Abstract
Objective:
To determine whether antibiograms for Veterans Affairs (VA) nursing homes (NHs), termed Community Living Centers, are similar to those from their affiliated acute care medical centers.
Design:
Descriptive study.
Setting and participants:
We compared the 2017 antibiograms for VA NHs to their affiliated VA medical centers (VAMCs). Antibiograms included antibiotic susceptibility rates for commonly observed bacteria in this setting (Staphylococcus aureus, Enterococcus spp, Escherichia coli, Klebsiella spp, Proteus mirabilis, and Pseudomonas aeruginosa).
Methods:
Antibiograms were considered to be in complete agreement when the overall susceptibility rate between the NH and affiliated VAMC was either at or above 80% or below 80% across all bacteria and antibiotics. Average percentage of bacteria-antibiotic comparisons in disagreement per facility pair, and number of facilities with agreement for specific bacteria-antibiotic comparisons were also assessed. The chi-square test was used to compare disagreement between NH-VAMC facilities based on geographic proximity of the NH to the VAMC, culture source, and bed size.
Results:
A total of 119 NH-VAMC affiliate pairs were included in this analysis, with 71% (84/119) on the same campus and 29% (35/119) on geographically distinct campuses. None of the NH-VAMC pairs demonstrated complete agreement (all bacteria vs all antibiotics) between their antibiograms. On average, 20% of the bacteria-antibiotic comparisons from the antibiogram disagreed clinically per NH-VAMC pair, and almost twice as often the nursing home had lower susceptibility (higher resistance) than the acute care facility. Some bacteria-antibiotic comparisons agreed in all facilities (eg, E coli–imipenem; S aureus–linezolid; S aureus–vancomycin), while others showed greater disagreement (eg, Klebsiella spp–cefazolin; Klebsiella spp–ampicillin-sulbactam; P aeruginosa–ciprofloxacin). Rates of clinical disagreement were similar by geographic proximity of the NH to the VAMC, culture source, and bed size.
Conclusions and implications:
Overall, this study showed a moderate lack of agreement between VA NH antibiograms and their affiliate VAMC antibiograms. Our data suggest that antibiograms of acute care
Keywords: Antibiogram, antimicrobial stewardship, Community Living Center, empiric antibiotic therapy, Veterans Affairs
Antibiograms are profile reports of antibiotic susceptibility rates of bacteria from a single facility over a duration of 1 calendar year.1 These reports are used to guide empiric antibiotic prescribing and to track emerging bacterial resistance within the facility. They are especially informative for antimicrobial stewardship practices that help guide appropriate empiric prescribing when waiting for culture results.2-5 Unfortunately, because of a lack of resources and a low number of clinical cultures, the creation of antibiograms in nursing home (NH) settings can often be challenging.6,7 To overcome these barriers, some NHs may use antibiograms from nearby or affiliated acute care facilties.8
The rationale for NH use of acute care facility antibiograms is that NH residents are often admitted to nearby acute care facilities, and there are high rates of bidirectional patient movement and pathogen transmission between the 2 settings.9-11 The same rationale supports the use of regional antibiograms when facility-specific antibiograms are unavailable.1,12 Despite this convention, there are no studies that assess whether antibiograms from acute care settings can be applied to NHs. Specifically, it is largely unknown if the susceptibly profiles among NHs and their affiliated acute care facilities are similar enough to produce the same empiric antibiotic treatment recommendations and if providers can use acute care antibiograms to make decisions about empiric antibiotic therapy in NHs. To address this question, we evaluated culture results from a national cohort from the Veterans Health Administration to develop antibiograms for individual Veterans Affairs (VA) Community Living Centers, herein termed VA NHs, and compared them to the antibiograms for their affiliated acute care VA medical centers (VAMCs).
Methods
VA NH and VAMC Pairs
Culture and susceptibility results from Veterans admitted to VA NHs and their affiliated VAMCs from January 1, 2017 through December 31, 2017 were included (n = 119 NH-VAMC pairs). NH campuses were classified by the project coordinator of this study as either being geographically similar or distinct from their affiliate VAMC based on an e-mail or telephone query, or both, of medical directors or chiefs of service. VA NHs that were reported to be in the same building or in a separate building contiguous with the affiliated VAMC were classified as “same” campus NH-VAMC pairs; all others were classified as “remote” campus NH-VAMC pairs.
