Abstract
Background
Active lifestyles are related to better cognitive aging outcomes, yet the unique role of different types of activity are unknown.
Objective
To examine the independent contributions of physical (PA) versus cognitive (CA) leisure activities to brain and cognitive aging.
Methods
Independent samples of non-demented older adults from University of California, San Francisco Hillblom Aging Network (UCSF; n = 344 typically aging) and University of California, Davis Diversity cohort (UCD; n = 485 normal to MCI) completed: 1) self-reported engagement in current PA and CA (UCSF: Physical Activity Scale for the Elderly and Cognitive Activity Scale; UCD: Life Experiences Assessment Form); 2) neuropsychological batteries; and 3) neuroimaging total gray matter volume, white matter hyperintensities, and/or global fractional anisotropy. PA and CA were simultaneously entered into multivariable linear regression models, adjusting for demographic characteristics and functional impairment severity.
Results
Brain outcomes: In UCSF, only PA was positively associated with gray matter volume and attenuated the relationship between age and fractional anisotropy. In UCD, only CA was associated with less white matter hyperintensities and attenuated the relationship between age and gray matter volume. Cognitive outcomes: In both cohorts, greater CA, but not PA, related to better cognition, independent of age and brain structure. In UCSF, CA attenuated the relationship between fractional anisotropy and cognition. In UCD, PA attenuated the association between white matter hyperintensities and cognition.
Conclusions
Although their specificity was not easily teased apart, both PA and CA are clearly related to better brain and cognitive resilience markers across cohorts with differing educational, racial, and disease statuses. PA and CA may independently contribute to converging neuroprotective pathways for brain and cognitive aging.
Keywords: Brain aging, cognitive aging, exercise, mental stimulation, reserve
INTRODUCTION
Age-related cognitive decline and Alzheimer’s disease (AD) are major public health issues, yet we lack effective strategies to optimize brain health with age. Modifiable lifestyle factors are estimated to account for >30% of dementia cases [1], and represent prime targets as a means to mitigate the growing dementia problem. However, little is known regarding the pathways underlying the relationship between different lifestyle behaviors and the brain, limiting our ability to identify the most at-risk patients at the earliest stages and development of personalized behavioral treatments.
Large-scale epidemiologic studies indicate that older adults who engage in more frequent physical (PA) or cognitive (CA) activities in daily life show reduced age-related cognitive decline and delayed dementia onset [2, 3]. For instance, in a population- based study in China, older adults who engaged in more aerobic exercise were 19% less likely to develop incident dementia over 6-years [4]. In the US, simply walking 10 blocks/day was associated with 13% lower odds of cognitive decline over a 6–8 year period [5]. Additionally, using an identical twin study design, the twin with greater intellectual complexity in their occupation demonstrated ~5× reduced risk of AD [6]. After adjusting for lifelong occupational complexity and education, late-life CA is consistently associated with better cognitive status in older adults [7, 8]. Randomized controlled trials further support directionality of these effects, demonstrating domain- specific cognitive and brain (e.g., functional and structural neuroimaging) improvements following cognitive or physical training [9–11]. However, most studies have examined PA or CA in isolation, despite evidence that they often co-occur in the healthiest of elders. Of the few that have examined their intersection, PA and CA appear to independently relate to cognitive outcomes, suggesting distinct pathways to brain health [12–14]. Such behaviorally-based strategies offer the potential to prevent or delay dementia onset at low cost and with high scalability. Development of a framework to disentangle their underlying mechanisms will help foster testable hypotheses and, ultimately, more efficacious prevention and treatment programs.
Through both human and animal studies, it has become clear that a subset of individuals demonstrate slower rates of brain or cognitive decline compared to similarly aged peers, and some individuals with changes in brain integrity do not show commensurate cognitive or functional decline [15, 16]. Both PA and CA are implicated as factors that may confer such resilience to aging and pathology. In the current study, we model the independent contributions of PA and CA to: 1) better brain integrity than predicted by age (brain resilience to age), 2) better cognition than predicted by age (cognitive resilience to age), and 3) better cognition than predicted by brain integrity (cognitive resilience to brain integrity). These concepts are tested separately with the underlying assumption that there may be differing and potentially discrete mechanisms that PA or CA may be acting on to drive each of these phenomena. For instance, PA appears to be associated with better brain health with age, as indicated by greater hippocampal growth [10], stabilization of immune homeostasis [17], glymphatic clearance [18], and lower amyloid plaque burden even among autosomal dominant AD gene carriers [19]. Intellectual enrichment is also associated with increased synaptic density and reduced Aβ aggregation in mice [20], supporting a potential role for CA in maintaining better brain health. However, in addition to its role in brain health, CA also accounts for variance in cognition above and beyond neuropathologic burden [21–24], suggesting that it may also promote cognitive resilience to age and/or age-related pathology. Given their positive, parallel associations with aging outcomes, examining the relative contributions of PA versus CA to brain and cognitive resilience to aging will both improve design of lifestyle interventions for targeted populations and support future mechanistic hypothesis testing of these constructs.
We tested parallel models across two independent samples of non-demented older adults from the University of California, San Francisco (UCSF) and the University of California, Davis (UCD) examining the relative contributions of PA and CA to age-related brain and cognitive performances. We hypothesized that greater current engagement in PA would be more directly associated with MRI indicators of age-related brain integrity and attenuate the effect of age on the brain (brain resilience to age), while greater current CA would attenuate the relationship between brain integrity and cognition (cognitive resilience to brain integrity).
