Abstract
Purpose
To assess whether certain CT chest features of patients with confirmed coronavirus disease 2019 (COVID-19) may have short-term prognostic value.
Materials and Methods
One hundred-twenty consecutive symptomatic patients with COVID-19 infection who had undergone chest CT were enrolled in this retrospective study. Patients were categorized into three groups: routine inward hospitalization, intensive care unit admission, and deceased based on a short-term follow-up. Detailed initial CT features and distributional evaluation were recorded.
Results
The mean age in the deceased group was 70.7 years, significantly higher than the other two groups (P < .05). Ninety-four percent (113/120) of the patients had ground-glass opacities (GGO). Peripheral and lower zone predilection was present in most patients. Subpleural sparing and pleural effusion were seen in approximately 23% (28/120) and 17% (20/120) of the patients, respectively. The combined intensive care unit group and deceased patients had significantly more consolidation, air bronchograms, crazy paving, and central involvement of the lungs compared with routinely hospitalized patients (all P < .05).
Conclusion
This study supports the previously described typical CT appearance of COVID-19 pneumonia with bilateral GGO, in peripheral distribution and lower lung zone predilection. Subpleural sparing and pleural effusion were seen approximately in one-fifth and one-sixth of the patients with COVID-19, respectively. Consolidation, air bronchograms, central lung involvement, crazy paving and pleural effusion on initial CT chest have potential prognostic values, the features more commonly observed in critically ill patients.
© RSNA, 2020
Summary
In the evaluation of initial CT chest features of 120 patients with confirmed cases of COVID-19, certain findings are statistically more common in critically ill and deceased patients after a short-term follow-up, including consolidation, air bronchograms, crazy paving, central lung involvement, and pleural effusion.
Key Points
■ Typical CT features of COVID-19 pneumonia include bilateral lung involvement in the form of ground-glass opacities, predominantly in peripheral posterior distribution with lower lung zone predilection.
■ Subpleural sparing and pleural effusion are seen approximately in one-fifth and one-sixth of the patients with COVID-19, respectively.
■ Consolidation, air bronchograms, central lung involvement, and pleural effusion on initial CT of the chest have prognostic value, seen significantly more in intensive care unit–admitted and deceased patients.
Introduction
The coronavirus disease 2019 (COVID-19) outbreak was first reported from Wuhan, China, in December 2019. The rapid spread of the COVID-19–associated pneumonia and its surging mortality rate drew intense attention, leading to the World Health Organization to announce a global health emergency on January 30, 2020. As of April 10, 2020, the total number of confirmed cases is 1,521,252 worldwide with a reported mortality rate of 6.1% (1), slightly higher than the preliminary reports. The total number of cases in the United States has reached 459,165 and all 50 states have reported cases based on the Centers for Disease Control and Prevention and progression daily report, as of April 10, 2020 (2).
Reverse-transcription polymerase chain reaction (RT-PCR) remains the standard diagnostic reference for COVID-19 infection, but the high false-negative rate and its restricted availability limit the prompt diagnosis, which can result in a growing number of cases given the contagiousness of the virus (3). Radiologists have played an important role in the era of COVID-19, although our knowledge about the imaging features of the associated pneumonia is expeditiously evolving (4). Imaging provides health care facilities with a feasible screening tool for the detection of early pneumonia in high-risk patients and aids physicians in the diagnosis before the RT-PCR results are available. Last, it could be useful in assessing the severity and course of disease progression (5). Timely recognition of the disease ensures immediate care and appropriate follow-up and halts the spread of disease in the community. Chest radiography is not a sensitive tool to detect viral pneumonia, as has been confirmed for COVID-19 in recent investigations (6,7). Therefore, CT is the major diagnostic radiologic tool in identifying the COVID-19–associated pneumonia among the suspected cases.
Recently performed studies suggest that CT could have higher sensitivity than RT-PCR in the diagnosis of COVID-19–associated pneumonia (8–10). Multiple recent studies from China and South Korea revealed that ground-glass opacities (GGOs) are the most common finding in COVID-19–associated pneumonia. Both lungs are involved in almost all patients and a peripheral subpleural distribution is the most reported location. Multiple other radiologic features have also been described, although imaging features are highly nonspecific (7,11–14).
Based on the Johns Hopkins Center for Systems Science and Engineering online dashboard, as of March 10, 2020, Iran is the third leading country in the number of cases and mortality rate (15). This study was the first reported cohort of COVID-19 cases from a single academic center from Kashan, Iran, which focuses on CT findings and assesses the short-term prognosis based on the primary radiologic features.
In this retrospective research study, we aimed to review the radiologic findings in our 120 laboratory-confirmed cohort of patients with COVID-19 pneumonia, one of the few first available reports out of the center of the disease and to compare with the prior studies from China. In addition, we followed the patients’ clinical course for a 1- to 2-week period after the hospitalization for assessing the short-term prognosis. We hypothesized that certain radiologic features may be more prevalent in critically ill and deceased patients compared with patients undergoing routine ward hospitalization.
