Abstract
Juvenile Neuronal Ceroid Lipofuscinosis (JNCL) is a lysosomal storage disease caused by autosomal recessive mutations in CLN3. Children with JNCL experience progressive visual, cognitive, and motor deterioration with a decreased life expectancy (late teens-early 20s). Neuronal loss is thought to occur, in part, via glutamate excitotoxicity; however, little is known about astrocyte glutamate regulation in JNCL. Spontaneous Ca2+ oscillations were reduced in murine Cln3Δex7/8 astrocytes, which were also observed following glutamate or cytokine exposure. Astrocyte glutamate transport is an energy-demanding process and disruptions in metabolic pathways could influence glutamate homeostasis in Cln3Δex7/8 astrocytes. Indeed, basal mitochondrial respiration and ATP production were significantly reduced in Cln3Δex7/8 astrocytes. These changes were not attributable to reduced mitochondria, since mitochondrial DNA levels were similar between WT and Cln3Δex7/8 astrocytes. Interestingly, despite these functional deficits in Cln3Δex7/8 astrocytes, glutamate transporter expression and glutamate uptake were not dramatically affected. Concurrent with impaired astrocyte metabolism and Ca2+ signaling, murine Cln3Δex7/8 neurons were hyper-responsive to glutamate, as reflected by heightened and prolonged Ca2+ signals. These findings identify intrinsic metabolic and Ca2+ signaling defects in Cln3Δex7/8 astrocytes that may contribute to neuronal dysfunction in CLN3 disease.
Keywords: Astrocyte, CLN3, mitochondrial dysfunction, calcium signaling
Introduction
Juvenile Neuronal Ceroid Lipofuscinosis (JNCL), or CLN3 disease, is a pediatric lysosomal storage disorder afflicting an estimated 1 in every 100,000 live births (The International Batten Disease Consortium 1995, Jalanko & Braulke 2009, Schultz et al. 2011). Children appear healthy until the onset of disease symptoms between the ages of 5-10 that initiates as vision loss, followed by seizures, dementia, and motor and cognitive decline, with premature death by the late teens to-early 20s (The International Batten Disease Consortium 1995, Aberg et al. 2000, Rakheja et al. 2007). JNCL is caused by autosomal recessive mutations in CLN3 (The International Batten Disease Consortium 1995, Williams & Mole 2012). Most common is a 1.02kb deletion that occurs in approximately 85% of mutated CLN3 alleles, which is considered to encode a truncated protein that is minimally expressed and/or rapidly degraded (The International Batten Disease Consortium 1995, Getty & Pearce 2011). While its function still remains unknown, CLN3 has been implicated in multiple cellular processes, including lysosomal acidification, amino acid transport, mitochondrial function, and intracellular Ca2+ regulation (Chandrachud et al. 2015, Chang et al. 2007, Fossale et al. 2004, Kyttala et al. 2006, Ramirez-Montealegre & Pearce 2005).
CLN3 pathology is characterized by the accumulation of lysosomal inclusions in all cell types, with neurons most dramatically affected (Haltia 2003, Williams et al. 2006). Current evidence suggests that inclusions are not a direct cause of neuron death, since many inclusion-positive neurons are not lost during the disease (Cooper et al. 2015, Cotman et al. 2002). In terms of alternative possibilities, previous studies have suggested that disruptions in glial function and loss of neuron homeostatic support may contribute to neuron dysfunction in JNCL. In particular, astrocytes have increased hemichannel (HC) opening during early disease that can serve as a conduit for ATP, Ca2+, and glutamate release (Burkovetskaya 2014, Orellana et al. 2011). Cln3-deficient astrocytes have also been reported to produce less neurotropic factors, which coincided with a negative impact on neuronal survival (Parviainen et al. 2017). In addition, Cln3Δex7/8 microglia exist in a primed pro-inflammatory state, producing exaggerated levels of several cytokines, such as IL-1β and TNF-α, that can potentiate astrocyte HC opening and augment glutamate release, disrupting cellular homeostasis (Bosch & Kielian 2014, Xiong & Kielian 2013). These data are supported by the finding that glutamate levels are elevated in the brains of both JNCL patients as well as Cln3 mouse models (Kovacs & Pearce 2008, Pears et al. 2005, Sitter et al. 2004, Anzai et al. 2006). The underlying mechanisms responsible for elevated glutamate in the JNCL brain remain to be identified, but collectively the available evidence suggests a disruption in vital astrocyte homeostatic functions is a contributing factor.
Astrocytes are the primary cell type responsible for regulating extracellular glutamate levels to maintain neuronal homeostasis (Sofroniew & Vinters 2010). Astrocyte projections surround the tripartite synapse and remove glutamate primarily through the Na2+-dependent glutamate symporters glutamate-aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1) (Beart & O'Shea 2007). Glutamate transporter action is an energy-demanding process, which requires the Na+/K+ ATPase to produce large quantities of ATP (Stobart & Anderson 2013). Disruption in any step of the glutamate uptake pathway can result in increased synaptic glutamate concentrations that are capable of inducing neuron excitotoxicity (Eid et al. 2004, Vercellino et al. 2007). In addition to CLN3 disease, alterations in glutamate homeostasis have been reported in several neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and other lysosomal storage disorders, such as Niemann-Pick type C (Assous et al. 2014, Byun et al. 2006, Kulijewicz-Nawrot et al. 2013, van der Hel et al. 2005). Besides maintaining CNS metabolic homeostasis, astrocytes control neurotransmitter release and neuronal signaling, in part, by regulating Ca2+ levels at the synapse (Eroglu & Barres 2010, Li et al. 2013, Ullian et al. 2001). Astrocytes interpret neuronal signaling patterns and communicate to surrounding cells via Ca2+ waves, which act as a glial signaling system for the propagation of both paracrine and distant signals in the CNS (Bazargani & Attwell 2016, Scemes & Giaume 2006). Strong evidence has emerged suggesting that perturbations in astrocyte signaling Ca2+ contribute to neuronal hyperactivity, loss of astrocyte homeostatic support, and disruption of the extracellular milieu (Manning & Sontheimer 1997, Seifert et al. 2006).
Here we present evidence of intrinsic abnormalities in Cln3Δex7/8 astrocytes that may account, in part, for neuroexcitotoxicity in the CLN3 brain. Specifically, Cln3Δex7/8 astrocytes displayed reduced Ca2+ oscillations, impaired mitochondrial activity, and ATP production. However, despite these intrinsic defects, glutamate transporter expression and glutamate uptake were not significantly different between Cln3Δex7/8 and WT astrocytes. Cln3Δex7/8 neurons were hyper-responsive to glutamate, exhibiting elevated and prolonged Ca2+ signals. Collectively, these findings reveal mechanisms that could impair astrocyte-neuron glutamate crosstalk and contribute to neuronal excitotoxicity during CLN3 disease progression.
Materials and Methods
Animals.
Male and female Cln3Δex7/8 mice (C57BL/6 background, The Jackson Laboratory; RRID:IMSR_JAX:004685), which harbor the same 1.02 kb deletion spanning exons 7 and 8 that occurs in approximately 85% of mutated CLN3 alleles in humans, were used for preparing primary astrocyte and neuron cultures (Cotman et al 2002). Both sexes of C57BL/6 mice were used as wild type (WT) controls (The Jackson Laboratory; RRID:IMSR_JAX:000664). Adult mice were bred under standard cage density conditions with 2 females and 1 male per cage with ad libitum access to food and water. For generating timed pregnant mice for primary neuronal cultures, the male was removed from females after a 24 h period, which was considered gestational day 1. Pregnancy was verified by the presence of vaginal plugs and/or weight gain. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and complies with the ARRIVE guidelines. The animal use protocol was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center (11-074-08-EP) and was not pre-registered. There was no randomization of animals for these studies; mouse embryos or pups were sacrificed from an individual timed pregnant female or litter, respectively, for preparing primary neuron and astrocyte cultures.
Experimental overview.
No blinding was performed during the preparation of primary cell cultures or during the course of experiments. There were no differences in sample sizes for each experiment between the beginning and end of the study.
Astrocyte and neuron cultures.