METHODS
Participants
UCSF
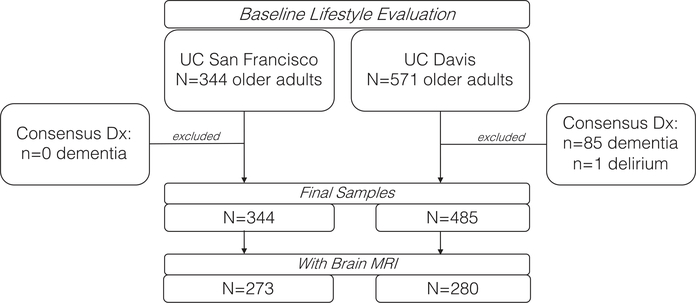
In this cross-sectional study, 344 adults who completed the lifestyle measures of interest as part of the UCSF Memory and Aging Center Hillblom Aging Network were included. Participants in this cohort represent a community-dwelling, convenience sample of older adults in the Bay Area recruited between 2000–2018. Given that the major aim of the Hillblom Aging Network is to study typical brain aging, upon initial entry into the study, participants were excluded for diagnoses of memory or other neurological conditions (e.g., epilepsy, stroke), and all participants demonstrated either no (98% CDR = 0) or minimal (2% CDR = 0.5) functional changes as operationalized by the Clinical Dementia Rating (CDR) scale via study partner interviews (Table 1). Most participants (79.4%, n = 273) had a brain MRI available within 6-months of their lifestyle evaluation.
Table 1.
Demographic and clinical characteristics of two independent study cohorts from University of California, San Francisco (UCSF) and Davis (UCD). Mean (standard deviation) reported unless otherwise specified
| Demographic and Clinical Characteristics | UCSF (n = 344) | UCD (n = 485) |
|---|---|---|
| Age, y | 73.5 (7.3) | 76.9 (7.1) |
| % Female (n) | 55.8% (192) | 59.6% (289) |
| Education, y | 17.5 (2.0) | 14.3 (4.1) |
| Race (%, n) | ||
| White | 90.7% (303) | 50.2% (243) |
| Asian | 7.3% (25) | 0.01% (3) |
| Black | 0.9% (3) | 23.1% (112) |
| Hispanic/Latino | 0.0% (0) | 21.6% (105) |
| Other | 0.9% (3) | 0.01% (3) |
| Not reported | 2.9% (10) | 0% |
| Clinical Dementia Rating (CDR) | ||
| %CDR Total >0(n) | 2.3% (8) | 48.1% (233) |
| Sum of boxes (median, IQR) | 0 (0,0), range: 0, 1.5 | 0.5 (0,1.5), range: 0,13 |
| Mini Mental Status Exam (median. | 29(28,30) | 28 (26, 29) |
| IQR) | . | |
| Physical Activity | ||
| PASE | 130.7 (68.5), range: 5,390.4 | — |
| LEAF-Physical activities | — | 19.4 (4.4), range: 6, 30 |
| Cognitive Activity | ||
| CAS | 18.6 (3.1). range: 9,25 | — |
| LEAF-Cognitive activities | — | 29.22 (5.5), range: 13,50 |
| Total gray matter volume (L3) | 0.60 (0.06) | 0.57 (0.52) |
| White matter hyperintensity volume | 2.25 (0.88, 5.93) | 5.71 (2.23, 15.28) |
| (mm3) (median, IQR) | ||
| Global fractional anisotropy | 0.43 (0.012) | — |
MMSE range, 0-30; CDR sum of boxes range, 0-18; IQR, interquartile range; y, years; PASE, Physical Activity Scale for the Elderly; CAS, Cognitive Activity Scale.
UCD
571 older adults from the UC Davis Diversity Cohort who completed a lifestyle evaluation were considered for inclusion in the current cross-sectional study. This cohort is heterogenous in race/ethnicity and educational attainment and spans a spectrum of cognitive function from normal to mildly impaired to demented. Cohort composition and recruitment methods are described in Hinton et al. [25] To increase comparability to UCSF, individuals diagnosed with dementia (n = 81) and 1 with delirium were excluded, leaving 485 non-demented older adults included in the primary analyses (Table 1); 58.1% (n = 280) of UCD participants also completed brain MRI within 6 months of evaluation.
In both studies, dementia was diagnosed using available clinical data via research consensus criteria as determined by a panel of neurologists and neuropsychologists (Fig. 1) [26].
Fig. 1.
Study flowchart outlining participant selection across study cohorts.
Current physical and cognitive activity lifestyle measurement
UCSF
Current levels of PA were assessed by the Physical Activity Scale for the Elderly (PASE). The PASE is an 11-item measure of physically demanding activities over the past 7 days across recreational, household, and work-related domains in which participants rated both the weekly frequency and daily duration. Activity scores were obtained by multiplying the activity frequency by the task-specific weight, according to the standardized scoring manual [27]. Activity scores were summed to calculate a total score with higher values indicating greater activity.
Current levels of CA were assessed by the Cognitive Activity Scale (CAS). The CAS is a 25-item self-reported measure of engagement in mentally stimulating leisure activities across the life course [22]. Given our interest in current lifestyle, we only included the 5 items from the CAS querying current activities (“in the presenttems included frequency of reading newspapers, magazines, and books, writing letters, and playing games. Activity scores were summed to calculate a total CAS score, with higher scores indicating greater activity engagement (Table 2).
Table 2.
Comparison of primary measurement approaches in University of California, San Francisco (UCSF) and Davis (UCD) studies
| UCSF | UCD | |
|---|---|---|
| Lifestyle Measures | ||
| Physical Activity | PASE: Composite reflecting frequency and duration of physical activities (“past 7 days”); 11-item | LEAF: Life experiences questionnaire; summation frequency of physical activities (“current”); 10-items |
| Cognitive Activity | CAS: Summation of frequency of cognitive activities (“present”); 5-item | LEAF: Life experiences questionnaire; summation frequency of cognitive activities (“current”); 10-items |
| Neuropsychological Domain | ||
| Episodic Memory | CVLT-II Long Delay Free Recall | SENAS Verbal Episodic Memory |
| CVLT-II Recognition | ||
| Discriminability | ||
| Modified Benson Figure Delay | ||
| Recall | ||
| Executive Functions | Digit Span backwards | SENAS Executive Functioning |
| Modified Trail Making Test | ||
| DKEFS Design Fluency, condition 1 | ||
| Lexical Fluency (D words/60”) | ||
| Stroop Inhibition | ||
| Processing Speed | ||
| Spatial | Computerized battery [53] | WAIS-IV Digit Symbol |
| Verbal | Computerized battery [53] | — |
| Semantic Processing | Animal fluency (60”) | SENAS Semantic Memory |
PASE, Physical Activity Scale for the Elderly; CAS, Cognitive Activity Scale; LEAF, Lifetime Experiences Assessment Form; CVLT-II, California Verbal Learning Test, second edition; DKEFS, Delis-Kaplan Executive Functioning System; SENAS, Spanish English Neuropsychological Assessment Scales;WAIS-IV,Weschler Adult Intelligence Scale, fourth edition.
