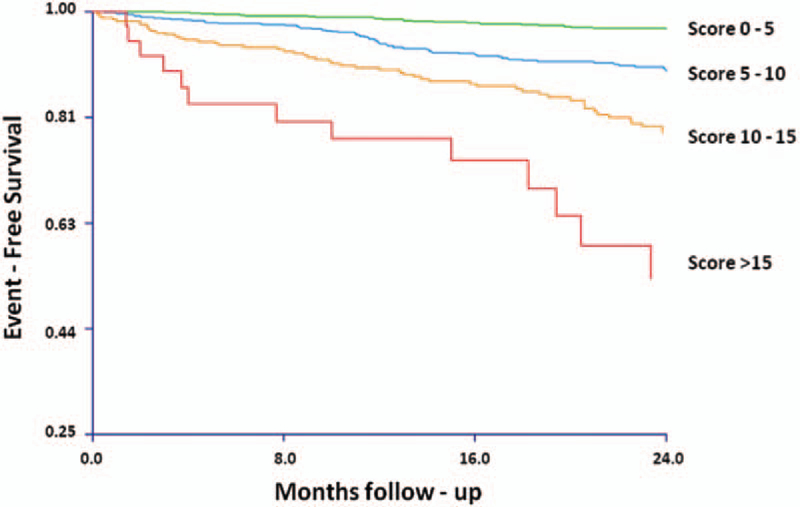
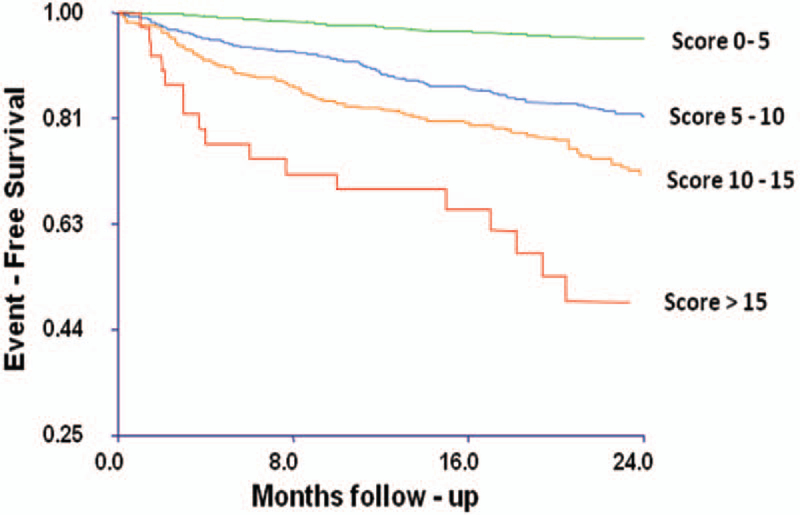
Overall mortality and the composite event-free Kaplan–Meier survival estimates according to summed score classifications are presented in Figures 1 and 2, respectively. There were significant stepwise increases in both mortality and composite outcome rates associated with increasing weighted summed scores. The estimated 1-year death rate was 12.2% for subjects with a summed score of >15 and only 1.2% for subjects with a summed score of <5. Similarly, whereas subjects with summed scores of >15 had estimated 1-year rates of death, transplantation, or LVAD of 17%, subjects with summed scores of <5 had rates of such events at 1 year of 2.8%. This stepwise increase in risk per-sisted in Kaplan–Meier subgroup analyses for both subjects with LVEF >30% and subjects with LVEF ≤30%, although subjects with LVEF >30% had lower overall event rates.
Figure 1.
Kaplan–Meier curves illustrating cumulative survival for increasing cardiopulmonary exercise test scores. P<0.01 by log-rank test.
Figure 2.
Kaplan–Meier curves illustrating event-free survival for the composite outcome (death, transplantation, and left ventricular assist device implantation) by increasing cardiopulmonary exercise test scores. P<0.01 by log-rank test.