Culture and Susceptibility Data
We evaluated the following pathogens: Staphylococcus aureus, Enterococcus spp, Escherichia coli, Klebsiella spp, Proteus mirabilis, and Pseudomonas aeruginosa. For each bacterial species, susceptibility rates to commonly used antibiotics were assessed (9 antibiotics for both gram-negative and gram-positive bacteria).
Antibiograms
Antibiograms were created for all individual VA NHs and VAMCs for the calendar year of 2017 according to Clinical and Laboratory Standards Institute recommendations for using the first clinical isolate cultured per patient per bacterial species (regardless of specimen source) for percentage susceptibility calculations.1 The percentage susceptibility was calculated by dividing the number of susceptible isolates by the total number of isolates tested against that antibiotic multiplied by 100. All isolates of the aforementioned bacterial species were included, regardless if there were <30 isolates per year, which are typically removed from antibiogram reports in clinical use as recommended by Clinical and Laboratory Standards Institute.1
Weighted national antibiograms were created for all VA NHs and VAMCs for 2017, and VA NH–VAMC differences in weighted percentage susceptibilities, as well as carbapenem resistance and multidrug resistance rates, were compared using chi-square tests. E coli, Klebsiella spp, and P mirabilis carbapenem-resistant isolates were defined as resistant to ≥1 carbapenem (doripenem, ertapenem, imipenem, or meropenem), with the same definition used for P aeruginosa with the exception of ertapenem, which is not an anti-pseudomonal carbapenem. E coli, Klebsiella spp, and P mirabilis multidrug-resistant isolates were defined as resistant to at least 1 drug in at least 3 of the following categories: (1) extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, ceftriaxone), (2) fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), (3) aminoglycosides (amikacin gentamicin, tobramycin), (4) carbapenems (imipenem, meropenem, doripenem, ertapenem), or (5) piperacillin group (piperacillin, piperacillin-tazobactam), with the same definition used for P aeruginosa with the exception of the antibiotics that do not have anti-pseudomonal activity (cefotaxime, ceftriaxone, moxifloxacin, doripenem).13
Analyses of Clinical Agreement Between Antibiograms
VA NH antibiograms were compared to each affiliate VAMC antibiogram for clinical agreement for each bacteria-antibiotic combination. Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) suggest that an antibiotic is appropriate for empiric treatment if the percentage susceptibility on an antibiogram is ≥80%.14,15 Therefore, NH-VAMC pairs were defined as agreeing clinically if the percentage susceptibility for the bacteria-antibiotic combination was ≥80% in both the NH and VAMC, or <80% in both the NH and VAMC. Complete agreement was defined as agreement for all bacteria-antibiotic comparisons per NH-VAMC pair.
Disagreement was defined as differences in the percentage susceptibility between the NH and VAMC for the bacteria-antibiotic combination, where the percentage susceptibility was ≥80% in 1 facility and <80% in the other facility. Average percentage of bacteria-antibiotic comparisons that clinically disagreed per facility were calculated. Clinical disagreement was assessed by type of disagreement (NH susceptibility ≥80% and VAMC susceptibility <80%, or NH susceptibility <80% and VAMC susceptibility ≥80%), geographic proximity of the NH to the VAMC, culture source, and bed size. Number of facilities with agreement for specific bacteria-antibiotic comparisons were also assessed.
The Chi-square test was used to compare the proportion of agreement between NH-VAMC facilities that were on the same campus vs those on geographically distinct campuses. Bonferroni corrections were performed to correct for multiple comparisons [eg, comparison of 9 antibiotic susceptibilities for E coli; P value (α) = .05/9 = .006]. To determine whether agreement was greater for certain antibiotics, kappa statistics were calculated for the percentage of agreement between facilities by antibiotic.
Results
A total of 119 NH-VAMC affiliate pairs were included in this analysis, with 71% (84/119) on the same campus and 29% (35/119) on geographically distinct campuses. None of the NH-VAMC pairs demonstrated complete agreement in their antibiograms. On average, 20% of the bacteria-antibiotic comparisons from the antibiogram disagreed clinically per NH-VAMC pair (Table 1). Disagreement, where NH susceptibility was <80% and VAMC susceptibility was ≥80%, accounted for 13% of the disagreement vs 7% where the resistance was higher in the VAMC. Rates of clinical disagreement were similar by geographic proximity of the NH to the VAMC, culture source, and bed size.