Materials and Methods
This retrospective study received institutional review board approval (IR.KAUMS.MEDNT.REC.1398.143), and the requirement for informed consent was waived by the ethics committee of Kashan University of Medical Sciences.
The medical records of all hospitalized patients with RT-PCR confirmation of COVID-19 who had undergone chest CT in Shahid Beheshti Hospital, Kashan, Iran, were reviewed from February 18, 2020, to March 2, 2020. The diagnosis of COVID-19 infection was confirmed by real-time RT-PCR assay with samples obtained from endotracheal aspirate, nasopharyngeal swab, or oropharyngeal swab.
A total of 120 symptomatic patients with confirmed COVID-19 infection (mean age, 54.9 years ± 17.07, 50 women and 70 men) were eligible to be enrolled in this study and available laboratory, radiologic, and epidemiologic data were collected. On the basis of a 1- to 2-week inpatient clinical follow-up, we categorized our patients into three groups: routine ward hospitalization, intensive care unit (ICU) admission, and deceased. All patients had at least one chest CT performed at initial presentation. In patients with more than one chest CT, the initial CT scan was considered for interpretation.
All CT studies were performed using a multidetector scanner (Alexion TSX-034A, Canon, Japan). A low-dose institutional protocol was applied with the detailed acquisition parameters as follows: tube voltage, 120 kVp; tube current, 50–90 mAs using automatic exposure control; slice thickness, 3 mm. CT images were acquired at full inspiration with the patient in the supine position and without administration of intravenous contrast medium. The mean CTDIvol was 4.9 mGy (range, 3.9–7.8 mGy).
Two radiologists (S.M.H.T., H.T.) (7 and 5 years of experience) reviewed the chest CT scans independently and resolved any discrepancies by consensus on a picture archiving and communication system workstation (MARCO PACS, Tehran, Iran). All CT images were viewed with both lung (width, 1600 HU; level, −550 HU) and mediastinal (width, 400 HU; level, 40 HU) window settings.
The major CT findings were described based on the Fleischner Society glossary of terms for thoracic imaging (16). The readers classified pulmonary lesions as GGO, consolidation, or a mixed pattern. Based on morphology, lesions were categorized as patchy/segmental, reticular/linear, crazy paving, reverse halo, or round/nodular. The laterality of the lesions was recorded, and the distribution of the lesions was classified as: (a) upper, middle, or lower zones, (b) peripheral or central, and (c) anterior or posterior. The peripheral lung was defined as the outer one-third of the lung, and the central lung was defined as the inner two-thirds of the lung. Anterior or posterior locations were defined using a horizontal line dividing the lungs into the anterior and posterior halves. The affected pulmonary lobes were also recorded. We also evaluated the percentage of total lung involvement by abnormalities, subdividing each lung into three zones (ie, upper, mid, and lower) and visually assessing the percentage of involvement in each zone, followed by calculation of the percentage of total lung involvement by averaging all of the six zones.
In addition, the readers assessed the presence of perilobular opacities, air bronchograms, cavitation, subpleural sparing, tree-in-bud nodules, mediastinal and hilar lymphadenopathy (defined as short axis measurement of ≥ 10 mm), pleural effusion, and splenomegaly.
Categorical variables were presented as numbers and percentages. Differences between groups were analyzed by χ2 for categorical variables and one-way ANOVA for the continuous variables. All statistical analyses were performed with SPSS software (version 24.0, IBM, Armonk, NY) and P values < .05 were considered statistically significant.
Results
Of the 120 patients, 70 were men and 50 were women. The patients’ ages ranged from 23 to 95 years with a mean of 54.9 years ± 17.1. All patients were symptomatic with laboratory confirmation of COVID-19.
In 1- to 2-week follow-up, 80% of patients (96/120) were hospitalized in the routine ward. Eleven patients were admitted to ICU and intubated, whereas 13 patients died. The mean age in the deceased group was 70.7 years, significantly higher than the other two groups (P < .05). On complete review of patients’ clinical data, we only had two immunosuppressed patients in our cohort, one in our ward hospitalization group and one in the ICU group.
