Abstract
Limited data exist evaluating the effect of blood pressure (BP) on clinical outcomes among patients with acute ischemic stroke with large vessel occlusion treated with mechanical thrombectomy (MT). We sought to evaluate the association of BP levels on clinical outcomes among patients with acute ischemic stroke with large vessel occlusion treated with MT. Studies were identified that reported the association of systolic BP (SBP) or diastolic BP levels before, during, or after MT on the outcomes of patients with acute ischemic stroke treated with MT. Unadjusted and adjusted analyses of studies reporting odds ratios (ORadj) per 10 mm Hg BP increment were performed. Our analysis included 25 studies comprising 6474 patients. Higher pre-MT mean SBP (P=0.008) and post-MT maximum SBP (P=0.009) levels were observed in patients who died within 3 months. Patients with 3-month functional independence were noted to have lower pre-MT (P<0.001) and post-MT maximum SBP levels (P<0.001). In adjusted analyses, increasing post-MT maximum SBP and diastolic BP levels were associated with 3-month mortality (ORadj, 1.19 [95% CI,1.00–1.43]; I2=78%, P value for Cochran Q test: 0.001) and symptomatic intracranial hemorrhage (ORadj, 1.65 [95% CI, 1.11–2.44]; I2=0%, P value for Cochran Q test: 0.80), respectively. Increasing pre- and post-MT mean SBP levels were associated with lower odds of 3-month functional independence (ORadj, 0.86 [95% CI, 0.77–0.96]; I2=18%, P value for Cochran Q test: 0.30) and (ORadj, 0.80 [95% CI, 0.72–0.89]; I2=0%, P value for Cochran Q test: 0.51), respectively. In conclusion, elevated BP levels before and after MT are associated with adverse outcomes among patients with acute ischemic stroke with large vessel occlusion.
Keywords: blood pressure, consensus, intracranial hemorrhages, odds ratio, thrombectomy
Large vessel occlusion (LVO) has been reported to occur in up to one-third of patients with acute ischemic stroke (AIS) and is associated with higher rates of poststroke dependence and mortality.1,2 Mechanical thrombectomy (MT) in patients with LVO improves functional outcomes and has profoundly changed the landscape of acute stroke therapy.3 During the hyperacute stage of AIS due to LVO, the fate of the ischemic penumbra largely depends on the maintenance of perfusion above the threshold for the infarct core. Among other factors that affect cerebral perfusion, optimizing blood pressure (BP) remains a potential target to improve neurological outcome in patients with LVO during hyperacute AIS stage. Elevated pre- and post-treatment BP levels have been adversely associated with AIS outcomes in patients treated with intravenous thrombolysis (IVT).4 Current American Heart Association/American Stroke Association guidelines recommend strict, though arbitrary, thresholds of systolic BP (SBP) <180 mm Hg and diastolic blood pressure (DBP) <105 mm Hg during and after MT.5 However, data regarding guidance for optimal BP management among patients with LVO-AIS treated with MT largely remain scarce.
Observational data indicate that elevated BP among patients with LVO treated with MT is associated with an increased risk of symptomatic intracranial hemorrhage (sICH)6,7 as well as mortality8,9 and reduce the odds of MT-induced recanalization10,11 and functional independence.6,9,12–14 Randomized data are lacking to render a clear consensus for the optimal BP control before, during, and after MT among patients with AIS with LVO. In view of these considerations, we conducted a systematic review and a pairwise meta-analysis seeking to evaluate the association of elevated acute BP levels before, during, and after MT on different clinical outcomes among patients with LVO treated with MT.
Methods
We adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement15 and reported in accordance to the Meta-analysis of Observational Studies in Epidemiology16 proposal. The protocol of the present systematic review and meta-analysis has been registered to PROSPERO (The International Prospective Register of Systematic Reviews) (CRD42019134621). The present manuscript also adheres to the American Heart Association Journals implementation of the Transparency and Openness Promotion guidelines.17 Authors declare that all supporting data are available within the article and its online-only Data Supplement.
Data Sources and Searches
Eligible studies were identified by systematically searching in Ovid MEDLINE, Ovid Embase, and Scopus databases. The combination of search strings used to query all the databases included: “mechanical thrombectomy”, “stroke”, “cerebral ischemia”, “blood pressure,” “systolic,” and “diastolic”. The search algorithm used for the MEDLINE database is available in the online-only Data Supplement. We restricted our search to articles in English language, and our search spanned from database inception to August 6, 2019. Additional manual search of conference abstracts and bibliographies of articles meeting study criteria for a comprehensive literature search was conducted.
Study Selection and Data Extraction
We identified all observational studies (prospective or retrospective) and post hoc studies of randomized controlled clinical trials that provided data on the association of acute BP levels with clinical outcomes in patients with AIS treated with MT. Per study protocol, we excluded studies that reported (1) outcomes not documented according to our predefined criteria such as parenchymal hematoma or asymptomatic intracranial hemorrhage, (2) treatment with intraarterial thrombolysis, (3) categorical or descriptive data for BP levels reported as median values, (4) studies reporting mean arterial pressure levels instead of SBP or DBP levels, and (5) case reports, case series, or conference abstracts. In case of overlapping data (same patient data used in >1 publication) for each outcome of interest, we used the study with the highest number of included patients. Reference lists of all articles that met the inclusion criteria and of relevant review articles were examined to identify studies that may have been missed by the initial database search. All retrieved studies were scanned independently by 2 reviewers (K. Malhotra, A. Filippatou), and in any case of disagreement, the third author (G. Tsivgoulis) was consulted to resolve any disagreements.