Table 1.
Percentage of Bacteria-Specific Antimicrobial Susceptibilities With Clinical Disagreement Between the Nursing Home and Affiliated Medical Center
| Groupings of NH-VAMC Affiliate Pairs |
Number of Nursing Home and Affiliated Medical Center Pairs |
Mean % (Standard Deviation) |
Median % (Interquartile Range) |
|---|---|---|---|
| All NH-VAMC pairs | 119 | 20 (8) | 19 (14-24) |
| NH susceptibility ≥80%, VAMC susceptibility <80% | 119 | 7 (5) | 5 (3-10) |
| NH susceptibility <80%, VAMC susceptibility ≥80% Campus | 119 | 13 (8) | 12 (6-17) |
| Remote campus | 35 | 19 (8) | 20 (13-24) |
| Same campus | 84 | 20 (8) | 19 (15-24) |
| Culture source | |||
| Urine | 117 | 21 (9) | 20 (15-27) |
| Skin and soft tissue | 114 | 23 (14) | 21 (14-30) |
| Respiratory tract | 57 | 24 (17) | 25 (13-33) |
| Blood | 73 | 26 (20) | 25 (13-38) |
| Nursing home bed size | |||
| <50 beds | 32 | 21 (9) | 21 (17-24) |
| 50-99 beds | 35 | 18 (7) | 17 (13-22) |
| 100-199 beds | 45 | 20 (7) | 19 (15-24) |
| >199 beds | 7 | 18 (9) | 17 (12-27) |
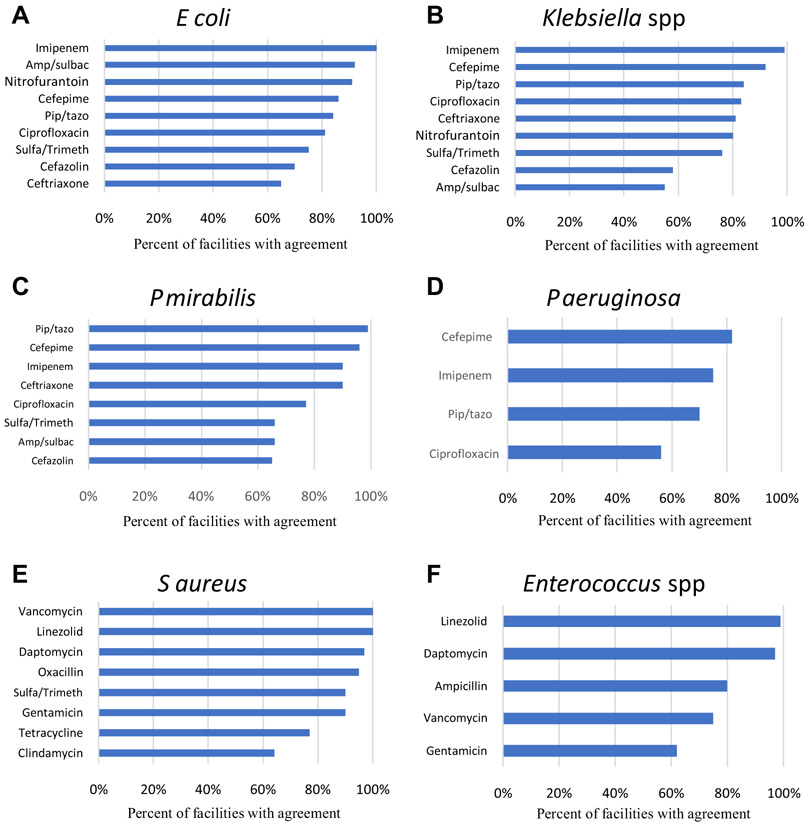
Some bacteria-antibiotic comparisons agreed in all facilities (E coli–imipenem; S aureus–linezolid; S aureus–vancomycin), whereas others showed greater disagreement (Klebsiella spp–cefazolin; Klebsiella spp–ampicillin-sulbactam; P aeruginosa–ciprofloxacin). Figure 1 shows the agreement rate for each antibiotic-bacteria combination assessed. Because not every NH or VAMC had a culture for each of the 6 organisms or 18 antibiotics assessed, the maximum number of VA NH–VAMC pairs available for inclusion for a specific bacteria-antibiotic combination in the comparisons was 114 and the minimum was 29. Greater clinical agreement was observed between VA NHs and VAMCs for imipenem (kappa statistic 0.73) and nitrofurantoin (0.71) among the gram-negatives, and linezolid (0.66) among the gram-positives. For the remaining antibiotics, antibiotic-specific clinical agreement was lower (kappa less than 0.50; Figure 1).