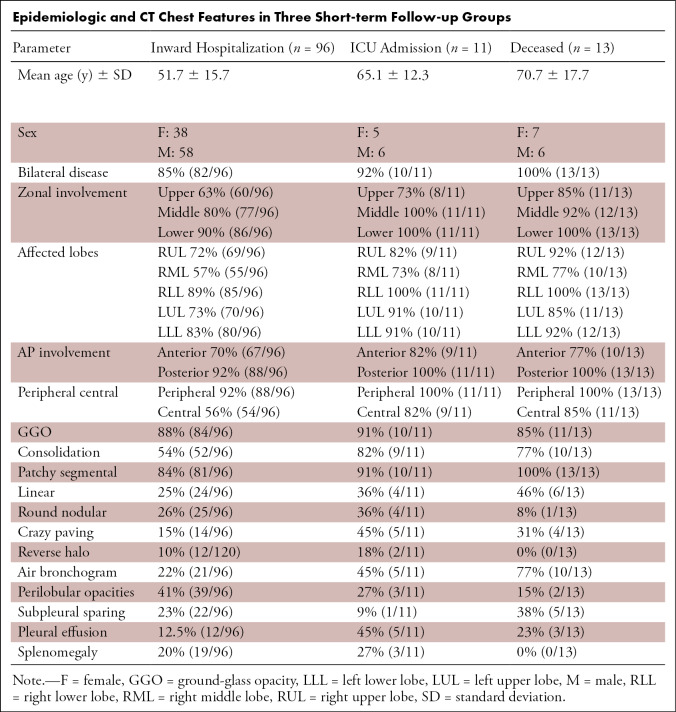
Of the 120 cases, six patients had normal chest CT. The CT features and distribution across the 120 reported patients are listed in the Table. A total of 105 out of 120 patients (87.5%) had bilateral lung disease, not significantly different between follow-up groups. The predominant feature was GGO (94%, 113/120) (Fig 1). Seventy-four percent (89/120) of patients had consolidation (Fig 2), significantly higher in the combined ICU and deceased cases compared with the routinely hospitalized patients (P < .05). The opacities were peripheral in 93% (112/120) of patients. Central involvement was significantly higher in the combined ICU admitted and deceased cases compared with the routinely hospitalized group (P < .05). Posterior involvement was seen in 93% (112/120) of patients with additional anterior involvement in 72% (86/120) of patients. Lower zone disease was seen in 92% (110/112) of patients, whereas middle and upper zones involvement was reported in 83% (100/120) and 66% (79/120) of patients, respectively. Right lower and left lower lobes involvement was reported in 91% (109/120) and 85% (102/120) of patients, respectively. Right middle lobe was the least common lobe involved, seen in only 61% (73/120) of patients. Patchy and segmental (Figs 1, 2) morphologic pattern was seen in 87.5% (105/120) of the patients, whereas linear/reticular (Fig 3]) and nodular (Fig 4) patterns were seen in 28% (34/120) and 25% (24/120) of patients, respectively, not significantly different between the follow-up groups.
Epidemiologic and CT Chest Features in Three Short-term Follow-up Groups
Figure 1:
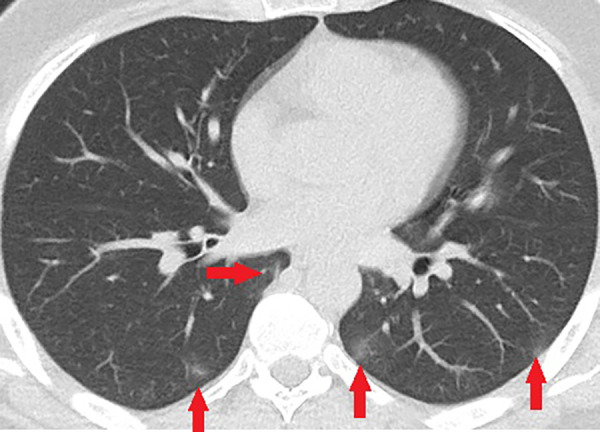
Unenhanced CT of the chest of a 43-year-old man with confirmed COVID-19 pneumonia. Few small peripheral patchy ground-glass opacities (arrows) are the only positive findings on the chest CT of this patient.
Figure 2:
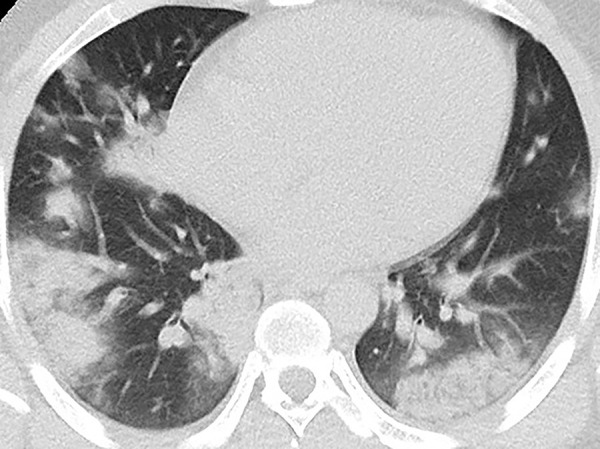
Unenhanced CT of the chest of a 28-year-old man with confirmed COVID-19 pneumonia. Multiple peripheral consolidations are shown bilaterally, with patchy and segmental morphology.