Predictive Accuracy of CPX Variables and Summed Score
The predictive accuracy of CPX responses and the weighted summed score for mortality and major events are presented in Table 3. The VE/VCO2 slope was the most accurate predictor of outcomes among individual CPX variables (C index, 0.70 for major events), followed by peak VO2, OUES, PetCO2, and HRR. The summed risk score was a more accurate predictor of outcomes than any individual CPX variable (C indexes, 0.70 for cardiac mortality and 0.72 for major events, respectively). The predictive accuracy of the summed score for mortality and the composite outcome was similar in subjects with ischemic and nonischemic cardiomyopathy.
Table 3.
Predictive Accuracy of CPX Parameters and Composite Risk Score
| Cardiac Mortality |
Major Events |
|
|---|---|---|
| Variable | C Index | C Index |
| Peak VO2≤14 mL·kg−1·min−1 | 0.61 | 0.67 |
| HHR≤6 beats at 1 min | 0.61 | 0.60 |
| VE/VCO2 slope ≥34 | 0.66 | 0.70 |
| OUES≤1.4 | 0.62 | 0.65 |
| PetCO2<33 mm Hg | 0.58 | 0.61 |
| Composite risk score | 0.70 | 0.72 |
CPX indicates cardiopulmonary exercise test; HRR, heart rate recovery at 1 min; OUES, oxygen uptake efficiency slope; and PetCO2, end-tidal carbon dioxide pressure.
Table 4 presents age-adjusted Akaike Information Criterion weights for each individual CPX response and the summed score. The summed score had the highest predictive value (0.73, indicating a 73% probability of being the strongest model). The score remained the most powerful after adjust-ment for β-blocker use and after applying different cut points for high risk.
Table 4.
Predictive Accuracy of Cardiopulmonary Exercise Testing Parameters and Composite Risk Score
| Mortality |
||
|---|---|---|
| Predictive Model* | AIC | AIC Weight (%) |
| A | ||
| Peak VO2≤14 mL·kg−1·min−1 | 922 | 0.02 |
| B | ||
| HRR≤6 beats at 1 min | 944 | 0 |
| C | ||
| VE/VCO2 slope ≥34 | 916 | 0.49 |
| D | ||
| OUES≤1.4 | 925 | 0.005 |
| E | ||
| PetCO2<33 mm Hg | 937 | 0 |
| F | ||
| Peak VO2≤14 mL·kg−1·min−1 and VE/VCO2 slope ≥ 34 | 908 | 26.7 |
| G | ||
| Weighted summed score | 906 | 72.7 |
AIC indicates Akaike information criterion; HRR, heart rate recovery; OUES, oxygen uptake efficiency slope; and PetCO2, end-tidal carbon dioxide pressure.
Age-adjusted.
Classification of Risk
Table 5 presents category-free NRI indexes for major cardio-vascular events at 36 months for individual components of the CPX score. The VE/VCO2 slope, OUES, end-tidal PetCO2, and HRR all provided significant overall incremental risk reclassification. The risk reclassification improvement pro-vided by HRR was specific to individuals without cardiovascular events; all other components of the CPX score provided significant risk reclassification improvement both for subjects with cardiovascular events and those without cardiovascu-lar events at 36 months. Table 6 presents category-free NRI for the CPX score in comparison with standard clinical risk factors (age, systolic blood pressure, HF pathogenesis, body mass index, diuretic use, and LVEF). The CPX score provided significant NRI for both cardiovascular-related mortality (NRI=0.65; 95% CI, 0.61–0.69) and for cardiovascular events (NRI=0.63; 95% CI, 0.59–0.68) at 36 months.
Table 5.