Fig. 1.
Percentage of nursing homes and affiliated medical centers with clinical agreement in bacteria-specific antimicrobial susceptibility. Agreement between NH-VAMC pairs was defined as NH and VAMC susceptibilities both ≥80% or both NH and VAMC susceptibilities <80%. Antibiotic-specific kappa statistics for gram-negative organisms in NHs vs VAMCs were as follows: ampicillin-sulbactam 0.38, cefazolin 0.29, cefepime 0.22, ceftriaxone 0.27, ciprofloxacin 0.48, imipenem 0.73, nitrofurantoin 0.71, piperacillin-tazobactam 0.07, and sulfamethoxazole-trimethoprim 0.43. Antibiotic-specific kappa statistics for gram-positive organisms in NHs vs VAMCs were as follows: ampicillin 0.22, clindamycin 0.12, gentamicin 0.50, linezolid 0.66, oxacillin 0.23, tetracycline 0.01, vancomycin 0.24. Antibiotic abbreviations: amp/sulb, ampicillin-sulbactam; pip/tazo, piperacillin-tazobactam; sulfa/trimeth, sulfamethoxazole-trimethoprim. (A) Findings for E coli. (B) Findings for Klebsiella spp. (C) Findings for P mirabilis. (D) Findings for P aeruginosa. (E) Findings for S aureus. (F) Findings for Enterococcus spp.
Statistically significant differences were observed in weighted percent susceptibilities between VA NHs and VAMCs, all in which the VA NHs had lower susceptibilities than the VAMCs (Tables 2 and 3). With the exception of P aeruginosa–ciprofloxacin, both the VA NH and VAMC susceptibilities either fell below the 80% susceptibility threshold or above the threshold and therefore would not have indicated different clinical decisions in most scenarios. Multidrug-resistant and carbapenem-resistant rates of the included gram-negative bacteria were found to be similar between VA NHs and VAMCs (Table 2).
Table 2.
Weighted Antibiotic Resistance and Susceptibility Rates for Gram-Negative Bacteria in VA NHs and VAMCs
| Gram-Negative Organisms |
% MDR | % Carbapenem Resistance |
Percentage Susceptible |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin- Sulbactam |
Cefazolin | Cefepime | Ceftriaxone | Ciprofloxacin | Imipenem | Nitrofurantoin | Piperacillin- Tazobactam |
Trimethoprim- Sulfamethoxazole |
|||
| Escherichia coli | |||||||||||
| NH | 8.4 | 1.2 | 46 | 66 | 89 | 80 | 53 | 100 | 94 | 92 | 67 |
| VAMC | 6.7 | 0.4 | 52 | 73 | 92 | 84 | 65 | 100 | 96 | 95 | 71 |
| Difference in % | 1.7 | 0.8 | −6* | −7* | −3 | −4 | −12* | 0 | −2 | −3* | −4 |
| Klebsiella spp | |||||||||||
| NH | 8.8 | 1.9 | 69 | 74 | 92 | 84 | 86 | 98 | 40 | 90 | 82 |
| VAMC | 5.2 | 1.1 | 73 | 76 | 92 | 86 | 90 | 98 | 47 | 91 | 86 |
| Difference in % | 3.6 | 0.8 | −4 | −2 | 0 | −2 | −4* | 0 | −7* | −1 | −4 |
| Proteus mirabilis | |||||||||||
| NH | 4.5 | 19.0 | 83 | 68 | 97 | 92 | 39 | 40 | – | 99 | 58 |
| VAMC | 5.1 | 18.6 | 83 | 65 | 96 | 90 | 59 | 35 | – | 99 | 66 |
| Difference in % | −0.6 | 0.4 | 0 | 3 | 1 | 2 | −20* | 5 | – | 0 | −8* |
| Pseudomonas aeruginosa | |||||||||||
| NH | 12.3 | 15.2 | – | – | 87 | – | 73 | 82 | – | 85 | – |
| VAMC | 9.1 | 13.4 | – | – | 89 | – | 80 | 83 | – | 88 | – |
| Difference in % | 3.2 | 1.8 | – | – | −2 | – | −7* | −1 | – | −3 | – |
MDR, multidrug resistant.