UCD
Current PA and CA participation were derived from the UCD Life Experiences Assessment Form (LEAF). The LEAF is a larger questionnaire querying multiple social and lifestyle determinants of health (e.g., place of birth, occupation, income, recreational activities). The physical activity subscale includes 6 items measuring frequency of current participation in light and heavy work (i.e., housework/yardwork) and exercise. The cognitive activity subscale comprises 10 items assessing frequency of current engagement in reading, complex cooking, writing, classes/learning new skills, performance arts, games/puzzles, cultural events, arts/crafts, socializing, and clubs/religious activities (Table 2).
Neuropsychological measures
We selected measures capturing episodic memory, executive functions, processing speed, and semantic processing across the two cohorts. Measures were converted onto a similar metric using sample-based z-scores and then averaged together to create a global cognitive composite.
UCSF
See Table 2 for the specific measures included per domain. The selected neuropsychological measures have been widely published and shown to be sensitive to age-related neurologic processes [28]. For domains with more than one measure, scores were blom rank transformed to achieve normality before conversion into sample-based z-scores.
UCD
The Spanish English Neuropsychological Assessment Scales (SENAS) is a comprehensive, extensively developed battery created with the aim of measuring diseases relevant to aging comparably in English and Spanish [29]. To be consistent with available UCSF neuropsychological data, we included verbal episodic memory, executive function, and semantic memory domains in analyses, as well as the WAIS-IV Digit Symbol test (processing speed).
Neuroimaging
Participants completed a 3-Tesla brain MRI from which global gray matter volumes, white matter hyperintensity volumes (UCD only), and fractional anisotropy (UCSF only) were extracted.
Gray matter volume (GMV)
UCSF
Participants completed 3T Magnetom Vision TIM Siemens Trio (Siemens, Iselin, NJ) magnetic resonance imaging (MRI). Before processing, all T1-weighted images were visually inspected for quality. Images with excessive motion or image artifact were excluded. Magnetic field bias was corrected using the N3 algorithm [30]. Tissue segmentation was performed using the unified segmentation procedure in SPM12 [31]. Each participant’s T1-weighted image was warped to create a study-specific template using Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) [32]; subsequently, the images were normalized and modulated in the study-specific template space using non-linear and rigid-body registration. Images were smoothed using an 8-mm Gaussian kernel. For registration with a brain parcellation atlas, linear and non-linear transformations between DARTEL’s space and ICBM space were applied.
UCD
T-1 images were processed using an inhouse pipeline previously described [33]. In brief, images were nonlinearly registered to a minimal deformation template (MDT) [34] adapted for ages >60 and registration was performed using cubic B- spline deformation [35]. Tissue segmentation was automatically initiated using an iterative maximal likelihood estimation of tissue classes (gray, white, cerebrospinal fluid) based on alternating voxel class assignment followed by tissue class parameter estimation. The class likelihood priors included terms designed to enhance accuracy at tissue boundaries.
In both UCSF and UCD, total intracranial volume was regressed from GMV and resulting residuals were included in all final analyses.
DTI fractional anisotropy
In the UCSF cohort only, we used the Oxford Center for Functional MRI of the Brain’s Software Library (FSL) to co-register diffusion direction images with the b = 0 image. A gradient direction with eddy current and distortion correction were applied. Diffusion tensors were calculated using nonlinear least-squares algorithm from Dipy [36]. Following quality control, participant tensors (4-dimensional image) were linearly and nonlinearly registered to a common space using DTI ToolKit [37]. Participants tensors were then moved into a group template. DTI scalar maps of fractional anisotropy was calculated from participants’ tensors in the group template space. Whole brain FA was extracted using ICBM- DTI-81 white matter atlas.
White matter hyperintensities
In the UCD cohort only, white matter hyperintensity volumes were obtained from T2-weighted FLAIR images using an automated method for quantification and localization of white matter hyperintensities. Total white matter hyperintensity volume was estimated by summing all the voxels classified as white matter hyperintensities, as previously described using the same pipeline [38].
Statistical analyses
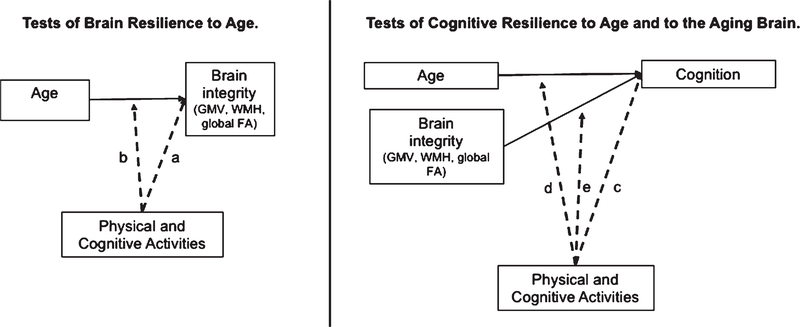
Pearson correlations and t-tests were conducted to examine bivariate relationships among leisure activities and demographics. Parallel multivariable linear regression models were conducted within the UCSF and UCD samples, separately, with standardized beta coefficients reported to facilitate model comparison (same-scaled estimates). Age, sex, and years of education were included as covariates in all models, as well as CDR sum of boxes (CDRsob) as an indicator of disease severity to adjust for potential reverse causality. Of note, the pattern of results was similar when CDRsob was excluded from models. We operationalized a lifestyle activity as supporting brain aging if that activity demonstrated an age-independent relationship with a brain structure outcome (gray matter, fractional anisotropy, or white matter hyperintensities) or moderated the relationship between age and brain structure (brain resilience to age). We operationalized a lifestyle activity as supporting cognitive aging if that activity related to cognition independent of age, moderated the relationship between age and cognitive performance (cognitive resilience to age), or moderated the relationship between brain structure and cognitive performance (cognitive resilience to brain integrity). All moderation effects were tested entering the main effect and interaction terms between variables of interest. See Fig. 1 for illustration of statistical models. For visualization purposes, we selected sample-specific levels of PA or CA as low (10th%ile) or high (90th%ile) to probe significant interactions [UCSF: PASE 10th%ile = 50.6, 90th%ile = 220.4 and CAS 10th%ile = 14, 90th%ile = 22; UCD: LEAF Physical Activity 10th%ile = 13, 90th%ile = 25; LEAF Cognitive Activity 10th%ile = 22, 90th%ile = 36).