Net Reclassification Improvement for Major Events at 36 Months According to Components of CPX Score
| Model | Event-free Survival Rate (All Subjects) | Event-free Survival Rate (Subjects Reclassified Up‡) | Event-free Survival Rate (Subjects Reclassified Down‡) | NRI, Subjects With Events | NRI, Subjects Without Events | NRI, All Subjects |
|---|---|---|---|---|---|---|
| Peak VO2+VE/VCO2 slope* | 0.87 | 0.77 (185) | 0.92 (293) | 0.31 (0.27 to 0.34) | 0.30 (0.29 to 0.31) | 0.61 (0.57 to 0.64) |
| Peak VO2+VE/VCO2 | 0.69 (131) | 0.93 (347) | 0.22 (0.18 to 0.25) | 0.55 (0.54 to 0.56) | 0.77 (0.73 to 0.80) | |
| slope+OUES† | ||||||
| Peak VO2+VE/VCO2 | 0.71 (188) | 0.93 (290) | 0.37 (0.33 to 0.40) | 0.29 (0.28 to 0.30) | 0.66 (0.62 to 0.69) | |
| slope+OUES+PetCO2† | ||||||
| Peak VO2+VE/VCO2 | 0.63 (79) | 0.92 (399) | −0.02 (−0.05 to 0.01) | 0.76 (0.76 to 0.78) | 0.74 (0.71 to 0.78) | |
| slope+OUES+PetCO2+HRR† | ||||||
CPX indicates cardiopulmonary exercise test; HRR, heart rate recovery at 1 min; OUES, oxygen uptake efficiency slope; PetCO2, end-tidal carbon dioxide pressure; and NRI, net reclassification improvement.
Compared with peak VO2 only.
Compared with model described in preceding row.
Numbers in parentheses represent numbers of subjects reclassified up or down.
Table 6.
Net Reclassification Improvement for Major Cardiovascular Events and Cardiovascular-Related Mortality at 36 Months According to CPX Score
| Event-free Survival Rate (All Subjects) | Event-free Survival Rate (Subjects Reclassified Up†) | Event-free Survival Rate (Subjects Reclassified Down†) | NRI, Subjects With Events | NRI, Subjects Without Events | NRI, All Subjects | |
|---|---|---|---|---|---|---|
| Major cardiovascular events | ||||||
| Clinical variables | 0.80 | … | … | … | … | … |
| Clinical variables+CPX score* | 0.69 (585) | 0.88 (826) | 0.36 (0.32–0.39) | 0.27 (0.26–0.29) | 0.63 (0.59–0.68) | |
| Cardiovascular-related mortality | ||||||
| Clinical variables | 0.88 | … | … | … | … | … |
| Clinical variables+CPX score* | 0.80 (568) | 0.93 (842) | 0.36 (0.33–0.40) | 0.29 (0.28–0.31) | 0.65 (0.61–0.69) | |
CPX indicates cardiopulmonary exercise test; and NRI, net reclassification improvement. Compared with model including clinical variables only: age, systolic blood pressure, heart failure etiology, body mass index, diuretic use, and left ventricular ejection fraction.
Numbers in parentheses represent numbers of subjects reclassified up or down.
Discussion
The current results provide a measure of validation for previously developed CPX score6 in a group of patients with HF that is significantly larger than the sample from which the score was developed. The multivariate score used common and easily derived CPX responses, and its application improved the classification risk for adverse events in patients with HF. The estimation of risk was incremental, with each component of the score adding progressively and independently to the pre-diction of outcomes. The composite CPX score was the most accurate predictor of outcomes among all the CPX responses considered, and accurately predicted risk for adverse events among patients with both ischemic and nonischemic cardio-myopathy and among both patients with LVEF >30% and ≤30%. The current data thus represent what is commonly termed a validation set or a measure of cross-validation for the original score.28 These findings further refine the application of CPX for the estimation of risk in patients with HF and may help to optimize the clinical decision-making process when evaluating these patients.
We used a novel index of risk classification, the NRI,15–17 to provide better insight into the individual components of CPX that are known to predict risk.4,6 The NRI reflects clinically meaningful improvement in risk classification achieved with each component of the score; it is calculated as the net change in risk among subjects after the addition of each marker to the baseline model (in the current case, standard clinical variables). Our results extend previous findings by demonstrating that each CPX response included in an integrated score (the VE/VCO2 slope, peak VO2, OUES, resting PetCO2, and HRR) added significant incremental improvement in risk reclassification. For example, adding individual components of the score to peak VO2 improved classification of subjects at risk (up or down) by ≈70% (Table 5); similarly, adding the inte-grated CPX score to clinical variables improved the classification of subjects at risk by >60% (Table 6). Thus, the addition of the score to a simple model of clinical risk factors significantly improved risk classification. In addition, these findings suggest that the individual components of the score reflect separate maladaptive pathways in HF and that each contributes to adverse event risk.