The percentage of MDR and carbapenem-resistant isolates are indicated in the corresponding columns. Otherwise, the columns indicate percentage susceptibility.
Statistically significant difference using Bonferroni correction for multiple comparisons.
Table 3.
Weighted Antibiotic Susceptibility Rates for Gram-Positive Bacteria in VA NHs and VAMCs
| Gram-Positive Organisms | Ampicillin | Clindamycin | Daptomycin | Gentamicin | Linezolid | Oxacillin | Tetracycline | Trimethoprim- Sulfamethoxazole |
Vancomycin |
|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | |||||||||
| NH | – | 58 | 99 | 93 | 100 | 39 | 86 | 93 | 100 |
| VAMC | – | 73 | 100 | 97 | 100 | 54 | 92 | 96 | 100 |
| Difference in % | – | −15* | −1 | −4* | 0 | −15* | −6* | −3 | 0 |
| Enterococcus spp | |||||||||
| NH | 88 | – | 99 | 58 | 99 | – | – | – | 84 |
| VAMC | 88 | – | 100 | 70 | 99 | – | – | – | 87 |
| Difference in % | 0 | – | −1 | −12* | 0 | – | – | – | −3 |
Statistically significant difference using Bonferroni correction for multiple comparisons.
Discussion
We found that none of the VA NH antibiograms and their affiliate VAMC antibiograms had complete clinical agreement, suggesting that antibiograms of acute care facilities should not be used to guide therapy in affiliated nursing homes. Our study demonstrated that clinical similarities and differences exist between annual antibiograms in NHs and affiliate medical centers. The average percentage clinical disagreement rate between the NH and affiliate medical center antibiograms was 20%, and the agreement rate varied greatly, from 55% to 100% among the specific bacteria-antibiotic combination assessed. These results suggest that NHs may have similar rates of susceptibility to their affiliate medical center that will result in similar empiric prescribing decisions in certain scenarios, but not all of the time. Moreover, higher rates of clinical agreement (82%-100%) were observed for most broad-spectrum intravenous antibiotics such as cefepime, linezolid, and daptomycin. These antibiotics are often reserved for treatment of severe acute infections that require inpatient hospitalization and are not commonly used in NHs.16 Lower rates of agreement (56%-83%) were observed for orally available antibiotics such as ciprofloxacin and sulfamethoxazole-trimethoprim. Because these agents tend to be commonly prescribed in the NH setting, this further suggests that antibiograms of acute care facilities should not be used to guide therapy in affiliated nursing homes.16-18
Describing the similarities helps to determine if utilizing affiliate medical center antibiograms is an appropriate practice within NHs that either cannot make antibiograms of their own or have few bacteria isolated. Studies have shown that antibiotic-resistant bacteria are more prevalent in NH populations than other populations, but it has been unclear if such differences would be large enough to change empiric prescribing recommendations.19 For example, 1 study found that when compared to the general community patients, those ≥65 years of age who resided in a single nursing home were found to have more methicillin-resistant Staphylococcus aureus (MRSA) from all culture sites and more resistant Enterobacteriaceae from urine cultures over a 5-year period.20 Additionally, other studies have argued the importance of creating antibiograms specific to special populations and facilities because of differences in susceptibility rates of specific bacteria to antibiotics.21-24 For example, community-acquired compared to nosocomial-acquired E coli infections in an 860-bed tertiary hospital in Zurich, Switzerland were found to be less susceptible to sulfamethoxazole-trimethoprim (susceptibility rate of 70% vs 67%, P = .006).25 Although the susceptibility rates differed statistically, the rates of both 70% and 67% would make sulfamethoxazole-trimethoprim a poor empiric therapy option for E coli coverage. Therefore, our study interprets the differences in NH and affiliate medical center antibiograms that would likely correlate with different empiric prescribing recommendations.