RESULTS
Relationship among leisure activities and clinical factors across cohorts
Within the UCSF sample, engagement in PA and CA were related to only a small degree, while in UCD, PA and CA were more strongly associated (Table 3). In both study groups, older age and less education were associated with less PA and CA. Women reported engaging in more CA than men in UCD (t = 7.7, p < 0.001), but sex was not significantly related to CA in UCSF, or PA in either sample (UCSF: CA t = 0.97, p = 0.34, PA t = −1.1, p = 0.29; UCD: PA t = 1.2, p = 0.22). Greater clinical severity (CDRsob) was associated with engagement in fewer PA and CA in UCD, but was not strongly associated with activity engagement in UCSF, which inherently had less variability in clinical severity by selection.
Table 3.
Correlation matrix reflecting associations among lifestyle activities, demographic, and functional variables (Pearson r) across study cohorts
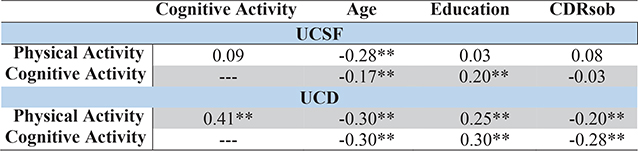 |
p < 0.01. CDRsob, Clinical Dementia Rating, sum of boxes; UCSF, University of California, San Francisco; UCD, University of California, Davis.
Brain integrity and brain resilience to age
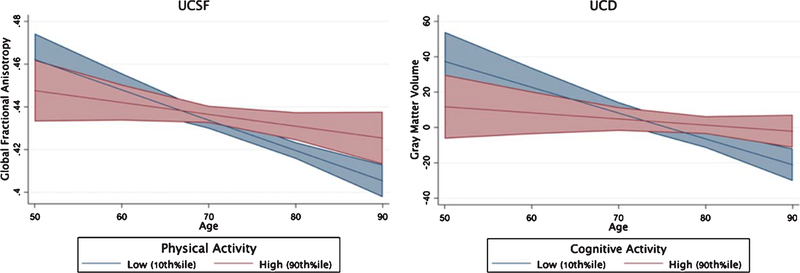
Entered simultaneously, greater PA but not CA was more strongly associated with larger gray matter volumes (Table 4; PA β = 0.17, p = 0.003; CA β = 0.02, p = 0.70), and PA uniquely attenuated the relationship between age and global fractional anisotropy in the UCSF sample (age*PA β = 0.15, p = 0.049; age*CA β =−0.07, p = 0.32; Fig. 3). On the other hand, in the UCD sample, greater CA but not PA was more strongly related to a lower white matter hyperintensity burden (CA β = −0.17, p = 0.009; PA β = −0.004, p = 0.85; Fig. 3), and CA attenuated the relationship between age and gray matter volumes (CA β = 0.17, p = 0.02; PA β = 0.06, p = 0.75). Interestingly, PA appeared to strengthen the association between age and white matter hyperintensities in UCD (age*PA β = 0.18, p = 0.02; age*CA β = 0.005, p = 0.98); examined more closely, greater PA was associated with overall less white matter hyperintensities in younger ages, an effect that reversed in the oldest ages (>80 years). Taken together, both PA and CA each appeared to relate to age-related brain integrity.
Table 4.
Brain Aging. Multivariable linear regression models illustrating the relationship of physical (PA) and cognitive (CA) activities on models of brain aging across independent cohorts (UCSF and UCD). Each column represents a regression model with omnibus model fit and parameter standardized beta values (β) reported. *p < 0.05; **p < 0.01
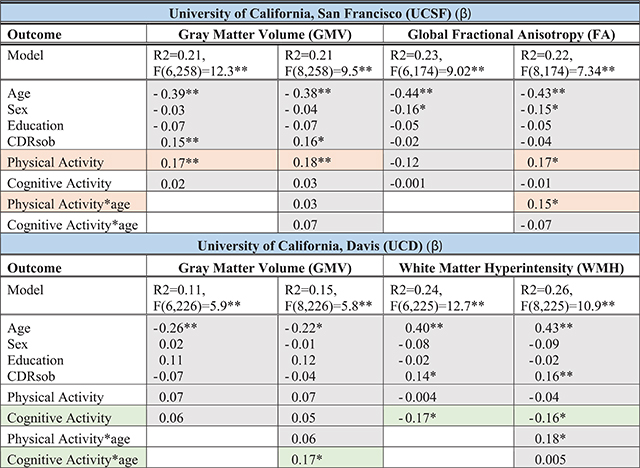 |
Orange reflects models supporting a role for physical activity for brain aging; Green reflects models supporting a role of cognitive activities for brain aging. GMV, total gray matter volume adjusted for total intracranial volume; CDRsob, Clinical Dementia Rating, sum of boxes. Sex: M = 1, F = 2.
Fig. 3.
Brain Resilience to Age. Physical and Cognitive Activities independently support brain resilience to aging. Age x lifestyle interactions modeled continuously with both physical and cognitive activity interactions simultaneous entered into multivariable regression models adjusting for age, sex, education, and CDR sum of boxes. Gray matter volume reflects the residual adjusting for total intracranial volume.