Although peak VO2 has been the most widely used CPX variable to predict risk, reliance on any single factor or statistic is generally known to have limited accuracy.15,29 For example, multivariate scores have long been recommended to enhance the diagnostic and prognostic accuracy of the standard exercise test and have been recommended in exercise testing guidelines.14 This is in part because of a growing awareness of the need to apply statistical techniques to develop evidence-based multivariable models for improving clinical decision making.10,12 The CPX score described herein is consistent with this approach, because it provides quantification of risk across the spectrum of abnormal responses. The performance of the score was similar to the original analysis6 both in terms of its predictive accuracy and overall risk using cumulative scores (Tables 3 and 4; Figures 1 and 2). Patients with a summed score of >15 had a >4-fold risk for cardiovascular mortality, a >12-fold risk for secondary events, and a >9-fold risk for total events. This is contrasted by the risk associated with, for example, an impaired peak VO2 alone, which had an age-adjusted hazard ratio of 2.0, and underscores the advantages of applying a multivariable approach as opposed to the commonly applied binary method. The CPX score yielded a 73% probability that the model was superior, as compared with the negligible probability when using any 1 of the variables alone, or even when compared with the combination of peak VO2 and the VE/VCO2 slope (Table 4).
In recent years, our group4,6,20,21,24,25 and others4,5,22,30 have demonstrated significant and independent prognostic value for each of the components of the score. One explanation for the strong and incremental prognostic power we observed is that the components of the score reflect different pathologies that are characteristic of HF. Although peak VO2 has long been recognized as an important prognostic marker in patients with HF, indices of ventilatory inefficiency have more recently been demonstrated to be important components of the risk para-digm,4,5 and their application is now advocated in guidelines on HF management.31,32 In particular, the VE/VCO2 slope has been widely studied and has been shown to be a more powerful predictor of risk than peak VO2,4,6,31 which is consistent with the current study (Tables 2 and 3). Abnormalities in ventilatory efficiency have been demonstrated to reflect ventilation/perfusion mismatching in the lungs (related, in part, to an impaired cardiac output response to exercise), early lactate accumulation, and abnormalities in respiratory control.4,5,31,32 Abnormal HRR is associated with autonomic dysfunction that is com-mon in HF (reflected by impaired vagal reactivation) and has been shown to provide prognostic power independent of peak VO2 and the VE/VCO2 slope.24
The present results extend previous findings from Aaronson et al11 who used peak VO2 along with several noninvasive clinical markers in a multivariate model to predict event-free survival in patients with HF (termed the Heart Failure Survival Score [HFSS]). Application of 7 noninvasive variables identified low- and high-risk groups with 93% and 43% 1-year event-free survival, respectively. The HFSS has outperformed peak VO2 alone in both US and European populations of patients with HF.11,33,34 Although the HFSS has been validated33 and widely used, it included only peak VO2 from CPX. Numerous recent studies have also incorporated indices of ventilatory inefficiency in addition to peak VO2 to predict prognosis in HF.4–6 All of these studies have demonstrated improved risk stratification by documenting inefficient ventilation in addition to impaired peak VO2. However, we are unaware of other multivariate scores focusing specifically on CPX responses.
Limitations
By design, the CPX score focuses primarily on the ventilatory gas exchange response to exercise and does not include other clinical markers of risk in HF. There are other CPX responses that predict risk, particularly oscillatory breathing,4,31 which were not included in the score. In addition, although the score was compared with simple clinical variables, it should be noted that there are many other variables and biomarkers that have been used to define risk in HF. A more complex score including some of these markers may provide better precision for estimating risk. Some patients had LVAD or transplantation as their end point, and given patients’ CPX responses likely influenced the decision to have these procedures, rais-ing a potential bias. Finally, the sample was 75% men, and the results may not be as applicable to women.