Primary WT and Cln3Δex7/8 astrocytes were prepared from mouse pups at postnatal days 2-3 as previously described (Esen et al 2007). Mouse pups were euthanized with an overdose of inhaled isoflurane and death was confirmed by the absence of heartbeat. For euthanasia, isoflurane was added to the bottom of a glass desiccator jar and pups separated from the irritating anesthetic by a 1 cm-thick porcelain base. Following isoflurane euthanasia, the cortex was dissected from both male and female pups and immediately placed in ice-cold phosphate buffered saline (PBS) supplemented with 2% fetal bovine serum (FBS; Atlanta Biologicals; Cat. #S11550). Cells were disassociated with trypsin and following several washes plated in 75mm2 flasks (one brain per flask) in Dulbecco Modified Eagle Medium (DMEM, 4.5 g/L glucose; Hyclone/ThermoFisher; Cat. #SH3002202) supplemented with 10% FBS, 200μM L-glutamine (Corning; Cat. #25-005-Cl), oxaloacetic acid/sodium pyruvate/insulin (OPI; Sigma; Cat. #O5003), 1X penicillin/streptomycin/fungizone (Corning; Cat. #30-004-Cl), and 100μM L-leucine methyl ester (L-LME; Sigma; Cat. #L1002) to induce microglial apoptosis and prevent their expansion. Upon reaching confluence (approximately 7-10 days in vitro; DIV), astrocytes were passaged every 3-4 days and were not used for experiments past three passages (DIV 30-40). To ensure the removal of residual, loosely adherent microglia, flasks were shaken at 200 rpm for 12 h prior to plating. This approach resulted in astrocyte purity of > 95% as revealed by glial fibrillary acidic protein (GFAP) staining.
Primary cultures of WT and Cln3Δex7/8 cortical neurons were prepared as previously described (Xiong & Kielian 2013). Timed pregnant mice were sacrificed using an overdose of inhaled isoflurane, as described above, and embryos (embryonic day 16; E16) were collected post-mortem. The cortex was dissected from E16 embryos and following trypsin treatment, cells were plated on polyethylenimine (PEI; Sigma; Cat. #P3143)-coated 10mm2 dishes at 106 cells/dish. Medium was changed 4 h after plating to remove non-adherent cells. Neurons were grown in Neurobasal medium (Life Technologies; Cat. #21103-0449) supplemented with L-glutamine, penicillin/streptomycin/fungizone, and B-27 supplement (ThermoFisher; Cat. #17504044). Cultures were treated with 1 μM cytosine arabinoside (AraC; Sigma; Cat. #C6645-100) to prevent glial expansion, beginning on DIV 3 and continuing until use in experiments. Every 3 days, half of the spent culture medium was replaced with fresh medium and cultures were not utilized until after DIV 10. Cultures contained a heterogeneous population of neurons in an effort to recapitulate neuronal heterogeneity within the cortex. The composition of excitatory and interneuron populations was not determined.
Seahorse mitochondrial stress and glycolysis assays.
Primary WT and Cln3Δex7/8 astrocytes were plated at 2x104 cells per well in 96-well plates and incubated for 12 h prior to treatment with combinations of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (PeproTech; Cat. # 314-10B and 211-11B, respectively; 10ng/ml each) or C6 ceramide (5μM; Sigma; Cat. #H6524) and neuronal lysate (1:5 dilution) for 24 h. Neuronal lysate was prepared from primary C57BL/6 wild type E16 neurons after multiple freeze-thaw cycles as previously described, since our prior report revealed no significant differences in microglial responses to neuronal lysates from WT or Cln3Δex7/8 mice (Xiong & Kielian 2013). Prior to the start of metabolic assays, culture medium was replaced with sodium bicarbonate- and serum-free medium. Seahorse XF Cell Mito Stress Test Kit (Cat. #103015-100) and Seahorse XFp Glycolysis Stress Test Kit (Cat. #103017-100) protocols were performed according to the manufacturer’s instructions (Agilent, Santa Clara, CA). For mitochondrial stress tests, oxygen consumption rate (OCR) was measured following sequential exposure to oligomycin (1μM; Sigma; Cat. #75351), carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP, 2μM; Sigma; Cat. #C2920), and rotenone (1μM; Sigma; Cat. #R8875). At the beginning of the assay, the Seahorse instrument measures changes in oxygen concentration in immediate proximity to cells to determine the baseline oxygen consumption rate. Next, oligomycin is injected to inhibit adenosine triphosphate (ATP) production by blocking ATP synthase. Following oligomycin treatment, FCCP is injected, which is a membrane uncoupler that causes an increase in oxygen consumption without ATP generation. Finally, the complex I inhibitor rotenone, is injected to completely block mitochondrial respiration. By sequentially inhibiting different stages of the electron transport chain, well-established algorithms can be used to calculate basal respiration, ATP production, maximal respiration, and non-mitochondrial oxygen consumption (Rogers et al. 2011, Tan et al. 2015).
To test mitochondrial function in neurons, primary WT and Cln3Δex7/8 neurons were plated at 5x104 cells per well in 96-well plates for 10 days prior to treatment for 24 h with combinations of TNF-α and IL-1β (10ng/ml each) or C6 ceramide (5μM) and neuronal lysate (1:5 dilution). For mitochondrial stress tests, oxygen consumption rate (OCR) was measured following sequential exposure to oligomycin (1μM), FCCP (1μM), and rotenone (1μM), whereupon mitochondrial respiration was calculated as described above for astrocytes.
To measure glycolytic activity, astrocytes were pre-incubated for 1 h in medium lacking sodium bicarbonate, serum, and glucose prior to initiating the assay to reduce intracellular glucose stores. Next, cells were sequentially exposed to glucose (2.5M; Sigma; Cat. #D6134), oligomycin (1μM), and 2-deoxy-D-glucose (100μM; Sigma; Cat. #D8375) and extracellular acidification rate (ECAR) measured. Total protein was quantified in each well following Seahorse assays to confirm lack of cell toxicity.
Mitochondrial DNA (mtDNA) content.
Total DNA was extracted from WT and Cln3Δex7/8 astrocytes or E16 neurons using a DNAeasy Blood and Tissue kit (Qiagen, Valencia, CA, Cat. #69504) according to the manufacturer’s instructions. mtDNA content was quantified using TaqMan primer-probe sets for the mitochondrial-specific genes NADH dehydrogenase 3 (ND3; Mm04225292_g1) and cytochrome c oxidase subunit-1 (Cox-1; Mm04225243_g1) as well as nuclear DNA (glyceraldehyde 3-phosphate dehydrogenase; GAPDH; Mm99999915_g1) (all from ThermoFisher) and analyzed using a BioRad CFX Connect Real Time system (BioRad). Results were expressed as the ratio of mtDNA (ND3 or Cox-1) to nuclear DNA (GAPDH).
Calcium signaling.
Primary WT and Cln3Δex7/8 neurons and astrocytes were prepared as described above and plated on 18mm PEI-coated glass coverslips. Cells were loaded for 30 min with the Ca2+ indicator dye 4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4′-methyl-2,2′-(ethylenedioxy)dianiline-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester (Fluo4-AM, 1μM; Life Technologies; Cat. #F14202) in the presence of artificial cerebral spinal fluid (ACSF; containing in mM: 124 NaCl, 26 NaHCO3, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 10 glucose, 0.5 ascorbic acid, 1.5 Na-pyruvate, 1 thiourea; pH 7.4, maintained by continuous bubbling with carbogen [95% O2 and 5% CO2]) and cells were continuously perfused with ACSF throughout the imaging period. For neurons, live cell imaging was performed for 1 min to acquire baseline fluorescence signals, whereupon cells were stimulated with 25nM glutamic acid (Sigma; Cat. #G8415) and images were captured every 5 sec for 10 min using AxioVision software (Zeiss; RRID:SCR_002677). For neurons, 3 dishes per experimental group were imaged (7-10 neurons per dish) and the experiment was replicated 3 times. For astrocytes, spontaneous Ca2+ oscillations with or without cytokine pre-treatment (TNF-α and IL-1β; 10ng/ml each for 24 h) were measured over a 5 min period with images taken every 5 sec for baseline measurements. Astrocytes were then stimulated with 10mM glutamate and intracellular Ca2+ signaling associated with the cell soma was quantified by changes in mean fluorescent intensity after normalization to baseline values. Three dishes per experimental group were imaged (20 astrocytes per dish) and the experiment was replicated 3-4 times.
Glutamate uptake assay.