Cognitive performance and cognitive resilience to age
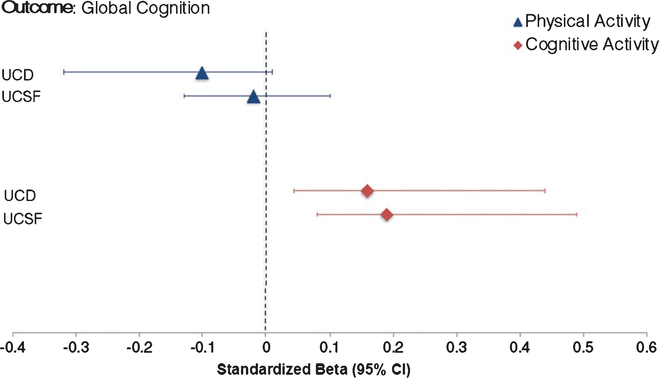
Entering both into the model, CA but not PA independently and consistently related to better cognitive performance across study cohorts beyond age (CA β range = 0.16 to 0.18; Table 5 and Fig. 4). Neither activity type moderated the effect of age on cognition in either sample (all p-values > 0.05).
Table 5.
Cognitive Aging. Multivariable linear regression models illustrating the relationship of physical (PA) and cognitive (CA) activities on models of cognitive aging across independent cohorts (UCSF and UCD). Each column represents a regression model with omnibus model fit and parameter standardized beta values *p < 0.05; **p < 0.01
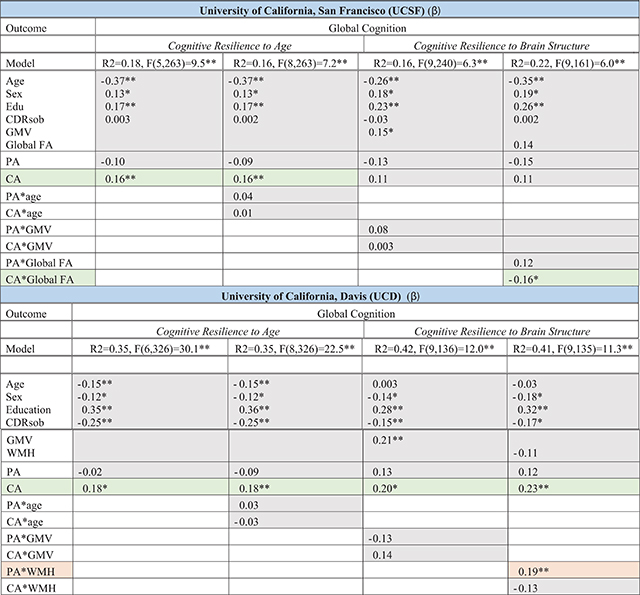 |
Orange reflects models supporting a role for physical activity for cognitive aging; Green reflects models supporting a role of cognitive activities for cognitive aging. PA, physical activity; CA, cognitive activity; CDRsob, Clinical Dementia Rating, sum of boxes; GMV, total gray matter volume adjusted for total intracranial volume; WMH, white matter hyperintensity volumes; global FA, whole brain fractional anisotropy. Sex: M = 1, F = 2.
Fig. 4.
Cognitive Aging. Cognitive but not physical activities related to cognition independent of age across study cohorts. Physical and cognitive activities simultaneously entered into multivariable linear regression models adjusting for age, sex, education, and CDR sum of boxes.
Cognitive resilience to brain integrity
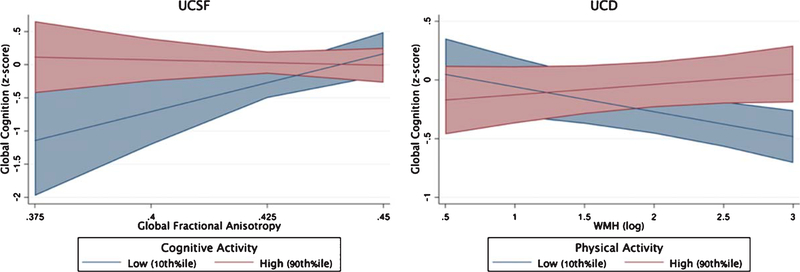
Within the UCSF sample, only CA but not PA significantly moderated the relationship between global fractional anisotropy and cognitive performance (global FA*CA β = −0.16, p = 0.039; global FA*PA β = 0.12, p = 0.12), such that adults with greater CA demonstrated disproportionately better global cognition given their white matter integrity compared to less active peers (Fig. 5). In the UCD sample, CA continued to demonstrate significant main effects with global cognition adjusting for demographics and brain structure (CA β range = 0.20 to 0.23). However, only PA but not CA significantly moderated the relationship between white matter hyperintensities and global cognitive performances (WMH*PA β = 0.19, p = 0.009; WMH*CA β = −0.13, p = 0.09). Adults with greater PA demonstrated an attenuated relationship between white matter hyperintensities and global cognition compared to their low activity peers. Models examining the moderating impact of leisure activities on gray matter volumes did not reach significance in either cohort (all p-values > 0.5; Table 5).
Fig. 5.
Cognitive Resilience to Brain Integrity. Physical and cognitive activities independently support cognitive resilience to the aging brain. Age x lifestyle interactions modeled continuously with both physical and cognitive activity interactions simultaneous entered into multivariable regression models adjusting for age, sex, education, CDR sum of boxes.
DISCUSSION
Examining physical (PA) and cognitive (CA) activities simultaneously across two independent cohorts, we hypothesized that PA would relate more directly to age-related markers of brain integrity, while CA would be more strongly associated with cognitive performances, even adjusting for age and brain integrity markers. Though our hypotheses were supported in the UCSF sample, results in the UCD sample indicated greater overlap in the nature of the effects of PA and CA. While PA was related to better brain structure in UCSF, CA was independently associated with better brain structure in the UCD cohort, and both activities attenuated the effect of age on brain structure suggesting a role in brain resilience to age (i.e., better brain integrity than less active peers the same age). Interestingly, CA (over PA) was consistently directly related to cognition independent of age or brain indicators in both samples. However, CA in UCSF, but PA in UCD, moderated the relationship between brain integrity and cognition, such that both types of leisure activities attenuated the relationship between brain and cognition, supporting their role in cognitive resilience to the aged brain. Taken together, though their specificity as related to brain versus behavior may not be evidently delineated, it is clear that both PA and CA robustly relate to better markers of age-related brain and cognitive health —a benefit consistently observed even across inherently disparate older adult cohorts representing different educational, racial/ethnic, and cognitive contexts.