As use of the affiliate hospital antibiogram is unreliable for commonly used antibiotics within the NH, potential other solutions to decide appropriate treatment may include extending the time period of the antibiogram data collection beyond 1 year, collapsed antibiograms specific to specimen site or infection type, and NH antibiograms including bacteria species regardless of low isolate numbers.8 Although some of the approaches would help circumvent the issue of low isolate numbers, they still may not be representative of the bacteria acquired within the NH, and it is not yet elucidated which of these approaches best inform empiric prescribing. Additionally, several other patient-specific considerations aside from just the antibiogram data, such as prior infection history, need to be considered when empirically prescribing an antibiotic.26,27 Exactly how to integrate the use of the antibiogram into clinical practice within an NH is also currently unknown.
This work is limited in that this is a Veteran population, which may differ from other NH populations considering that many facilities were on the same campus as their affiliate medical center, and it is a unique closed health care system. Additionally, only culture data that were entered into the VA microbiology system were included, and as such, cultures obtained from facilities outside the VA may not be completely captured if they were not manually entered into that resident’s electronic medical record. Although some NH-VAMC pairs use the same microbiology laboratory, others use different laboratories. Culture practices are not standardized throughout the VA; therefore, some may have implemented the 2010 Clinical and Laboratory Standards Institute updated breakpoints for Enterobacteriaceae and P aeruginosa whereas others may not have.28 Tested antibiotics and bacteria combinations also may have differed, and overall the susceptibility interpretations of the testing microbiology laboratories were relied on. Another limitation is that an 80% susceptibility cut-off was used in this study, and other clinicians may use different cut-offs to guide empiric antibiotic choices within their institutions. Further, positive cultures of any source (eg, skin and soft tissue, urine, and respiratory cultures) could represent colonization and/or contamination rather than infection. Lastly, many of the nursing homes had few cultures to assess, depending on the organism, which affects the accuracy of the susceptibility estimate.
Conclusions and Implications
Overall, this study showed a lack of complete agreement between VA NH antibiograms and their affiliate VAMC antibiograms, and a wide range of agreement among the specific bacteria-antibiotic combination assessed. Although this work was limited to the Veteran population, we demonstrated that even in a closed health care system, where the majority of NH patients may come from a single acute care facility, the extent of disagreement limits the use of susceptibility data from acute care hospitals. This may result from differences in the patient populations, culture practices, length of stay, and antibiotic use between NHs and hospitals. Other nursing homes outside the VA system, even those that receive the majority of their patients from a single acute care facility, should reconsider using that acute care hospital’s susceptibility data. Our data suggest that antibiograms of acute care facilities should be used with caution (if at all) in guiding empiric antibiotic therapy within nursing homes.
Acknowledgments
We acknowledge Stefanie I. Gidmark, our project coordinator, for her assistance and contributions in carrying out this study.
This work was supported in part by the Veterans Affairs (VA) Health Services and Research Merit Award no. 15-120. This work was supported in part by funds and facilities provided by the Cleveland Department of Veterans Affairs (VA), the Cleveland Geriatric Research Education and Clinical Center (GRECC), and the Specialty Care Center of Innovation. The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs.
A.C. has received research funding from Pfizer, Merck (Cubist), and The Medicines Company. D.D. has funded projects from the Veterans Administration, the National Institutes of Aging,and Meals on Wheels of America. R.J. is the principal investigator on research grants from Pfizer and Accelerate. K.L has received research funding or acted as a scientific advisor for Merck, Ocean Spray, Pfizer, and The Medicines Company.