Primary WT and Cln3Δex7/8 astrocytes were seeded at 2x104 cells per well in 96-well plates and incubated for 12 h prior to treatment with combinations of TNF-α and IL-1β (10ng/ml each) or C6 ceramide (5μM) and neuronal lysate (1:5 dilution) for 24 h. To examine glutamate uptake efficiency, astrocytes were exposed to 1mM glutamic acid in phenol red-free DMEM (ThermoFisher; Cat. #31053-028), whereupon supernatants were collected at 30 min and 2 h following glutamate treatment. Glutamate concentrations were immediately analyzed using an Amplex Red Glutamic Acid assay kit according to the manufacturer’s instructions (ThermoFisher, Cat. #A12221) with values normalized to the respective 1mM glutamate control at each time point.
Statistical analysis.
A Student’s t-test was used to analyze paired data sets of Cln3Δex7/8 and WT astrocytes for Ca2+ oscillations, Seahorse metabolic assays, mtDNA content, and neuronal Ca2+ oscillations using GraphPad Prism version 6.04 (San Diego, CA; RRID:SCR_002798). An assessment of data normality and a test to identify outliers were not performed for the datasets. For all analysis p < 0.05 was considered statistically significant.
Results
CLN3 mutation perturbs astrocyte Ca2+ oscillations.
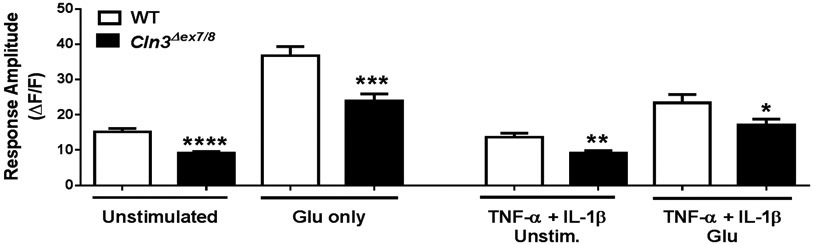
Astrocytes utilize Ca2+ signaling to communicate with surrounding cells as well as regulate synaptic firing. Glutamate released from neurons activates astrocyte glutamate transporters and triggers an increase in astrocytic intracellular Ca2+ (Dani et al. 1992, Volterra et al. 2014). Elevated intracellular Ca2+ in astrocytes can activate multiple glutamate regulatory pathways, including trafficking of GLAST transporters to the membrane, mobilizing mitochondria to increase energy production, and increased Na+-Ca2+ exchange pump activity to reduce K+ levels and neuronal firing (Bazargani & Attwell 2016, Mashimo et al. 2010, Stephen et al. 2014, Wang et al. 2012). Unchecked neuronal activity from impaired astrocyte regulation can increase glutamate concentrations, further potentiating glutamate dysregulation. To determine whether Ca2+ transients are perturbed in Cln3Δex7/8 astrocytes, live-cell Ca2+ imaging was performed. Cln3Δex7/8 astrocytes displayed decreased spontaneous Ca2+ oscillations under resting conditions, which was also observed when cells were exposed to proinflammatory cytokines that have previously been shown to be over-produced by Cln3Δex7/8 microglia (Figure 1) (Xiong & Kielian 2013). Following the assessment of basal spontaneous Ca2+ activity, astrocytes were exposed to glutamate to model extracellular neuronal signaling. Glutamate treatment increased intracellular Ca2+ in both WT and Cln3Δex7/8 astrocytes compared to baseline; however, levels were still significantly reduced in Cln3Δex7/8 cells (Figure 1). A single astrocyte is capable of simultaneously regulating multiple synapses, while at the same time one neuron may have its synapses enveloped by multiple astrocytes. Therefore, the reduction in Cln3Δex7/8 astrocyte Ca2+ signaling may not only have intrinsic effects or regulate local neuronal activity, but could also disrupt larger signaling networks.
Figure 1. CLN3 mutation alters astrocyte Ca2+ responses.
Primary WT and Cln3Δex7/8 astrocytes were unstimulated or treated with TNF-α and IL-1β (10ng/mL each) for 24 h and loaded with the Ca2+ indicator dye Fluo4-AM. Following a 5 min period for baseline readings, cells were exposed to 10mM glutamate (Glu) and the amplitude of the first Ca2+ response was calculated. Results are presented as the mean ± standard error of the mean (SEM) combined from three independent experiments with a total of 9 biological replicates (*, p < 0.05; **, p < 0.01; ****, p < 0.0001; Student’s t-test).
Mitochondrial respiration is impaired in Cln3Δex7/8 astrocytes.
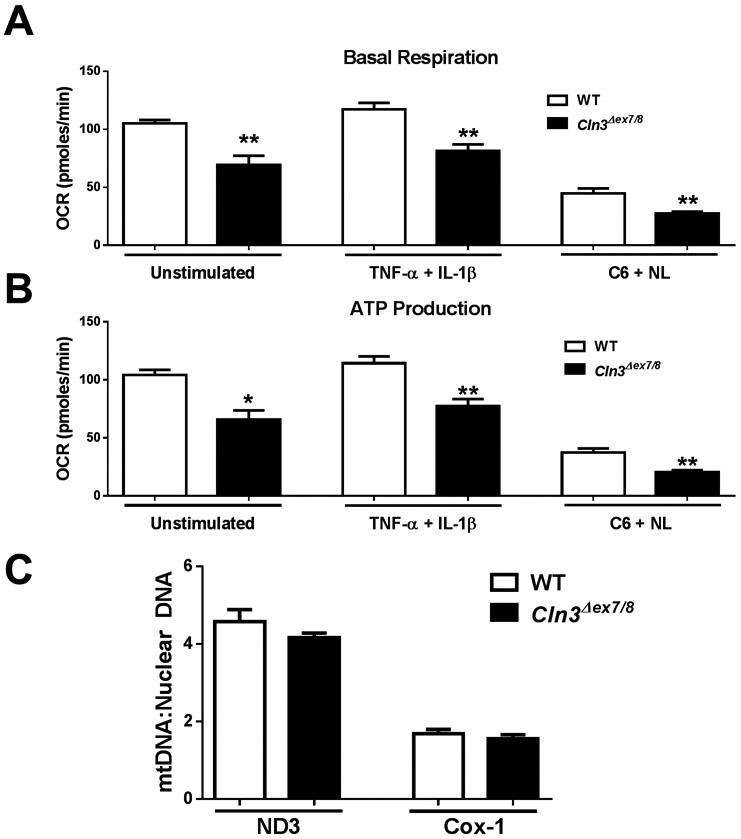
During periods of robust neuronal activity, astrocyte energy demands increase to maintain homeostatic functions (Stobart & Anderson 2013). Although neurons possess the highest metabolic requirements in the CNS, astrocytes account for approximately 20% of the energy usage in the brain during consciousness (Allaman et al. 2015). Astrocytes must maintain mitochondrial and glycolytic function to regulate neurotransmitter levels, ion homeostasis, and synaptic activity. Previous studies support mitochondrial dysfunction in JNCL, including increased mitochondrial oxidative stress molecules, mitochondrial membrane depolarization, and alterations in mitochondrial morphology (Cao et al. 2011, Chandrachud et al. 2015, Fossale et al. 2004, Hong et al. 2016, Kang et al. 2013). However, most of these studies were conducted with an immortalized Cln3Δex7/8 cerebellar cell line and none have examined mitochondrial activity in real-time in astrocytes. Here we utilized Seahorse bioassays to determine whether Cln3Δex7/8 astrocytes display defects in mitochondrial respiration. Under resting conditions, both basal respiration and ATP production were significantly reduced in Cln3Δex7/8 astrocytes (Figure 2A and B, respectively). Mitochondrial defects in Cln3Δex7/8 astrocytes were also evident when cells were exposed to JNCL danger signals (ceramide + neuronal lysate) or proinflammatory cytokines (TNF-α + IL-1β; Figure 2A and B). These mitochondrial defects could be attributed, in part, to dampened intracellular Ca2+ in Cln3Δex7/8 astrocytes, since mitochondrial respiration and ATP production are Ca2+-dependent and the failure to raise intracellular Ca2+ in Cln3Δex7/8 cells would be expected to interfere with mitochondrial function. Importantly, total protein concentrations were equivalent between WT and Cln3Δex7/8 astrocytes pre/post assay, indicating lack of toxicity (data not shown).