Though maintaining physically and cognitively active lifestyles are clearly important indicators of brain and cognitive aging, these factors are commonly studied in isolation, and their individual contributing impact is not well understood, limiting potency in their implementation. Our data build on the growing literature suggesting potential unique and converging effects of PA and CA in aging. Using an epidemiological approach, Yaffe et al. (2009) demonstrated that both exercise and education/work/volunteering were independently associated with maintaining stable cognition over an 8-year period [12]. Extending this work, Najar et al. (2019) recently showed that greater midlife physical and cognitive activities each uniquely predicted ~30% reduced risk of dementia 44-years later [13]. Similarly, there are increasing randomized controlled interventions simultaneously targeting both PA and CA. While some of these latter studies show synergistic benefits [39–42], others suggest gains from either physical or cognitive training that appear largely coinciding and indistinct [41]. For example, using a factorial design, Shah and colleagues (2014) demonstrated benefits in both cognition and cerebral metabolism after physical or cognitive training that were disproportionately greater when combining the two modalities, suggesting at least some independence in their pathways of benefit. On the other hand, using a similar design, the Mental Activity and eXercise (MAX) Trial showed that either singular or combined trainings may improve cognitive performances equally [42]. The overlapping impact of these activities on global markers of brain integrity and cognition are supported by our data as well, and their measurement, particularly in observational studies, are likely not inherently discrete entities —that is, active individuals are likely to be active across domains of life, and physically-demanding activities often incorporate active thinking (and to a lesser extent, vice-versa). To this end, we found that in the UCD cohort, physical and cognitive behaviors were strongly correlated (r = 0.41); however, these behaviors were not as strongly associated within the UCSF sample (r = 0.08), suggesting that the larger UCD correlation may reflect shared method variance and/or was driven by greater cognitive variance in UCD. Perhaps not surprisingly then, we observed more discrete patterns of associations for physical versus cognitive behaviors in UCSF. Given the UCSF cohort was overall less impaired, this sample may be capturing the discrete impact of lifestyle activity types in an earlier disease state that appear to converge within the more impaired UCD sample. Additionally, it is important to note that we focused on downstream outcomes (i.e., global brain structure and cognitive function) that reflect an amalgamation of neurobiological processes. Although both PA and CA appear to converge and relate to both brain and cognitive end-points, it is possible that they tap into discrete upstream mechanisms not quantified here (e.g., immune activation for PA and synaptic turnover for CA). Our data do support this latter hypothesis given that in almost all models examined, both PA and CA were not simultaneously significant, potentially suggesting independent pathways to similar outcomes.
Regarding mechanisms, decades of animal and more recent clinical works demonstrate the benefit of both enriched physical and cognitive environments for neuro- and synapto-genesis, white matter connectivity, and brain size [9, 10, 43], supporting their dynamic role in brain health as demonstrated here. Animal studies modeling aging and AD also implicate a host of pleiotropic neuroprotective mechanisms as well, including release of trophic factors [10, 44], modulated glial activation and immuno-vascular homeostasis [45, 46], and glymphatic clearance [18] following physical or cognitive enrichment; perhaps theses are specific mechanisms by which lifestyle activities support brain resilience to the aging process. Though relatively fewer mechanistic studies have examined both in conjunction, those that have indicate a particularly potent effect of CA, over PA, in decreasing aberrant protein aggregation in both animals [20] and adults [14, 47]. Moving forward, advanced molecular measurement tools, such as biofluid analytes and novel PET imaging ligands, will be incredibly useful to more directly translate these animal models and uncover the mechanistic underpinnings of active lifestyles for the brain in humans.
Mechanisms underlying links among PA, CA, and cognitive resilience to the aging brain are less well understood but a critical area of study given their high therapeutic potential (i.e., secondary prevention approach). In the current study, both CA and PA attenuated the impact of brain integrity on cognitive performance. Boros and colleagues (2017) recently demonstrated that clinically normal adults who reached neuropathologically significant AD at autopsy (i.e., cognitive resilience to AD) had significantly greater dendritic spine density and complexity compared to peers with clinically evident AD [48]. These data suggest that structural integrity and morphology of the synapse may be a critical mechanism supporting the ability to “outperform” pathology burden. Similarly, Stern and colleagues identified functional neural synchrony (via fMRI) as a mediator explaining the discrepancy between brain structure and cognition [49, 50]. Taken together, these data suggest that shape and functioning of the synaptic unit, perhaps regardless of pathology or other structural changes may integrally underlie the phenomenon of cognitive resilience to aging and age-related pathologies. A recent study by Hohman et al. (2017) applied a predicted gene expression tool (PrediXcan) to identify genes related to cognition among older adults with and without cerebral amyloid. This study identified expression patterns reflecting angiogenic and heme biosynthesis (i.e., HMBS, PROK1 expressed in heart tissue) as other candidate pathways related to the maintenance of cognitive performance in the face of pathology [51]. These latter findings underscore the importance of considering the brain as only one of the interconnected organs within the aging body, which is particularly relevant for factors like PA. Future work considering systemic factors that may contribute to brain and cognitive aging may be highly fruitful avenues to identify novel and important resilience pathways. Ultimately, disentangling the active mechanisms by which lifestyle behaviors support the brain will need to take a translational approach integrating systems models with clinical trials, which has the potential to yield incredibly valuable information for our rapidly aging population.
Although this study presents novel findings for the systematic characterization of independent contributions of PA and CA to brain and cognitive aging in two independent cohorts, there are several limitations to note. First, the two study samples represented largely different groups of people: UCD was more educationally, racially, and cognitively diverse, while UCSF was composed of largely cognitively healthy, majority White, highly educated adults. These cohort differences were likely reflected in some of the differential associations observed both between PA and CA, and between lifestyle activities and brain/cognitive outcomes. There is a need for future research that is adequately powered to explicitly examine the roles of educational attainment, race/ethnicity, and disease severity in these associations. Nonetheless, the consistent, positive associations observed between active lifestyles and brain/cognitive health across disparate samples illustrates the overall robustness of the relationship between lifestyle and aging.