References
- 1.Clinical and Laboratory Standards Institute (CLSI). Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data Approved guideline M39eA4. 4th ed. Wayne, PA: CLSI; 2014. p. 34. [Google Scholar]
- 2.Centers for Disease Control and Prevention. The Core Elements of Antibiotic Stewardship for Nursing Homes Appendix A: Policy and Practice Actions to Improve Antibiotic Use, 2017. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. Available at:http://www.cdc.gov/longtermcare/index.html. Accessed September 1, 2019. [Google Scholar]
- 3.Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 4.Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: A new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007;44:867–873. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez J, Vazquez F. The importance of cumulative antibiograms in diagnostic stewardship. Clin Infect Dis; 2019. January 30. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE, Bentley DW, Garibaldi R, et al. Antimicrobial use in long-term-care facilities. SHEA Long-Term-Care Committee. Infect Control Hosp Epidemiol 2000;21:537–545. [DOI] [PubMed] [Google Scholar]
- 7.Kanwar A, Singh M, Lennon R, et al. Frailty and health-related quality of life among residents of long-term care facilities. J Aging Health 2013;25:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolg MA, Dosa DM, Jump RLP, et al. Antimicrobial stewardship in long-term care facilities: Approaches to creating an antibiogram when few bacterial isolates are cultured annually. J Am Med Dir Assoc 2018;19:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahvecioglu D, Ramiah K, McMaughan D, et al. Multidrug-resistant organism infections in US nursing homes: A national study of prevalence, onset, and transmission across care settings, October 1, 2010-December 31, 2011. Infect Control Hosp Epidemiol 2014;35:S48–S55. [DOI] [PubMed] [Google Scholar]
- 10.Wang HE, Shah MN, Allman RM, Kilgore M. Emergency department visits by nursing home residents in the United States. J Am Geriatr Soc 2011;59: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Dool C, Haenen A, Leenstra T, Wallinga J. The role of nursing homes in the spread of antimicrobial resistance over the healthcare network. Infect Control Hosp Epidemiol 2016;37:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Var SK, Hadi R, Khardori NM. Evaluation of regional antibiograms to monitor antimicrobial resistance in Hampton Roads, Virginia. Ann Clin Microbiol Antimicrob 2015;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antimicrobial Resistant Phenotype Definitions. National Healthcare Satety Network. Available at: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/aur/ar-phenotype-definitions-508.pdf. Accessed June 1, 2019.
- 14.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103–e120. [DOI] [PubMed] [Google Scholar]
- 16.Daneman N, Gruneir A, Newman A, et al. Antibiotic use in long-term care facilities. J Antimicrob Chemother 2011;66:2856–2863. [DOI] [PubMed] [Google Scholar]
- 17.Lim CJ, Kong DC, Stuart RL. Reducing inappropriate antibiotic prescribing in the residential care setting: Current perspectives. Clin Interv Aging 2014;9:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latour K, Catry B, Broex E, et al. Indications for antimicrobial prescribing in European nursing homes: Results from a point prevalence survey. Pharmacoepidemiol Drug Saf 2012;21:937–944. [DOI] [PubMed] [Google Scholar]
- 19.van Buul LW, van der Steen JT, Veenhuizen RB, et al. Antibiotic use and resistance in long term care facilities. J Am Med Dir Assoc 2012;13:568.e1–568.e13. [DOI] [PubMed] [Google Scholar]
- 20.Xie C, Taylor DM, Howden BP, Charles PG. Comparison of the bacterial isolates and antibiotic resistance patterns of elderly nursing home and general community patients. Intern Med J 2012;42:e157–e164. [DOI] [PubMed] [Google Scholar]
- 21.Swami SK, Banerjee R. Comparison of hospital-wide and age and location-stratified antibiograms of S. aureus, E. coli, and S. pneumoniae: Age- and location-stratified antibiograms. Springerplus 2013;2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boggan JC, Navar-Boggan AM, Jhaveri R. Pediatric-specific antimicrobial susceptibility data and empiric antibiotic selection. Pediatrics 2012;130: e615–e622. [DOI] [PubMed] [Google Scholar]
- 23.Bosso JA, Sieg A, Mauldin PD. Comparison of hospitalwide and custom antibiograms for clinical isolates of Pseudomonas aeruginosa. Hosp Pharm 2013;48: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen S, Zurayk M, Yeung S, et al. Emergency department urinary antibiograms differ by specific patient group. J Clin Microbiol 2017;55:2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuster SP, Ruef C, Zbinden R, et al. Stratification of cumulative antibiograms in hospitals for hospital unit, specimen type, isolate sequence and duration of hospital stay. J Antimicrob Chemother 2008;62:1451–1461. [DOI] [PubMed] [Google Scholar]
- 26.Linsenmeyer K, Strymish J, Gupta K. Two simple rules for improving the accuracy of empiric treatment of multidrug-resistant urinary tract infections. Antimicrob Agents Chemother 2015;59:7593–7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2007;27:1306–1312. [DOI] [PubMed] [Google Scholar]
- 28.Dudley MN, Ambrose PG, Bhavnani SM, et al. Background and rationale for revised clinical and laboratory standards institute interpretive criteria (Breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and aztreonam. Clin Infect Dis 2013;56:1301–1309. [DOI] [PubMed] [Google Scholar]