Figure 3a:
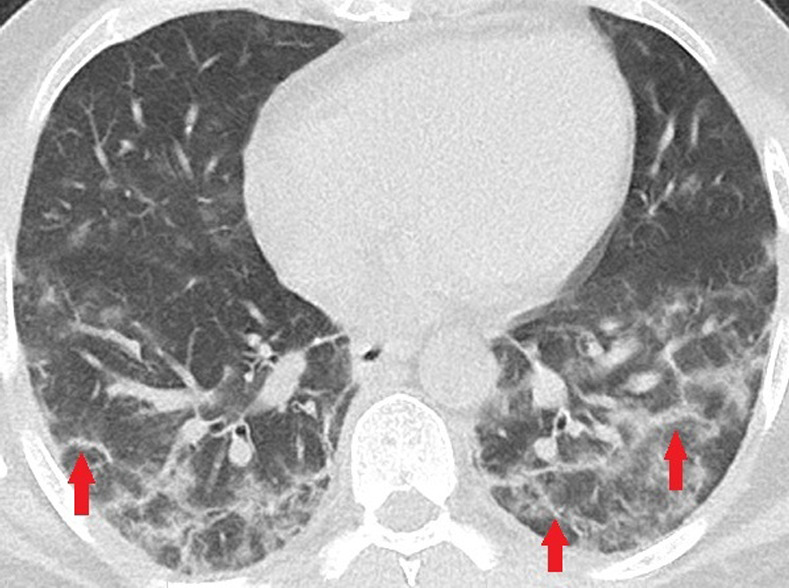
Unenhanced CT of the chest of two patients with confirmed COVID-19 pneumonia. (a, b) Multiple bilateral linear and reticular opacities (arrows) in a 50-year-old man. Note the linear opacities with an arc pattern (arrowheads) on the left side, representing perilobular opacities, called perilobular sign which is a typical sign of organizing pneumonia. Note some of the mentioned opacities on b outline borders of secondary pulmonary lobules (arrowheads), representing interstitial pneumonia. (c) A peripheral linear consolidation (arrow) in the left lung as well as ground-glass opacities and consolidations in some other parts of both lungs in an 81-year-old woman.
Figure 4a:
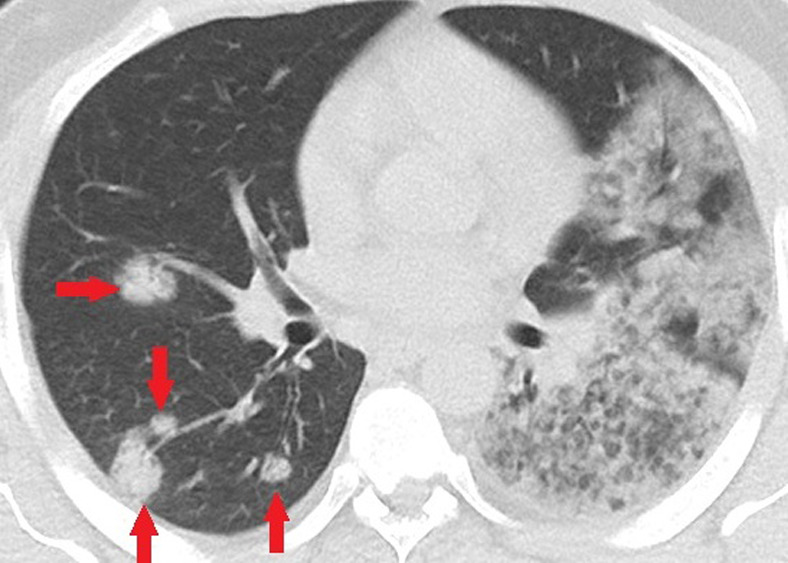
Unenhanced CT of the chest of a 32-year-old man who died because of confirmed COVID-19 pneumonia. (a, b) Multiple consolidations with nodular/round configuration in the right lung at two different levels. Note the extensive mixed consolidation and ground-glass opacities in the left lung.
Figure 3b:
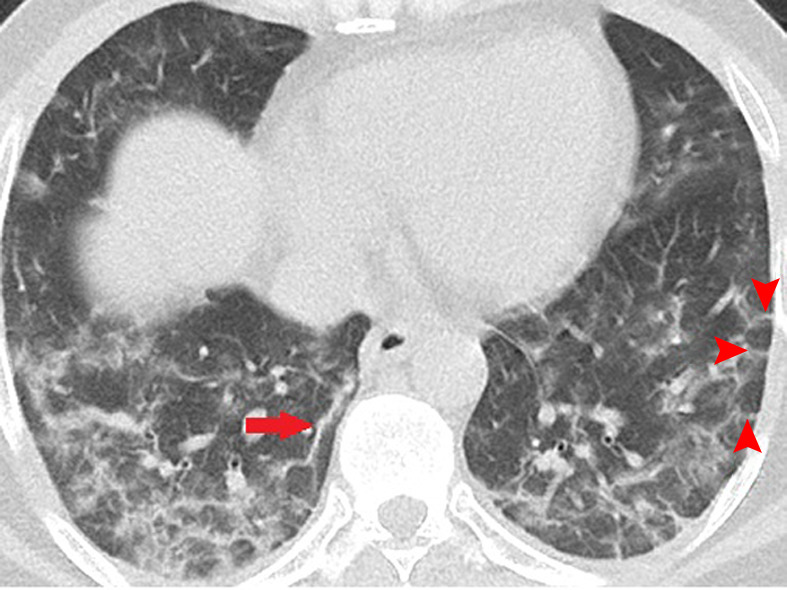
Unenhanced CT of the chest of two patients with confirmed COVID-19 pneumonia. (a, b) Multiple bilateral linear and reticular opacities (arrows) in a 50-year-old man. Note the linear opacities with an arc pattern (arrowheads) on the left side, representing perilobular opacities, called perilobular sign which is a typical sign of organizing pneumonia. Note some of the mentioned opacities on b outline borders of secondary pulmonary lobules (arrowheads), representing interstitial pneumonia. (c) A peripheral linear consolidation (arrow) in the left lung as well as ground-glass opacities and consolidations in some other parts of both lungs in an 81-year-old woman.