Figure 2. Mitochondrial respiration is impaired in Cln3Δex7/8 astrocytes.
Primary WT and Cln3Δex7/8 astrocytes were unstimulated or treated with TNF-α and IL-1β (10ng/mL each) or C6 ceramide (5μM) and neuronal lysate (NL) for 24 h, whereupon oxidative phosphorylation (Ox Phos) activity was examined using Seahorse Bioscience assays. (A) Basal mitochondrial respiration and (B) ATP production was determined based on oxygen consumption rate (OCR). Results are representative of three independent experiments with a total of 3 biological replicates (mean ± standard error of the mean (SEM). (C) Mitochondrial biomass was determined by quantiating NADH dehydrogenase 3 (ND3) and cytochrome c oxidase subunit-1 (Cox-1) expressed as a ratio to genomic DNA. Results are presented as the mean ± SEM of 4 biological replicates. Significant differences between WT and Cln3Δex7/8 astrocytes are denoted by asterisks (*, p < 0.05; **, p < 0.01; Student’s t-test).
To determine whether defective mitochondrial activity in Cln3Δex7/8 astrocytes was due to alterations in mitochondrial abundance, mtDNA was quantified. Examination of two mitochondrial-specific genes, ND3 and Cox-1, revealed no significant differences in mtDNA content between WT and Cln3Δex7/8 astrocytes (Figure 2C). These results further support the significance of the observed oxidative phosphorylation (Ox Phos) defects in Cln3Δex7/8 astrocytes, which are independent of mitochondrial abundance. Without sufficient ATP generation in the face of high neuronal activity, Cln3Δex7/8 astrocytes may be unable to regulate the CNS extracellular milieu.
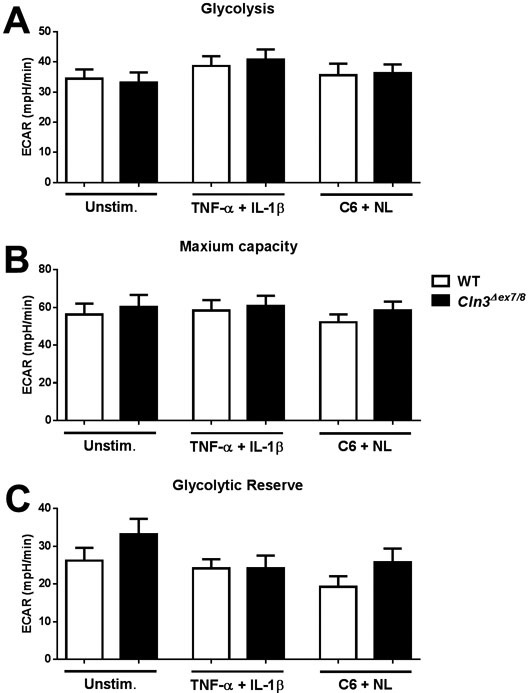
In instances of increased neuronal activity or distress, astrocytes augment glycolysis to produce more ATP. Since mitochondrial metabolism and ATP production were reduced in Cln3Δex7/8 astrocytes, we next examined whether cells shift to a more glycolytic profile to compensate. No significant differences in glycolytic rates were observed between Cln3Δex7/8 and WT astrocytes (Figure 3). This indicates that Cln3Δex7/8 astrocytes do not compensate for the reduction in mitochondrial ATP production by augmenting glycolytic pathways.
Figure 3. CLN3 mutation does not affect astrocyte glycolytic activity.
Primary WT and Cln3Δex7/8 astrocytes were unstimulated or treated with TNF-α and IL-1β (10ng/mL each) or C6 ceramide (5μM) and neuronal lysate (NL) for 24 h, whereupon glycolytic activity was examined using Seahorse Bioscience assays. (A) Glycolysis, (B) Maximum capacity, and (C) Glycolytic reserve was determined based on extracellular acidification rate (ECAR). Results are representative of three independent experiments with a total of 3 biological replicates (mean ± standard error of the mean (SEM).
Properly functioning mitochondria and metabolic pathways are critical for neuronal functions, such as neurotransmission and Ca2+ signaling. To determine if CLN3 mutation alters mitochondrial activity in neurons, Seahorse assays were performed using primary cortical neurons from WT and Cln3Δex7/8 mice. No significant changes in basal respiration or ATP production were detected under resting conditions between Cln3Δex7/8 and WT neurons (Supplemental Figure 1A and B). Similar results were observed when neurons were pre-treated for 24 h with cytokines (TNF-α + IL-1β) or danger signals (ceramide + neuronal lysate) (Supplemental Figure 1A and B). While a trend towards reduced mitochondrial activity was observed in Cln3Δex7/8 neurons, this was inconsistent and should be viewed with caution, since mtDNA levels were reduced in Cln3Δex7/8 neurons compared to WT (Supplemental Figure 1C).
Glutamate transporter expression and uptake are not significantly altered in Cln3Δex7/8 astrocytes.
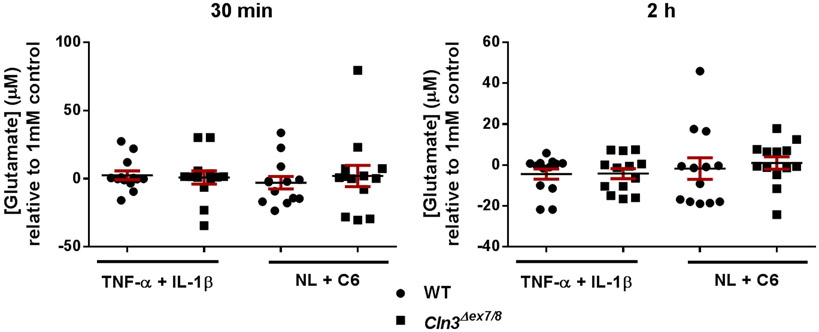
The excitatory neurotransmitter glutamate is tightly regulated in the CNS and the pathways responsible for maintaining glutamate homeostasis are highly conserved (Kanai & Hediger 2003, Kim et al. 2011). Disruptions in glutamate regulation have been implicated in CLN3 disease, where glutamate levels are elevated in Cln3 mouse models and treatment with NMDA or AMPA receptor antagonists improved motor function (Assous et al. 2014, Byun et al. 2006, Kovacs & Pearce 2008, Kovacs et al. 2012, Pears et al. 2005, Sitter et al. 2004). To explore the possibility of intrinsic alterations in glutamate transport in Cln3Δex7/8 astrocytes, we first examined glutamate transporter expression by Western blot. No significant differences in either GLT-1 or GLAST expression were detected between WT and Cln3Δex7/8 astrocytes under resting conditions or following cytokine (TNF-α + IL-1β) or glutamate treatment (data not shown). To determine whether glutamate uptake was affected in Cln3Δex7/8 astrocytes, cells were assessed for their ability to remove glutamate from the extracellular milieu using an Amplex Red glutamic acid assay. Following a 24 h pre-treatment period with danger signals (ceramide + neuronal lysate) or cytokines (TNF-α + IL-1β), astrocytes were exposed to glutamic acid (1mM), whereupon the amount of glutamate remaining in the supernatant at 30 min and 2 h was measured. Extracellular glutamate levels were similar between Cln3Δex7/8 and WT astrocytes in all treatment conditions (Figure 4). Collectively, the decreases in Ca2+ signaling and ATP production in Cln3Δex7/8 astrocytes do not significantly impact intrinsic glutamate regulatory pathways, instead suggesting possible defects in cell-cell communication with neurons.
Figure 4. Glutamate uptake is not altered in Cln3Δex7/8 astrocytes.
Primary WT and Cln3Δex7/8 astrocytes were treated with TNF-α and IL-1β (10ng/mL each) or C6 ceramide (5μM) and neuronal lysate (NL) for 24 h. Cells were then exposed to 1mM glutamate, whereupon supernatants were collected 30 min and 2 h later to evaluate residual extracellular glutamate concentrations. Results were normalized to the 1mM glutamate control and are presented as the mean ± standard error of the mean (SEM) combined from three independent experiments with a total of 3 biological replicates.