Summary
The current results extend the many recent studies demonstrating the strong prognostic value of CPX. Our findings validate a composite CPX score6 for predicting risk of adverse events in patients with HF; individual components of the score improved reclassification of risk for mortality and adverse events. The simple summation of easily derived responses from CPX can be applied to more accurately estimate risk in patients with HF.
Clinical Perspective.
The cardiopulmonary exercise test (CPX) has been widely used in recent years to stratify risk in patients with heart failure. However, the optimal method of applying CPX responses remains a topic of debate. We recently developed a multivariate CPX score that integrated the additive prognostic information from 5 CPX responses. The purpose of this study was to validate the score in a larger, independent sample of patients with heart failure. We studied 2625 adults with heart failure who underwent CPX and were followed for a mean of 29±30 months. The score was derived by weighting the age-adjusted prognostic power of 5 CPX variables using a summary of point-based risk scores. The VE/VCO2 slope (≥34) was attributed a relative weight of 7, with weighted scores for abnormal heart rate recovery at 1 minute, oxygen uptake efficiency slope, resting end-tidal CO2 pressure, and peak VO2 having scores of 5, 3, 3, and 2, respectively. A summed score of >15 was associated with an annual mortality rate of 12.2% and a relative risk of 9.2 for total events, whereas a score of <5 was associated with an annual mortality of 1.2%. The composite score was the most accurate predictor of cardiovascular events among all CPX responses considered. Each individual component of the score provided significant net reclassification improvement compared with peak VO2, and the score provided significant net reclassification improvement above clinical risk factors. These results validate the application of a simple, integrated multivariable CPX score; the score markedly improved risk classification when added to clinical variables, peak VO2, and other CPX responses.
Footnotes
Disclosures
None.
Contributor Information
Jonathan Myers, Division of Cardiology, VA Palo Alto Healthcare System, Palo Alto, CA; Cardiovascular Medicine, Stanford University, Palo Alto, CA.
Ricardo Oliveira, Rio de Janeiro State University, Rio de Janeiro, Brazil.
Frederick Dewey, Cardiovascular Medicine, Stanford University, Palo Alto, CA.
Ross Arena, Physical Therapy Program, Department of Orthopedics and Rehabilitation, and Division of Cardiology, Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
Marco Guazzi, Division of Cardiology, University of Milano, Milan, Italy.
Paul Chase Med, Lebauer Cardiovascular Research Foundation, Greensboro, NC.
Daniel Bensimhon, Lebauer Cardiovascular Research Foundation, Greensboro, NC.
Mary Ann Peberdy, Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA.
Euan Ashley, Cardiovascular Medicine, Stanford University, Palo Alto, CA.
Erin West, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA.
Lawrence P. Cahalin, Department of Physical Therapy, Leonard M. Miller School of Medicine, University of Miami, Miami, FL.