Figure 3c:
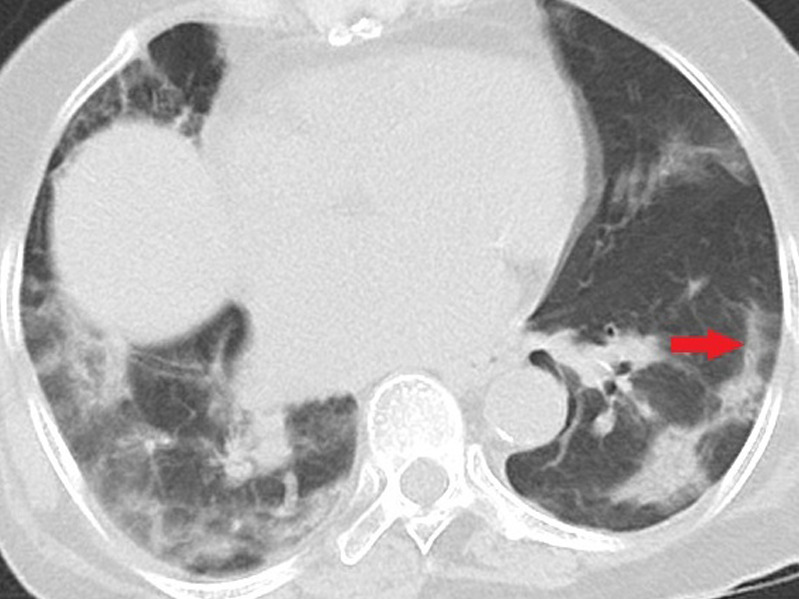
Unenhanced CT of the chest of two patients with confirmed COVID-19 pneumonia. (a, b) Multiple bilateral linear and reticular opacities (arrows) in a 50-year-old man. Note the linear opacities with an arc pattern (arrowheads) on the left side, representing perilobular opacities, called perilobular sign which is a typical sign of organizing pneumonia. Note some of the mentioned opacities on b outline borders of secondary pulmonary lobules (arrowheads), representing interstitial pneumonia. (c) A peripheral linear consolidation (arrow) in the left lung as well as ground-glass opacities and consolidations in some other parts of both lungs in an 81-year-old woman.
Figure 4b:
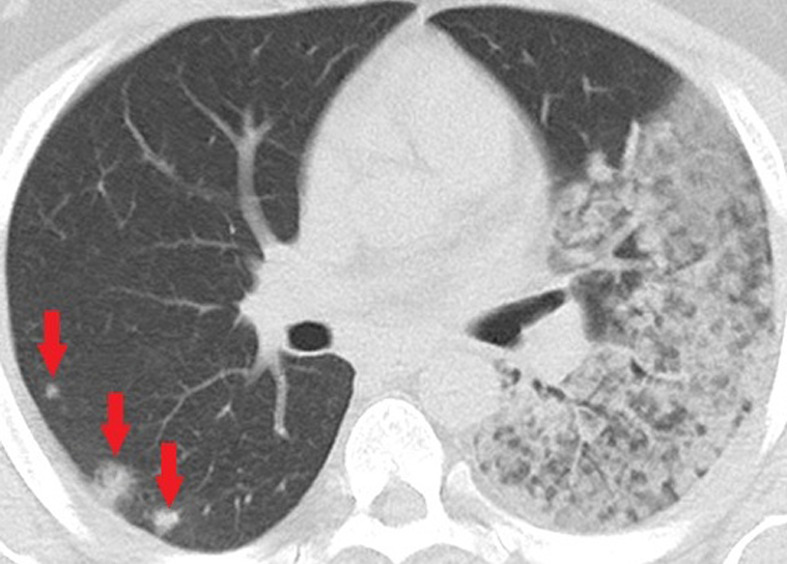
Unenhanced CT of the chest of a 32-year-old man who died because of confirmed COVID-19 pneumonia. (a, b) Multiple consolidations with nodular/round configuration in the right lung at two different levels. Note the extensive mixed consolidation and ground-glass opacities in the left lung.
Percentage of total lung involvement in the combined ICU and deceased group (36.52% ± 19.2) was significantly higher than routinely hospitalized patients (20.54% ± 17.1) (P < .05).