Another important limitation is the observational and self-report nature of the data. Both activity measures were self-report and queried different facets of each activity type. For example, the UCD cognitive activity questionnaires included several items capturing social engagement. These differences in measurement tools may have also contributed to differential patterns of results, particularly given the posited specific role of socially-engaging activities for brain health [52]. Relatedly, as noted above, the two study cohorts represented quite distinct cohorts of older adults (i.e., UCD was racially, educationally, and cognitively more diverse than UCSF); it is therefore difficult to determine if the slightly different pattern of results observed in each cohort represents true overlapping heterogeneity of the effects of PA and CA on neurobehavioral outcomes versus an artifact simply reflecting cohort differences. Additionally, while we statistically adjusted for disease severity via CDRsob, the CDR is a limited marker of disease staging. Therefore, the potential for reverse causality cannot be excluded, particularly in the more cognitively affected UCD cohort. Particularly within UCD, activity levels were significantly associated with clinical severity (CDR). More frail individuals are also inherently more likely to have both poorer cognition and less activity, further limiting causal inferences due to potential unmeasured confounding effects. Lastly, our study did not aim to examine specific brain or cognitive networks, or include other important brain measures, including perfusion, metabolism, proteinopathy, or other biofluid markers (e.g., inflammation). Future directions building on this initial work using data-driven approaches (e.g., voxel-based morphometry) to identify and model these questions, as well as multimodal tools will be important to help better parse out the potential targetable networks and upstream biology related to PA versus CA.
Nonetheless, these results further add to the growing literature indicating the benefits of active lifestyles for age-related brain and cognitive health, and their potential modifying impact on brain risk factors. The next important steps will be to more deeply phenotype lifestyle behavior characteristics in order to parse out the specific active components and identify sensitive outcomes/mechanisms of this relationship. Ultimately, these advances will help target the most at-risk individuals, promote development of more sensitive monitoring techniques for intervention efficacy, and ultimately, increase the potency of lifestyle modifications for age-related brain health.
Fig. 2.
Schematic of multivariable linear regression models conducted testing the contribution of physical and cognitive lifestyle activities to age-related brain or cognitive resilience. Tests of Brain Resilience to Age. Greater activity participation is hypothesized to be directly associated with greater brain integrity than predicted by age (path “a”) and/or attenuate the negative impact of advanced age on brain integrity (path “b”; interaction model). Tests of Cognitive Resilience to Age and Brain Integrity. Greater activity participation was hypothesized to be associated with better cognition independent of age and brain (path “c”) and/or attenuate the impacts of advanced age (path “d”; interaction model) or poor brain integrity (path “e”; interaction model) on cognition. Greater brain integrity was operationalized as greater total gray matter volume (GMV), lower whole brain white matter hyperintensity volume (WMH), and higher mean fractional anisotropy (FA) across white matter tracts. Reported engagement in physical and cognitive activities were entered simultaneously in all models.
ACKNOWLEDGMENTS
This work was supported by and occurred as part of the 2018 Advanced Psychometric Methods in Cognitive Aging Research conference funded by the National Institute on Aging (NIA) (R13 AG030995, D Mungas, PI). This work also was supported by NIA grants: K23AG058752 (K Casaletto, PI), R01AG032289 (JH Kramer, PI), R01AG048234, R01AG046928, R01AG054617, P30 AG10129, R01 AG021028, R01 AG047827 (C DeCarli, PI), R01 AG10220 (D Mungas, PI), R01/RF1 AG031563 (B Reed/D Mungas, PI), R01 AG031252 (S Tomaszewski Farias, PI), R01AG046928 and R01AG054617 (J Pa, PI), and K01AG050723 (S Tom, PI). NMA is supported by the NIA Intramural Research Program. Our work was also supported by Larry L. Hillblom Network and Fellowship Grants (2014-A-004-NET; 2017-A-004-FEL).
Footnotes
Authors’ diclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1114r2).
REFERENCES
- [1].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2017) The Lancet Commissions Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- [2].Hörder H, Johansson L, Guo X, Grimby G, Kern S, Östling S, Skoog I (2018) Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women. Neurology 90, e1298–e1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J (2009) Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73, 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou Z, Fu J, Hong YA, Wang P, Fang Y (2017) Association between exercise and the risk of dementia: Results from a nationwide longitudinal study in China. BMJ Open 7, e017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K (2001) A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch Intern Med 161, 1703–1708. [DOI] [PubMed] [Google Scholar]
- [6].Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B, Gatz M (2005) Complexity of work and risk of Alzheimer’s disease: A population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 60, P251–258. [DOI] [PubMed] [Google Scholar]
- [7].Finkel D, Andel R, Gatz M, Pedersen NL (2009) The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging 24, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jonaitis E, La Rue A, Mueller KD, Koscik RL, Hermann B, Sager MA (2013) Cognitive activities and cognitive performance in middle-aged adults at risk for Alzheimer’s disease. Psychol Aging 28, 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, Heinze HJ, Düzel E, Schmiedek F, Lindenberger U (2010) Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia 48, 3878–3883. [DOI] [PubMed] [Google Scholar]
- [10].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, Mcauley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chapman SB, Aslan S, Spence JS, Hart JJ, Bartz EK, Didehbani N, Keebler MW, Gardner CM, Strain JF, DeFina LF, Lu H (2015) Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex 25, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, Harris TB, Health ABC Study (2009) Predictors of maintaining cognitive function in older adults: The Health ABC Study. Neurology 72, 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Najar J, Östling S, Gudmundsson P, Sundh V, Johansson L, Kern S, Guo X, Hällström T, Skoog I (2019) Cognitive and physical activity and dementia. Neurology 92, e1322–e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ (2014) Neuroprotective pathways: Lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 35, 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Geula C, Marsel Mesulam M (2013) Youthful memory capacity in old brains: Anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci 25, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA (2013) Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci 5, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB (2004) Physical activity, exercise, and inflammatory markers in older adults: Findings from The Health, Aging and Body Composition Study. J Am Geriatr Soc 52, 1098–1104. [DOI] [PubMed] [Google Scholar]