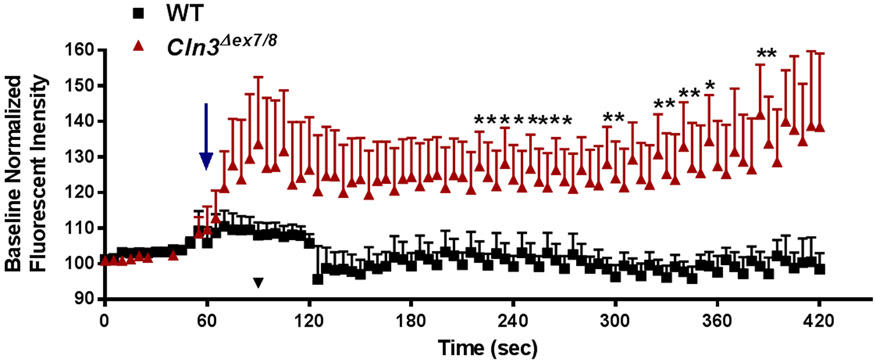
Cln3Δex7/8 neurons are hyper-excitable to extracellular glutamate.
Neurons are the principle cell type lost in CLN3 disease, which is thought to result, in part, from glutamate excitotoxicity (Haltia 2003, Williams et al. 2006, Chattopadhyay et al. 2002, Pears et al. 2005, Pontikis et al. 2005). Hyper-excitable or uninhibited excitatory neurons are a primary cause of seizures, which is a hallmark of disease in CLN3 patients (Cavus et al. 2016). To investigate the sensitivity of Cln3Δex7/8 neurons to physiological glutamate concentrations at the synaptic cleft (i.e. ~25nM) (Dzubay & Jahr 1999, Herman & Jahr 2007, Moussawi et al. 2011), live-cell Ca2+ imaging was performed. Glutamate application evoked a significant increase in intracellular Ca2+ in Cln3Δex7/8 neurons, reflecting their hyper-sensitivity to glutamate (Figure 5). After a 1 min stimulation period, glutamate washout was performed, which returned intracellular Ca2+ levels to baseline in WT but not Cln3Δex7/8 neurons, which is often used as an indication of impending excitotoxicity (Arundine & Tymianski 2004, Berdichevsky et al. 1983, Mattson 2007). Prior cytokine exposure (TNF-α and IL-1β) had no additive effect on the exaggerated Ca2+ flux in Cln3Δex7/8 neurons following glutamate stimulation (Supplemental Figure 2). Collectively, these studies suggest the existence of autonomous and non-cell autonomous mechanisms that may lead to glutamate dysregulation in CLN3 disease via impaired neuron and astrocyte activity. By extension, since Cln3Δex7/8 neurons are hyper-responsive to glutamate this could perpetuate glutamate release, which would ultimately lead to excitotoxicity and neuron death that is reminiscent of CLN3 disease.
Figure 5. Ca2+ signaling is disrupted in Cln3Δex7/8 neurons.
Primary WT and Cln3Δex7/8 neurons were loaded with the Ca2+ indicator dye Fluo4-AM. Following a 5 min period for baseline recordings, neurons were treated with 25nM L-glutamic acid and evaluated for a 420 sec period. Measurements were obtained using AxioVision software with fluorescent intensity normalized to baseline values. Results are presented as the mean ± standard error of the mean (SEM) combined from three independent experiments with a total of 3 biological replicates (*, p < 0.05; Student’s t-test). The arrow depicts the point of L-glutamic acid addition.
Discussion
Although CLN3 mutations were identified as the cause of JNCL in 1995, the precise function of CLN3 remains unknown (The International Batten Disease Consortium 1995, Kyttala et al. 2006). To date, CLN3 has been implicated in multiple processes critical for maintaining cellular homeostasis as well as dampening microglial proinflammatory activity (Chandrachud et al. 2015, Chang et al. 2007, Fossale et al. 2004, Kyttala et al. 2006, Ramirez-Montealegre & Pearce 2005, Xiong & Kielian 2013). Earlier studies have focused on the accumulation of lysosomal storage material within neurons, a hallmark of disease pathology and progression (The International Batten Disease Consortium 1995, Haltia 2003, Williams et al. 2006). However, there is little evidence directly implicating lysosomal inclusions as the cause of neuronal death (Bronson et al. 1993, Finn et al. 2011). For this reason, we chose to investigate other pathways that could contribute to neurodegeneration that is a hallmark of CLN3 disease.
One such pathway is glutamate regulation, which has been implicated as one mode of neurotoxicity in JNCL (Kovacs & Pearce 2008, Pears et al. 2005, Sitter et al. 2004, Anzai et al. 2006). However, not much is known about the mechanisms contributing to aberrant glutamate regulation in JNCL or the role of astrocytes in this process. It is likely that astrocyte activity influences neuronal survival in JNCL based on previous studies reporting that activated astrocytes coincide with brain regions where neurons are eventually lost in Cln3 mouse models (Pontikis et al. 2004, Pontikis et al. 2005) and recent evidence that Cln3Δ1-6 astrocytes negatively impact neuron survival in vitro (Parviainen et al. 2017). Likewise, it has been shown that increased astrocyte hemichannel activity and reduced expression of the glutamate transporter GLAST and glutamine synthetase are apparent in the Cln3Δex7/8 brain, which supports the concept of disrupted astrocyte glutamate regulation and homeostatic functions in JNCL (Burkovetskaya 2014). Here we present a potential model to account for increased extracellular glutamate in the JNCL brain, primarily centered on astrocyte-neuron crosstalk, since intrinsic astrocyte glutamate pathways are not dramatically affected (Figure 6). Our findings differ from a recent study that reported impaired glutamate uptake in Cln3Δ1-6 astrocytes (Parviainen et al. 2017). This discrepancy might be explained by the different Cln3 mouse models used or that Parviainen et al. examined glutamate uptake under resting conditions, whereas our study examined astrocytes that were exposed to JNCL-relevant stimuli. Specifically, our laboratory utilized Cln3Δex7/8 knock-in mice that harbor the same mutation that is observed in approximately 85% of mutated CLN3 alleles in JNCL patients (Cotman et al. 2002). In contrast, Parviainen et al. used Cln3Δex1-6 mice that lack the first six exons of CLN3, which may lead to some variations. Second, differences in astrocyte culture conditions can influence phenotypes. Along these lines, Parviainen et al. isolated astrocytes from mixed glial cultures in the presence of macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas our culture method enriched for astrocytes at the outset by limiting microglial expansion with L-leucine methyl ester, a widely used approach (Jebelli et al. 2015, Thiele et al. 1983), in the absence of growth factor supplementation. Therefore, the presence of growth factors during the culture period and initial contact with other glia may contribute to the differences in astrocyte glutamate phenotypes between the studies. In terms of the glutamate uptake assay, Parviainen et al. treated astrocytes with a higher concentration of glutamate (2mM) for a longer period of time (2 h), whereas in our study astrocytes were exposed to less glutamate (1mM) for a shorter period (1 h), and it is possible that our approach did not sufficiently stress the cells to detect differences. Nevertheless, it is important to note that both Parviainen et al. and our study reported Ca2+ defects in Cln3 astrocytes. Although our prior report documented reduced glutamate transporter expression, this was assessed in vivo with the full complement of interacting cell types, and phenotypes were evident in Cln3Δex7/8 mice at 3-6 months of age (Burkovetskaya 2014). It is possible that glutamate dysregulation may be observed in Cln3Δex7/8 astrocytes isolated from the adult brain, although this was not examined in the current study. Although we attempted to model disease-like conditions by treating astrocytes with mediators reportedly elevated post-mortem in the brains of CLN3 patients (i.e. ceramide and cytokines), this does not completely replicate the disease milieu in the brain. Nevertheless, we did identify several novel metabolic phenotypes in Cln3Δex7/8 astrocytes that may precede changes in glutamate regulation. The extracellular signals responsible for potential defects in astrocyte-neuron communication in Cln3Δex7/8 cells remain to be identified. Based on the existing literature, glutamate is a likely candidate, since glutamate levels are reportedly elevated in the CLN3 brain post-mortem as well as in Cln3 mouse models (Brockmann et al. 1996, Kovacs & Pearce 2008, Pears et al. 2005, Salek et al. 2011). Our study has identified defects in both Cln3Δex7/8 astrocytes and neurons in response to glutamate, namely decreased Ca2+ signaling in astrocytes coupled with neuronal hyper-excitability. Besides glutamate sensitivity, a reduction in growth factors or molecules responsible for stabilizing neuronal synapses might be altered, such as thrombospondin-1 that has been implicated in other neurological disorders (Christopherson et al. 2005, Crawford et al. 2012, Liauw et al. 2008, Risher & Eroglu 2012). Additional studies are needed to identify the key molecules in astrocyte-neuron crosstalk that are perturbed in the context of CLN3 mutation.