Twenty-three percent (28/120) of patients had subpleural sparing (Fig 5). Twenty patients demonstrated pleural effusion, all small (Fig 6), significantly different among the follow-up groups (P < .05) with more cases in ICU and deceased patients. Mild splenomegaly was seen in 22 patients. No cavitation, tree-in-bud nodularity, or mediastinal/hilar lymphadenopathy was seen in our cohort of patients. Reverse halo sign (Figs 5, 7) was seen in 10% (12/120) of patients, and crazy paving (Fig 8) was present in 19% (23/120) of patients, with significantly more crazy paving in ICU admitted and deceased patients (P < .05). Seventy-seven percent (10/13) of deceased cases had air bronchograms (Fig 9), significantly different from ICU admitted and routinely hospitalized patients (P < .05). Perilobular opacities were seen in 41% (39/96) of patients in the routinely hospitalized group with a relatively higher incidence than the ICU or deceased groups (Fig 3).
Figure 5:
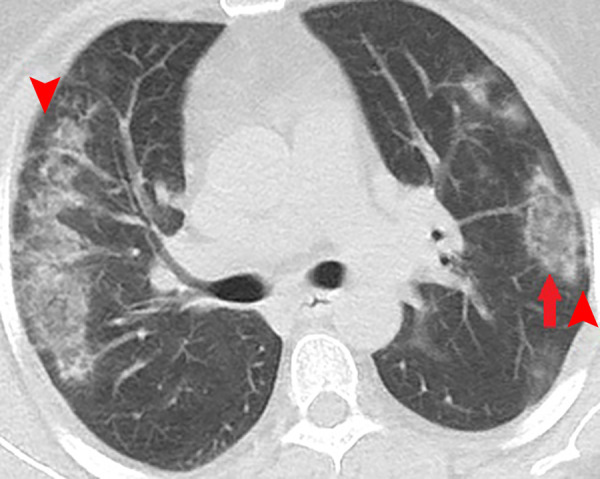
Unenhanced CT of the chest of a 53-year-old woman with confirmed COVID-19 pneumonia. Bilateral peripheral patchy mixed consolidation and ground-glass opacities are shown, with areas of subpleural sparing (arrowheads). Note the classic reverse halo sign on the left side (arrow).
Figure 6a:
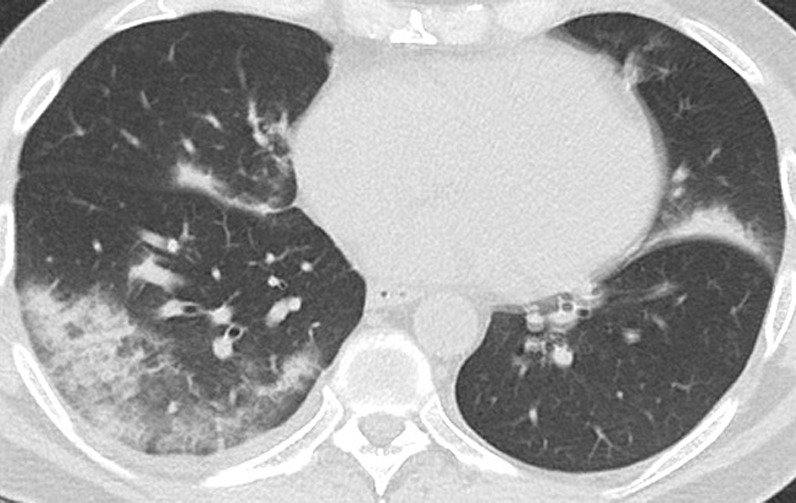
Unenhanced CT of the chest of a 53-year-old man with confirmed COVID-19 pneumonia who was admitted to the intensive care unit and intubated. (a) Lung window shows bilateral mixed consolidation and ground-glass opacities. (b) Mediastinal window at the same level shows minimal pleural effusion on the right side (arrow).
Figure 7:
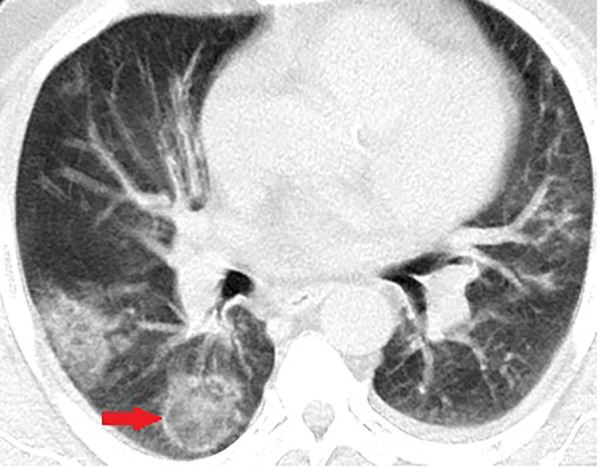
Unenhanced CT of the chest of a 56-year-old man with confirmed COVID-19 pneumonia. Typical reverse halo sign is seen in the right lower lobe (arrow).