- [18].He X, Liu D, Zhang Q, Liang F, Dai G, Zeng J, Pei Z, Xu G, Lan Y (2017) Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci 10, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Müller S, Preische O, Sohrabi HR, Gräber S, Jucker M, Ringman JM, Martins RN, McDade E, Schofield PR, Ghetti B, Rossor M, Fox NN, Graff-Radford NR, Levin J, Danek A, Vöglein J, Salloway S, Xiong C, Benzinger T, Buckles V, Masters CL, Sperling R, Bateman RJ, Morris JC, Laske C; Dominantly Inherited Alzheimer Network (DIAN) (2018) Relationship between physical activity, cognition, and Alzheimer pathology in autosomal dominant Alzheimer’s disease. Alzheimers Dement 14, 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cracchiolo JR, Mori T, Nazian SJ, Tan J, Potter H, Arendash GW (2007) Enhanced cognitive activity-over and above social or physical activity-is required to protect Alzheimer’s mice against cognitive impairment, reduce Aβ deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem 88, 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC (2009) The Nun study: Clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology 73, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA (2013) Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H (2004) Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable β-amyloid deposition. Neuroreport 15, 1751–1754. [DOI] [PubMed] [Google Scholar]
- [24].Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Machulda M, Lowe VJ, Mielke MM, Roberts RO, Gunter JL, Senjem ML, Geda YE, Rocca WA, Petersen RC, Jack CR (2016) Effect of intellectual enrichment on AD biomarker trajectories. Neurology 86, 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D (2010) Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord 24, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Washburn RA, Smith KW, Jette AM, Janney CA(1993) The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol 46, 153–162. [DOI] [PubMed] [Google Scholar]
- [28].Casaletto KB, Marx G, Dutt S, Neuhaus J, Saloner R, Kritikos L, Miller B, Kramer JH (2017) Is “Learning” episodic memory? Distinct cognitive and neuroanatomic correlates of immediate recall during learning trials in neurologically normal aging and neurodegenerative cohorts. Neuropsychologia 102, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mungas D, Reed BR, Haan MN, González H (2005) Spanish and English neuropsychological assessment scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology 19, 466–475. [DOI] [PubMed] [Google Scholar]
- [30].Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17, 87–97. [DOI] [PubMed] [Google Scholar]
- [31].Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- [32].Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. [DOI] [PubMed] [Google Scholar]
- [33].Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D (2018) Brain volume change and cognitive trajectories in aging. Neuropsychology 32, 436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P Regional spatial normalization: Toward an optimal target. J Comput Assist Tomogr 25, 805–816. [DOI] [PubMed] [Google Scholar]
- [35].Rueckert D, Aljabar P, Heckemann RA, Hajnal JV, Hammers A (2006) Diffeomorphic registration using B-splines. Med Image Comput Comput Assist Interv 9(Pt 2), 702–709. [DOI] [PubMed] [Google Scholar]
- [36].Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, Nimmo-Smith I, Dipy Contributors (2014) Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang H, Yushkevich PA, Alexander DC, Gee JC (2006) Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal 10, 764–785. [DOI] [PubMed] [Google Scholar]
- [38].DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ (2005) Anatomical mapping of white matter hyperintensities (WMH). Stroke 36, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jak AJ (2012) The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci 10, 273–291. [DOI] [PubMed] [Google Scholar]
- [40].Yoon JE, Lee SM, Lim HS, Kim TH, Jeon JK, Mun MH (2013) The effects of cognitive activity combined with active extremity exercise on balance, walking activity, memory level and quality of life of an older adult sample with dementia. J Phys Ther Sci 25, 1601–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shah T, Verdile G, Sohrabi H, Campbell A, Putland E, Cheetham C, Dhaliwal S, Weinborn M, Maruff P, Darby D, Martins RN (2014) A combination of physical activity and computerized brain training improves verbal memory and increases cerebral glucose metabolism in the elderly. Transl Psychiatry 4, e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K (2013) The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med 173, 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ambrogini P, Cuppini R, Lattanzi D, Ciuffoli S, Frontini A, Fanelli M (2010) Synaptogenesis in adult-generated hippocampal granule cells is affected by behavioral experiences. Hippocampus 20, 799–810. [DOI] [PubMed] [Google Scholar]
- [44].Novkovic T, Mittmann T, Manahan-Vaughan D (2015) BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus 25, 1–15. [DOI] [PubMed] [Google Scholar]
- [45].Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 30, 464–472. [DOI] [PubMed] [Google Scholar]
- [46].Markham JA, Greenough WT (2004) Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol 1, 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, Wilson RS, Jagust WJ (2012) Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol 69, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boros BD, Greathouse KM, Gentry EG, Curtis KA, Birchall EL, Gearing M, Herskowitz JH (2017) Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann Neurol 82, 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Habeck C, Hilton HJ, Zarahn E, Flynn J, Moeller J, Stern Y (2003) Relation of cognitive reserve and task performance to expression of regional covariance networks in an event- related fMRI study of nonverbal memory. Neuroimage 20, 1723–1733. [DOI] [PubMed] [Google Scholar]
- [50].Steffener J, Stern Y (2012) Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta 1822, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hohman TJ, Dumitrescu L, Cox NJ, Jefferson AL, Alzheimer’s Neuroimaging Initiative (2017) Genetic resilience to amyloid related cognitive decline. Brain Imaging Behav 11, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kremen WS, Lachman ME, Pruessner JC, Sliwinski M, Wilson RS (2012) Mechanisms of age-related cognitive change and targets for intervention: Social interactions and stress. J Gerontol A Biol Sci Med Sci 67, 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kerchner GA, Racine CA, Hale S, Wilheim R, Laluz V, Miller BL, Kramer JH (2012) Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS One 7, e50425. [DOI] [PMC free article] [PubMed] [Google Scholar]