Figure 6. Proposed mechanism for aberrant astrocyte-neuron crosstalk during CLN3 disease.
Glutamate uptake by astrocytes is an energy-demanding process and ATP production was significantly decreased in Cln3Δex7/8 astrocytes, which coincided with impaired Ca2+ signaling. Cln3Δex7/8 neurons were hyper-sensitive to physiological concentrations of glutamate, which elicited heightened and prolonged Ca2+ signals that with time may contribute to neuronal dysfunction and/or death. Gln, glutamine; Glu, glutamate; GLAST, glutamate-aspartate transporter; GLT-1, glutamate transporter 1.
An interesting attribute of CLN3 disease that distinguishes it from other forms of Batten disease is the protracted nature of pathology (occurs over a period of 10-15 years) and the fact that disease symptoms do not typically manifest until around 5-10 years of age (Goebel & Wisniewski 2004, Mole et al. 1999). This suggests the existence of compensatory mechanisms to maintain CNS homeostasis until a disease threshold is achieved. Although the mechanisms responsible for this prolonged disease course are unknown, one possibility may be progressive glutamate accumulation that initially leads to excessive neuronal activity and ultimately cell death. This is also an attractive possibility from a clinical perspective, since seizures are often an early hallmark of CLN3 disease, which may be attributed to glutamate hyperactivity, followed by neuronal death that could be due to glutamate excitotoxicity (Augustine et al. 2015, Coulter & Eid 2012, Stobart & Anderson 2013). We found that Cln3Δex7/8 neurons were hyper-reactive in response to physiological glutamate concentrations at the synaptic cleft (Arundine & Tymianski 2004, Berdichevsky et al. 1983, Mattson 2007). By extension, not only do Cln3Δex7/8 neurons require less glutamate to be activated, but increased activation would augment glutamate release, perpetuating the pathological circuit (Figure 6). Cln3Δex7/8 neurons also maintained higher levels of intracellular Ca2+ following glutamate exposure, indicative of a longer activation state (Rueda et al. 2016). In addition, whereas intracellular Ca2+ levels in WT neurons returned to baseline within 1 min of stimulation, Cln3Δex7/8 neurons maintained exaggerated intracellular Ca2+ levels, which has been shown to precede excitotoxicity (Manning & Sontheimer 1997, Seifert et al. 2006). We recently reported that neuronal activity was exaggerated in the hippocampus and cortex of Cln3Δex7/8 mice between 1 and 4 months of age (Burkovetskaya et al. 2017), supporting the heightened neuronal responses presented here. A recent report also described neuronal perturbations in Cln3-deficient mice, but this was only examined at late stage disease (i.e. 14 months) (Grunewald et al. 2017). Based on heightened Ca2+ responses to glutamate, Cln3Δex7/8 neurons were expected to exhibit metabolic deficits. Interestingly, we found no significant differences in basal respiration or ATP production between WT and Cln3Δex7/8 neurons. This differs from a prior study with a Cln3Δex7/8 cerebellar granular cell line that reported significantly reduced ATP levels (Cao et al. 2011). Although a trend towards decreased mitochondrial function was observed, the results were inconsistent between experiments and the slight reductions may be explained by decreased mtDNA content in Cln3Δex7/8 neurons. Alternatively, it is possible that the heterogeneous population of cortical neurons examined here masked a subset-specific phenotype that might manifest on a single cell basis. This is supported by previous studies demonstrating that CLN3 mutation effects certain neuron populations (i.e. GABAergic interneurons) and brain regions (i.e. thalamus and somatosensory barrel field cortex) more than others (Pears et al. 2005, Pontikis et al. 2004, Pontikis et al. 2005).
Glutamate regulation is critical for maintaining optimal neuron-astrocyte signaling networks (Fonnum 1984, Li et al. 2013). This is an extremely energy-demanding process, requiring a constant supply of ATP that becomes even more pronounced during times of heightened neuronal activity (Stobart & Anderson 2013), as suggested by our findings of hyper-reactive Cln3Δex7/8 neurons. Using Seahorse metabolic assays, mitochondrial basal respiration and ATP production were significantly lower in Cln3Δex7/8 astrocytes, even at the resting state. Mitochondrial function was also impaired when Cln3Δex7/8 astrocytes were exposed to JNCL-relevant stimuli. Importantly, these changes were independent of mitochondrial numbers, since mtDNA content was similar between WT and Cln3Δex7/8 astrocytes. To the best of our knowledge, this is the first report to demonstrate disruptions in astrocyte mitochondrial function in the context of CLN3 mutation.
Astrocytes utilize Ca2+ signaling to communicate with surrounding astrocytes and neurons, sense extracellular cues, and regulate synaptic activity (Eroglu & Barres 2010, Li et al. 2013, Parpura et al. 1994, Ullian et al. 2001). Here we show that Cln3Δex7/8 astrocytes have lower spontaneous Ca2+ oscillations under resting conditions and following exposure to danger signals/proinflammatory cytokines. Ca2+ transients were also significantly reduced in Cln3Δex7/8 astrocytes in response to extracellular glutamate. This finding agrees with a recent study showing decreased Ca2+ waves in Cln3Δ1-6 astrocytes following ATP exposure (Parviainen et al. 2017), although responses to the JNCL-relevant stimuli tested here (i.e. glutamate and inflammatory cytokines) were not examined. Previous studies have shown that Cln3Δex7/8 astrocytes have significantly lower connexin expression coupled with decreased hemichannel activity in vivo as the disease progresses (Burkovetskaya et al. 2014). Since hemichannels from adjacent cells join to form gap junctions that are known to propagate Ca2+, this may account for the significant decrease in Ca2+ oscillations in Cln3Δex7/8 astrocytes. By extension, if Cln3Δex7/8 astrocytes are less responsive to extracellular signals, unable to propagate Ca2+ signals to surrounding astrocytes, and are not able to properly regulate synaptic activity, Cln3Δex7/8 neurons would continue to fire and release more glutamate, resulting in the pathological propagation of a dysfunctional glutamate circuit in CLN3 disease.
In summary, our study supports the following model to account for neuronal hyper-excitability in the context of CLN3 mutation (Figure 6). First, CLN3 loss leads to cell autonomous changes in neurons, resulting in hyper-sensitivity to low glutamate concentrations. Excessive excitatory activity in Cln3Δex7/8 neurons triggers heightened glutamate release into the synaptic cleft and the surrounding extracellular milieu. Persistent elevations in extracellular glutamate are known to further induce pre-existing neuroinflammatory pathways that trigger the release of cytotoxic mediators, inhibit cellular homeostatic functions, and induce apoptosis (Munhoz et al. 2008, Takaki et al. 2012, Vesce et al. 2007). Mitochondrial basal respiration, ATP production, and spontaneous Ca2+ oscillations were significantly reduced in Cln3Δex7/8 astrocytes, which can have a major impact on cell signaling and synaptic activity. Without proper astrocytic regulation, synaptic firing can escalate in Cln3Δex7/8 neurons, which may augment glutamate release and perpetuate the pathological cycle. Ultimately, this could be one mechanism to account for eventual neuronal loss associated with CLN3 disease.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the UNMC Dean’s Pediatric Research Fund and the UNMC Department of Pediatrics (to T.K.). M.E.B. was supported by a predoctoral fellowship from the National Institute of Neurological Disorders and Stroke (F31 NS093884-01A1). The authors thank Rachel Fallet for mouse colony management.