Figure 8:
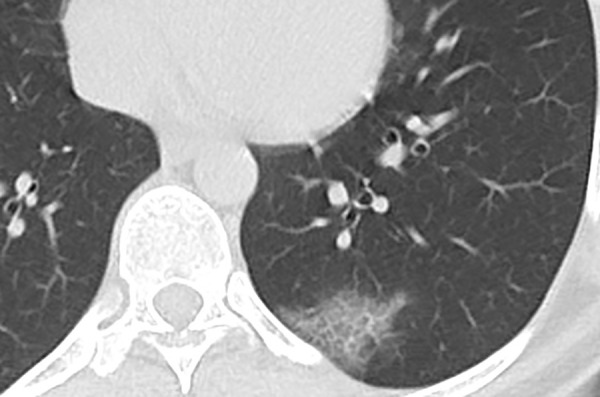
Unenhanced CT of the chest of a 62-year-old woman with confirmed COVID-19 pneumonia shows patchy ground-glass opacity with thickened intralobular septa, creating typical crazy paving appearance.
Figure 9:
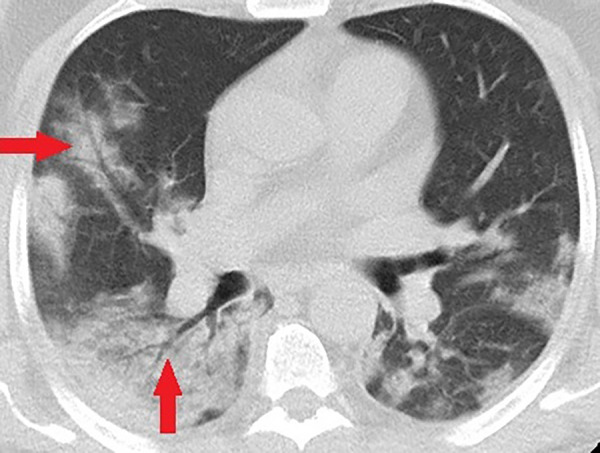
Unenhanced CT of the chest of a 58-year-old man with confirmed COVID-19 pneumonia who was admitted to the intensive care unit and intubated. Bilateral mixed consolidation and ground-glass opacities are shown, with an air bronchogram appearance (arrows) on the right side.
Figure 6b:
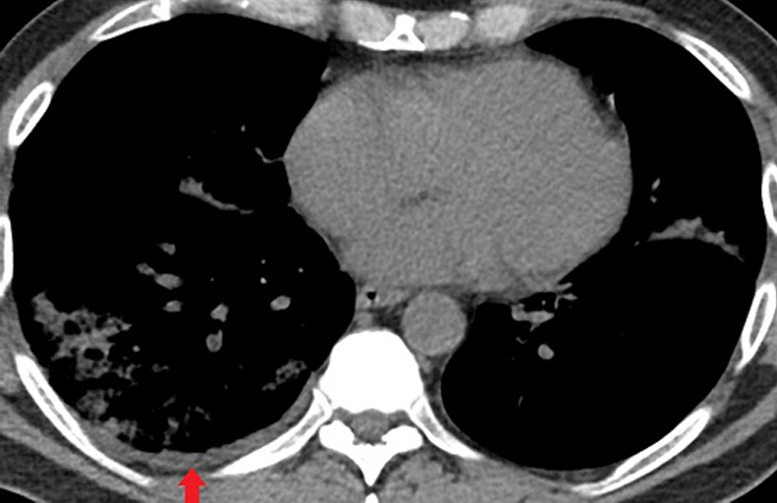
Unenhanced CT of the chest of a 53-year-old man with confirmed COVID-19 pneumonia who was admitted to the intensive care unit and intubated. (a) Lung window shows bilateral mixed consolidation and ground-glass opacities. (b) Mediastinal window at the same level shows minimal pleural effusion on the right side (arrow).
Discussion
COVID-19 pneumonia poses a huge threat to public health because of its high incidence and rapidly spreading nature. Hence, accurate and early recognition of the disease is crucial. Given the feasibility, promptness, and high sensitivity, chest CT is the key tool for the detection of COVID-19 pneumonia. We reviewed the first cohort of patients from Kashan, Iran, one of the current epidemic areas in the country and analyzed the imaging features of COVID-19 pneumonia. Our study was based on a low-dose institutionally designed CT chest protocol for suspected COVID-19 pneumonia during the national outbreak.
Although chest CT findings of COVID-19 pneumonia overlap with various other infectious or inflammatory lung processes, certain imaging characteristics and patterns exist in recently published data (11–14). Our study, one of the few available investigations outside of China, supports the previously identified typical CT features of COVID-19 pneumonia with most patients demonstrating bilateral lung involvement in the form of GGOs, predominantly in a peripheral posterior distribution with lower lung zone predilection. A recent study by Bernheim et al showed that GGO, peripheral distribution, and vascular thickening were the most distinguishing CT features of COVID-19 pneumonia (11). Pertinent negative features in our study included the absence of tree-in-bud nodules, cavitation, and mediastinal/hilar lymphadenopathy. Unlike a few prior studies (6,11,13), our cohort showed subpleural sparing and pleural effusion as two important findings in COVID-19 pneumonia, seen approximately in one-fifth and one-sixth of the cases, respectively. We also reported mild splenomegaly, a nonspecific finding not well evaluated in prior COVID-19 studies but proved to be present in many viral diseases.