Daniel E. Forman, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mus-solino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics−−2010 update: a report from the American Heart Association. Circulation. 2010;121:e46. [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Car-diol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 3.Heart Failure Society of America 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:476–506. [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–269. [DOI] [PubMed] [Google Scholar]
- 5.Ingle L Theoretical rationale and practical recommendations for cardio-pulmonary exercise testing in patients with chronic heart failure. Heart Fail Rev. 2007;12:12–22. [DOI] [PubMed] [Google Scholar]
- 6.Myers J, Arena R, Dewey F, Bensimhon D, Abella J, Hsu L, Chase P, Guazzi M, Peberdy MA. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156:1177–1183. [DOI] [PubMed] [Google Scholar]
- 7.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. [DOI] [PubMed] [Google Scholar]
- 8.Morise AP, Jalisi F. Evaluation of pretest and exercise test scores to as-sess all-cause mortality in unselected patients presenting for exercise testing with symptoms of suspected coronary artery disease. J Am Coll Cardiol. 2003;42:842–850. [DOI] [PubMed] [Google Scholar]
- 9.Froelicher V, Morrow K, Brown M, Atwood E, Morris C. Prediction of atherosclerotic cardiovascular death in men using a prognostic score. Am J Cardiol. 1994;73:133–138. [DOI] [PubMed] [Google Scholar]
- 10.Ashley E, Myers J, Froelicher V. Exercise testing scores as an ex-ample of better decisions through science. Med Sci Sports Exerc. 2002;34:1391–1398. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to pre-dict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. [DOI] [PubMed] [Google Scholar]
- 12.Swets JA, Dawes RM, Monahan J. Better decisions through science. Sci Am. 2000;283:82–87. [DOI] [PubMed] [Google Scholar]
- 13.Fearon WF, Gauri AJ, Myers J, Raxwal VK, Atwood JE, Froelicher VF. A comparison of treadmill scores to diagnose coronary artery disease. Clin Cardiol. 2002;25:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr.; American College of Cardiology/American Heart Association Task Force on Prac-tice Guidelines (Committee to Update the 1997 Exercise Testing Guide-lines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207. [DOI] [PubMed] [Google Scholar]
- 16.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, Pencina MJ, Schoonmaker C, Wilson PW, D’Agostino RB, Vasan RS. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Car-diology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–82. [DOI] [PubMed] [Google Scholar]
- 19.Myers J, Buchanan N, Walsh D, Kraemer M, McAuley P, Hamilton-Wessler M, Froelicher VF. Comparison of the ramp versus standard exer-cise protocols. J Am Coll Cardiol. 1991;17:1334–1342. [DOI] [PubMed] [Google Scholar]
- 20.Arena R, Guazzi M, Myers J, Ann Peberdy M. Prognostic character-istics of cardiopulmonary exercise testing in heart failure: comparing American and European models. Eur J Cardiovasc Prev Rehabil. 2005;12:562–567. [DOI] [PubMed] [Google Scholar]
- 21.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124:720–727. [DOI] [PubMed] [Google Scholar]
- 22.Bard RL, Gillespie BW, Clarke NS, Egan TG, Nicklas JM. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J Heart Lung Transplant. 2006;25:589–595. [DOI] [PubMed] [Google Scholar]
- 23.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–1572. [DOI] [PubMed] [Google Scholar]
- 24.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151:851 e7–851.13. [DOI] [PubMed] [Google Scholar]
- 25.Arena R, Guazzi M, Myers J. Prognostic value of end-tidal carbon dioxide during exercise testing in heart failure. Int J Cardiol. 2007;117:103–108. [DOI] [PubMed] [Google Scholar]
- 26.Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev. 2004;11:192–196. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net re-classification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisser S Predictive Inference. New York, NY: Chapman and Hall; 1993. [Google Scholar]
- 29.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, El-kind MS, Go AS, Harrell FE Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC Jr, Wilson PW; American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies LC, Wensel R, Georgiadou P, Cicoira M, Coats AJ, Piepoli MF, Francis DP. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. Eur Heart J. 2006;27:684–690. [DOI] [PubMed] [Google Scholar]
- 31.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 32.Piepoli MF, Corrà U, Agostoni PG, Belardinelli R, Cohen-Solal A, Ham-brecht R, Vanhees L. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction. Recommendations for performance and interpretation. Task Force of the Italian Work-ing Group on Cardiac Rehabilitation and Prevention. Eur J Cardiovasc Prev Rehabil. 2006;13:300–311.16926657 [Google Scholar]
- 33.Lund LH, Aaronson KD, Mancini DM. Validation of peak exercise ox-ygen consumption and the Heart Failure Survival Score for serial risk stratification in advanced heart failure. Am J Cardiol. 2005;95:734–741. [DOI] [PubMed] [Google Scholar]
- 34.Zugck C, Krüger C, Kell R, Körber S, Schellberg D, Kübler W, Haass M. Risk stratification in middle-aged patients with congestive heart failure: prospective comparison of the Heart Failure Survival Score (HFSS) and a simplified two-variable model. Eur J Heart Fail. 2001;3:577–585. [DOI] [PubMed] [Google Scholar]