ABBREVIATIONS
- ACSF
artificial cerebral spinal fluid
- AraC
cytosine arabinoside
- ARRIVE
Animal Research: Reporting of In Vivo Experiments
- ATP
adenosine triphosphate
- Ca2+
calcium
- CLN3
ceroid lipofuscinosis 3
- Cox-1
cytochrome c oxidase subunit-1
- DIV
day in vitro
- DMEM
Dulbecco Modified Eagle Medium
- E16
embryonic day 16
- ECAR
extracellular acidification rate
- FBS
fetal bovine serum
- FCCP
carbonilcyanide p-triflouromethoxyphenylhydrazone
- Fluo4-AM
4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4′-methyl-2,2′-(ethylenedioxy)dianiline-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- GLAST
glutamate-aspartate transporter
- Gln
glutamine
- Glu
glutamate
- GLT-1
glutamate transporter 1
- HC
hemichannel
- IL-1β
interleukin-1β
- JNCL
juvenile neuronal ceroid lipofuscinosis
- L-LME
L-leucine methyl ester
- mtDNA
mitochondrial DNA
- ND3
NADH dehydrogenase 3
- OCR
oxygen consumption rate
- Ox Phos
oxidative phosphorylation
- PEI
polyethylenimine
- PBS
phosphate buffered saline
- TNF-α
tumor necrosis factor-α
- WT
wild type
Footnotes
CONFLICT OF INTEREST
Tammy Kielian is a Deputy Chief Editor for the Journal of Neurochemistry.
References
- (The International Batten Disease Consortium) Isolation of a novel gene underlying Batten disease, CLN3. (1995) Cell, 82, 949–957. [DOI] [PubMed] [Google Scholar]
- Aberg L, Liewendahl K, Nikkinen P, Autti T, Rinne JO and Santavuori P (2000) Decreased striatal dopamine transporter density in JNCL patients with parkinsonian symptoms. Neurology, 54, 1069–1074. [DOI] [PubMed] [Google Scholar]
- Allaman I, Belanger M and Magistretti PJ (2015) Methylglyoxal, the dark side of glycolysis. Front Neurosci, 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai Y, Hayashi M, Fueki N, Kurata K and Ohya T (2006) Protracted juvenile neuronal ceroid lipofuscinosis--an autopsy report and immunohistochemical analysis. Brain Dev, 28, 462–465. [DOI] [PubMed] [Google Scholar]
- Arundine M and Tymianski M (2004) Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cellular and molecular life sciences : CMLS, 61, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Had-Aissouni L, Gubellini P, Melon C, Nafia I, Salin P, Kerkerian-Le-Goff L and Kachidian P (2014) Progressive Parkinsonism by acute dysfunction of excitatory amino acid transporters in the rat substantia nigra. Neurobiology of disease, 65, 69–81. [DOI] [PubMed] [Google Scholar]
- Augustine EF, Adams HR, Beck CA et al. (2015) Standardized assessment of seizures in patients with juvenile neuronal ceroid lipofuscinosis. Developmental medicine and child neurology, 57, 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N and Attwell D (2016) Astrocyte calcium signaling: the third wave. Nature neuroscience, 19, 182–189. [DOI] [PubMed] [Google Scholar]
- Beart PM and O'Shea RD (2007) Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. British journal of pharmacology, 150, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky E, Riveros N, Sanchez-Armass S and Orrego F (1983) Kainate, N-methylaspartate and other excitatory amino acids increase calcium influx into rat brain cortex cells in vitro. Neuroscience letters, 36, 75–80. [DOI] [PubMed] [Google Scholar]
- Bosch M and Kielian T (2014) Hemichannels in neurodegenerative diseases: is there a link to pathology? Frontiers in cellular neuroscience, 8, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann K, Pouwels PJ, Christen HJ, Frahm J and Hanefeld F (1996) Localized proton magnetic resonance spectroscopy of cerebral metabolic disturbances in children with neuronal ceroid lipofuscinosis. Neuropediatrics, 27, 242–248. [DOI] [PubMed] [Google Scholar]
- Bronson RT, Lake BD, Cook S, Taylor S and Davisson MT (1993) Motor neuron degeneration of mice is a model of neuronal ceroid lipofuscinosis (Batten's disease). Annals of neurology, 33, 381–385. [DOI] [PubMed] [Google Scholar]
- Burkovetskaya M, Karpuk N and Kielian T (2017) Age-dependent alterations in neuronal activity in the hippocampus and visual cortex in a mouse model of Juvenile Neuronal Ceroid Lipofuscinosis (CLN3). Neurobiology of disease, 100, 19–29. [DOI] [PubMed] [Google Scholar]
- Burkovetskaya M, Karpuk N, Xiong J, Bosch M, Boska M, Takeuchi H, Suzumur A, and Kielian T (2014) Evidence for aberrant astrocyte hemichannel activity in Juvenile Neuronal Ceroid Lipofuscinosis (JNCL). PLoS One. [DOI] [PMC free article] [PubMed]
- Byun K, Kim J, Cho SY et al. (2006) Alteration of the glutamate and GABA transporters in the hippocampus of the Niemann-Pick disease, type C mouse using proteomic analysis. Proteomics, 6, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Cao Y, Staropoli JF, Biswas S, Espinola JA, MacDonald ME, Lee JM and Cotman SL (2011) Distinct early molecular responses to mutations causing vLINCL and JNCL presage ATP synthase subunit C accumulation in cerebellar cells. PLoS One, 6, e17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavus I, Romanyshyn JC, Kennard JT et al. (2016) Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: Microdialysis study of 79 patients at the yale epilepsy surgery program. Annals of neurology, 80, 35–45. [DOI] [PubMed] [Google Scholar]
- Chandrachud U, Walker MW, Simas AM et al. (2015) Unbiased Cell-based Screening in a Neuronal Cell Model of Batten Disease Highlights an Interaction between Ca2+ Homeostasis, Autophagy, and CLN3 Protein Function. The Journal of biological chemistry, 290, 14361–14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Choi H, Kim HJ, Jo DG, Jeon YJ, Noh JY, Park WJ and Jung YK (2007) Neuronal vulnerability of CLN3 deletion to calcium-induced cytotoxicity is mediated by calsenilin. Human molecular genetics, 16, 317–326. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Ito M, Cooper JD, Brooks AI, Curran TM, Powers JM and Pearce DA (2002) An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Human molecular genetics, 11, 1421–1431. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC et al. (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell, 120, 421–433. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Tarczyluk MA and Nelvagal HR (2015) Towards a new understanding of NCL pathogenesis. Biochimica et biophysica acta, 1852, 2256–2261. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Vrbanac V, Lebel LA et al. (2002) Cln3(Deltaex7/8) knock-in mice with the common JNCL mutation exhibit progressive neurologic disease that begins before birth. Human molecular genetics, 11, 2709–2721. [DOI] [PubMed] [Google Scholar]
- Coulter DA and Eid T (2012) Astrocytic regulation of glutamate homeostasis in epilepsy. Glia, 60, 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Jiang X, Taylor A and Mennerick S (2012) Astrocyte-derived thrombospondins mediate the development of hippocampal presynaptic plasticity in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32, 13100–13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A and Smith SJ (1992) Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron, 8, 429–440. [DOI] [PubMed] [Google Scholar]
- Dzubay JA and Jahr CE (1999) The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. The Journal of neuroscience : the official journal of the Society for Neuroscience, 19, 5265–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD et al. (2004) Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet, 363, 28–37. [DOI] [PubMed] [Google Scholar]
- Eroglu C and Barres BA (2010) Regulation of synaptic connectivity by glia. Nature, 468, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R, Kovacs AD and Pearce DA (2011) Altered sensitivity of cerebellar granule cells to glutamate receptor overactivation in the Cln3(Deltaex7/8)-knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. Neurochemistry international, 58, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F (1984) Glutamate: a neurotransmitter in mammalian brain. Journal of neurochemistry, 42, 1–11. [DOI] [PubMed] [Google Scholar]
- Fossale E, Wolf P, Espinola JA et al. (2004) Membrane trafficking and mitochondrial abnormalities precede subunit c deposition in a cerebellar cell model of juvenile neuronal ceroid lipofuscinosis. BMC neuroscience, 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty AL and Pearce DA (2011) Interactions of the proteins of neuronal ceroid lipofuscinosis: clues to function. Cellular and molecular life sciences : CMLS, 68, 453–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH and Wisniewski KE (2004) Current state of clinical and morphological features in human NCL. Brain pathology, 14, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald B, Lange MD, Werner C et al. (2017) Defective synaptic transmission causes disease signs in a mouse model of juvenile neuronal ceroid lipofuscinosis. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia M (2003) The neuronal ceroid-lipofuscinoses. Journal of neuropathology and experimental neurology, 62, 1–13. [DOI] [PubMed] [Google Scholar]