Six asymptomatic patients had a normal initial chest CT. All of them had a history of exposure to confirmed cases. This emphasizes the importance of the combination of chest CT and RT-PCR, and follow-up chest CT for timely diagnosis in clinically suspected individuals.
We also investigated the CT features in our short-term follow-up groups. Patients admitted to ICU or deceased as a result of COVID-19 pneumonia, were significantly older and had more consolidation pattern, central involvement of the lungs (peribronchovascular), developed air bronchograms, had more crazy paving appearance and pleural effusion, all findings which may reflect the virulence of COVID-19. Therefore, these radiologic features have the potential to represent prognostic imaging markers in patients with COVID-19 pneumonia. Our study supports the fact that critically ill patients develop extensive consolidative opacities with air bronchograms, which is different from well-described GGOs associated with COVID-19 pneumonia, a finding which should also raise the concern when seen in CT chest. Clinically unstable patients also demonstrated a central pattern of involvement, in addition to the classic peripheral subpleural distribution. Conversely, there was a higher incidence of reverse halo sign and perilobular opacities in the stable patients, both signs representative of underlying organizing pneumonia. These finding could support a higher incidence of diffuse alveolar damage in the clinically unstable patients in the ICU and deceased groups, and a higher prevalence of organizing pneumonia pattern in stable patients who were routinely hospitalized in the ward. Finally, ICU and deceased deceased patients had more cases of pleural effusion, a nonspecific finding which has also been reported more frequently in emergent cases of the study by Zhao et al (17).
Our study had a few limitations. Our cohort of patients only consisted of the hospitalized patients and may not have reflected the accurate disease burden and imaging features in the community. We did not evaluate follow-up chest CT in patients to assess the temporal changes in lung parenchyma during the disease course, a process that has been better evaluated in multiple studies (12,18,19). Our follow-up groups were designed based on a short-term check of the patients’ clinical conditions and did not correlate with long-term prognosis. As a future direction, larger studies should be performed to evaluate the prognostic value of the initial CT features that were significantly more prevalent in our critically ill and deceased patients. In addition, longer follow-ups are warranted for the further evolution of the lung findings in COVID-19 pneumonia which may help predict worse outcomes. We also did not assess the role of chest CT in monitoring the response to treatment.
Chest CT helps in early diagnosis, guides the physicians, and provides potential prognostic markers and follow-up assessment of disease progression. Health care measures are evolving daily as new cases are identified. Future studies will be warranted in ascertaining how patients with COVID-19 pneumonia respond to treatment based on imaging surveillance.
Acknowledgments
Acknowledgments
We would like to thank Dr Reza Razzaghi, Dr Maedeh Najafi Zade, and Dr Ahmad Najafi in infectious disease department and Dr Milad Mokfi in radiology department for their assistance in data collection.
Current addresses: 1Radiology Department, Faculty of Medicine, Kashan University of Medical Sciences, Kashan, Iran
2Psychiatry Department, Faculty of Medicine, Kashan University of Medical Sciences, Kashan, Iran
3Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, Mass
Disclosures of Conflicts of Interest: S.M.H.T. disclosed no relevant relationships. H.T. disclosed no relevant relationships. F.M. disclosed no relevant relationships. H.R. disclosed no relevant relationships.
Abbreviations:
- COVID-19
- coronavirus disease 2019
- GGO
- ground-glass opacity
- ICU
- intensive care unit
- RT-PCR
- reverse-transcription polymerase chain reaction
References
- 1.World Health Organization . Novel Coronavirus (COVID-19) situation. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed April 10, 2020.
- 2.Centers for Disease Control and Progression . Corona virus 2019 disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html. Accessed April 10, 2020.
- 3.Yang Y, Yang M, Shen C, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. [preprint] Posted February 17, 2020. Accessed March 2020.
- 4.Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L. Radiology Perspective of Coronavirus Disease 2019 (COVID-19): Lessons From Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome. AJR Am J Roentgenol 2020 Feb 28:1–5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Yang R, Li X, Liu H, et al. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol Cardiothorac Imaging 2020;2(2):e200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng M, Lee EY, Yang J, et al. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol Cardiothorac Imaging 2020;2(1):e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon SH, Lee KH, Kim JY, et al. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19): Analysis of Nine Patients Treated in Korea. Korean J Radiol 2020;21(4):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020 Feb 26:200642 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Y, Zhang H, Xie J, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020 Feb 19:200432 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology 2020 Feb 12:200343 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernheim A, Mei X, Huang M, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020 Feb 20:200463 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan F, Ye T, Sun P, et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology 2020 Feb 13:200370 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung M, Bernheim A, Mei X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020;295(1):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020 Feb 21:200490 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns Hopkins Center for Systems Science and Engineering. Coronavirus COVID-19 global cases. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Published 2020. Accessed March 10, 2020.
- 16.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246(3):697–722. [DOI] [PubMed] [Google Scholar]
- 17.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol 2020 Mar 3:1–6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020 Feb 13 [Epub ahead of print] 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20(4):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]