- Herman MA and Jahr CE (2007) Extracellular glutamate concentration in hippocampal slice. The Journal of neuroscience : the official journal of the Society for Neuroscience, 27, 9736–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Song KD, Lee HK, Yi S, Lee YS, Heo TH, Jun HS and Kim SJ (2016) Fibrates inhibit the apoptosis of Batten disease lymphoblast cells via autophagy recovery and regulation of mitochondrial membrane potential. In Vitro Cell Dev Biol Anim, 52, 349–355. [DOI] [PubMed] [Google Scholar]
- Jalanko A and Braulke T (2009) Neuronal ceroid lipofuscinoses. Biochimica et biophysica acta, 1793, 697–709. [DOI] [PubMed] [Google Scholar]
- Jebelli J, Piers T and Pocock J (2015) Selective Depletion of Microglia from Cerebellar Granule Cell Cultures Using L-leucine Methyl Ester. J Vis Exp, e52983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y and Hediger MA (2003) The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. European journal of pharmacology, 479, 237–247. [DOI] [PubMed] [Google Scholar]
- Kang S, Seo JH, Heo TH and Kim SJ (2013) Batten disease is linked to altered expression of mitochondria-related metabolic molecules. Neurochemistry international, 62, 931–935. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP et al. (2011) Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. Journal of cellular physiology, 226, 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD and Pearce DA (2008) Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Experimental neurology, 209, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Saje A, Wong A, Ramji S, Cooper JD and Pearce DA (2012) Age-dependent therapeutic effect of memantine in a mouse model of juvenile Batten disease. Neuropharmacology, 63, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulijewicz-Nawrot M, Sykova E, Chvatal A, Verkhratsky A and Rodriguez JJ (2013) Astrocytes and glutamate homoeostasis in Alzheimer's disease: a decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN neuro, 5, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A, Lahtinen U, Braulke T and Hofmann SL (2006) Functional biology of the neuronal ceroid lipofuscinoses (NCL) proteins. Biochimica et biophysica acta, 1762, 920–933. [DOI] [PubMed] [Google Scholar]
- Li D, Agulhon C, Schmidt E, Oheim M and Ropert N (2013) New tools for investigating astrocyte-to-neuron communication. Frontiers in cellular neuroscience, 7, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw J, Hoang S, Choi M et al. (2008) Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab, 28, 1722–1732. [DOI] [PubMed] [Google Scholar]
- Manning TJ Jr. and Sontheimer H (1997) Spontaneous intracellular calcium oscillations in cortical astrocytes from a patient with intractable childhood epilepsy (Rasmussen's encephalitis). Glia, 21, 332–337. [PubMed] [Google Scholar]
- Mashimo M, Okubo Y, Yamazawa T, Yamasaki M, Watanabe M, Murayama T and Iino M (2010) Inositol 1,4,5-trisphosphate signaling maintains the activity of glutamate uptake in Bergmann glia. The European journal of neuroscience, 32, 1668–1677. [DOI] [PubMed] [Google Scholar]
- Mattson MP (2007) Calcium and neurodegeneration. Aging Cell, 6, 337–350. [DOI] [PubMed] [Google Scholar]
- Mole SE, Mitchison HM and Munroe PB (1999) Molecular basis of the neuronal ceroid lipofuscinoses: mutations in CLN1, CLN2, CLN3, and CLN5. Hum Mutat, 14, 199–215. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S and Kalivas PW (2011) Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci, 5, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C and Leza JC (2008) Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res, 41, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C and Saez JC (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. Journal of neurochemistry, 118, 826–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S and Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature, 369, 744–747. [DOI] [PubMed] [Google Scholar]
- Parviainen L, Dihanich S, Anderson GW et al. (2017) Glial cells are functionally impaired in juvenile neuronal ceroid lipofuscinosis and detrimental to neurons. Acta Neuropathol Commun, 5, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears MR, Cooper JD, Mitchison HM, Mortishire-Smith RJ, Pearce DA and Griffin JL (2005) High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. The Journal of biological chemistry, 280, 42508–42514. [DOI] [PubMed] [Google Scholar]
- Pontikis CC, Cella CV, Parihar N et al. (2004) Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain research, 1023, 231–242. [DOI] [PubMed] [Google Scholar]
- Pontikis CC, Cotman SL, MacDonald ME and Cooper JD (2005) Thalamocortical neuron loss and localized astrocytosis in the Cln3Deltaex7/8 knock-in mouse model of Batten disease. Neurobiology of disease, 20, 823–836. [DOI] [PubMed] [Google Scholar]
- Rakheja D, Narayan SB and Bennett MJ (2007) Juvenile neuronal ceroid-lipofuscinosis (Batten disease): a brief review and update. Current molecular medicine, 7, 603–608. [DOI] [PubMed] [Google Scholar]
- Ramirez-Montealegre D and Pearce DA (2005) Defective lysosomal arginine transport in juvenile Batten disease. Human molecular genetics, 14, 3759–3773. [DOI] [PubMed] [Google Scholar]
- Risher WC and Eroglu C (2012) Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol, 31, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA and Murphy AN (2011) High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One, 6, e21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda CB, Llorente-Folch I, Traba J et al. (2016) Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochimica et biophysica acta, 1857, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Salek RM, Pears MR, Cooper JD, Mitchison HM, Pearce DA, Mortishire-Smith RJ and Griffin JL (2011) A metabolomic comparison of mouse models of the Neuronal Ceroid Lipofuscinoses. J Biomol NMR, 49, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E and Giaume C (2006) Astrocyte calcium waves: what they are and what they do. Glia, 54, 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz ML, Tecedor L, Chang M and Davidson BL (2011) Clarifying lysosomal storage diseases. Trends in neurosciences, 34, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Schilling K and Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nature reviews. Neuroscience, 7, 194–206. [DOI] [PubMed] [Google Scholar]
- Sitter B, Autti T, Tyynela J et al. (2004) High-resolution magic angle spinning and 1H magnetic resonance spectroscopy reveal significantly altered neuronal metabolite profiles in CLN1 but not in CLN3. Journal of neuroscience research, 77, 762–769. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV and Vinters HV (2010) Astrocytes: biology and pathology. Acta neuropathologica, 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen TL, Gupta-Agarwal S and Kittler JT (2014) Mitochondrial dynamics in astrocytes. Biochemical Society transactions, 42, 1302–1310. [DOI] [PubMed] [Google Scholar]
- Stobart JL and Anderson CM (2013) Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Frontiers in cellular neuroscience, 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y and Sato K (2012) L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the 'collusion' hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. Journal of neuroinflammation, 9, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Xiao H, Li F, Zeng L and Yin Y (2015) The profiles of mitochondrial respiration and glycolysis using extracellular flux analysis in porcine enterocyte IPEC-J2. Anim Nutr, 1, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele DL, Kurosaka M and Lipsky PE (1983) Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. Journal of immunology, 131, 2282–2290. [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS and Barres BA (2001) Control of synapse number by glia. Science, 291, 657–661. [DOI] [PubMed] [Google Scholar]
- van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW and de Graan PN (2005) Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology, 64, 326–333. [DOI] [PubMed] [Google Scholar]
- Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL, Mutani R, Giordana MT and Cavalla P (2007) Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. Journal of neuropathology and experimental neurology, 66, 732–739. [DOI] [PubMed] [Google Scholar]
- Vesce S, Rossi D, Brambilla L and Volterra A (2007) Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol, 82, 57–71. [DOI] [PubMed] [Google Scholar]
- Volterra A, Liaudet N and Savtchouk I (2014) Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nature reviews. Neuroscience, 15, 327–335. [DOI] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L and Nedergaard M (2012) Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+. Sci Signal, 5, ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RE, Aberg L, Autti T, Goebel HH, Kohlschutter A and Lonnqvist T (2006) Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochimica et biophysica acta, 1762, 865–872. [DOI] [PubMed] [Google Scholar]
- Williams RE and Mole SE (2012) New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology, 79, 183–191. [DOI] [PubMed] [Google Scholar]
- Xiong J and Kielian T (2013) Microglia in juvenile neuronal ceroid lipofuscinosis are primed toward a pro-inflammatory phenotype. Journal of neurochemistry, 127, 245–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.