We primarily documented maximum, minimum, or mean SBP and DBP levels reported as mean±SD before, during, and after MT. During the post-MT interval, we recorded BP levels documented within 2 to 4 hours following MT. If these data were unavailable, BP levels within 24 to 72 hours of MT were used. Additional data on BP variability (BPV) including SD, coefficient of variation (CV), and successive variation (SV) were collected if available. In case of missing data, the authors of relevant studies were contacted, and previously unpublished data was occasionally provided according to their discretion.
Outcomes
The primary efficacy outcomes were defined as the unadjusted and adjusted for potential confounders likelihood of 3-month functional independence, defined as a modified Rankin Scale (mRS) score of 0 to 2 at 3 months or discharge. The primary safety outcomes were defined as unadjusted and adjusted for potential confounders likelihood of sICH (according to the definition provided in each study; Table S1 in the online-only Data Supplement).
Secondary outcomes included 3-month mortality, successful recanalization (defined as Thrombolysis in Cerebral Infarction scores of 2b or 3 at the end of MT), and functional improvement (assessed with ordinal [shift] analysis of the mRS scores at 3 months or at discharge).
Risk of Bias Assessment
We used the Newcastle-Ottawa Scale4 and the ROBINS-E (Risk of Bias in Nonrandomized Studies of Exposures)18 to assess the quality and explore the sources of bias amongst the included cohort studies. The quality control and bias identification were performed independently by 2 reviewers (K. Malhotra and A. Filippatou), and all potential disagreements were resolved by a third tie-breaking evaluator (G. Tsivgoulis).
Data Synthesis and Statistical Analysis
In the current pairwise meta-analysis, both unadjusted and adjusted for potential confounders analyses for pre- and post-MT SBP/DBP levels were handled as continuous variables, while the outcomes of interest were handled as dichotomous variables. Differences in maximum, minimum, or mean pre- and post-MT BP levels according to the outcomes of interest were reported in the form of standardized mean differences (SMDs) in all unadjusted analyses as previously described.3,16 We also conducted adjusted odds ratios (ORadj) of these associations evaluating the association of pre- and post-MT BP levels with different clinical outcomes. The ORadj of these associations are all presented per 10 mm Hg increments in SBP or DBP levels and derived as available from the original studies. For each of these associations (unadjusted and adjusted for potential confounders), the individual study effects were estimated using the random-effects model (DerSimonian and Laird).19 We used inverse variance method to calculate SMD for continuous variables. SMDs were interpreted using a general rule of thumb reported by Cohen,20 in which an SMD of 0.2 represents a small effect, an SMD of 0.5 represents a medium effect, and an SMD of 0.8 or larger represents a large effect.
As per the Cochrane Handbook for Systematic Reviews of Interventions,21 we assessed for heterogeneity between the included studies using Cochran Q and I2 statistics. For the qualitative interpretation of heterogeneity, I2>50% and I2>75% indicated substantial and considerable heterogeneity, respectively. We performed subgroup analyses on the outcomes of interest by including only studies that provided data on the association of BP levels and clinical outcomes in patients with LVO who achieved successful recanalization following MT.
Publication bias across individual studies was graphically evaluated using a funnel plot,21 while funnel plot asymmetry was assessed using the Egger linear regression test with P<0.10 significance level. For all other outcomes of interest, we performed equivalent z test for each pooled estimate and a 2-tailed P level <0.05 was considered statistically significant.
All statistical analyses were carried out with Cochrane Collaboration’s Review Manager Software Package (RevMan 5.3) and the Comprehensive Meta-analysis version 2 software (Biostat, Englewood, NJ, https://www.meta-analysis.com).
Results
Study Selection and Study Characteristics
A systematic search of all the databases yielded 303 articles. After removing the duplicates, the titles and abstracts from the remaining 287 studies were screened and 43 potentially eligible studies for the meta-analysis were retained. After retrieving the full-text version of the aforementioned 43 studies, 18 studies22–39 were excluded (Table S2) due to nonavailability of intended data and use of endovascular reperfusion procedures other than MT (eg, Intraarterial thrombolysis). One multicenter study39 was excluded due to overlapping data with other previous studies that were already included in our meta-analysis. After careful evaluation and without disagreements among the 2 reviewers, 25 studies6–14,40–54 were included that met the study protocol’s inclusion criteria. The detailed flow chart of the current meta-analysis is presented in Figure S1.
The included 25 studies comprising 6474 patients with their baseline characteristics are summarized in Table S3. Two studies were post hoc analyses51,54 of randomized-controlled clinical trial including 278 patients with AIS and the remaining 23 were retrospective observational6–14,40–50,52,53,55). Thirteen studies were conducted in the United States, 4 in South Korea, 3 in France, 2 in Germany, and 1 each in China, Czech Republic, and Denmark.
Study Quality Assessment
We assessed the risk of bias among the included studies using Newcastle-Ottawa scale (Table S4) and Risk of Bias in Nonrandomized Studies of Exposures (Table S5). The evaluation of the risk of bias using the Newcastle-Ottawa scale disclosed that the risks of selection and comparability biases were considered low in all included studies. Outcome bias was quantified as moderate since the majority of included studies did not report data on patients lost to follow-up or outcome assessment. The overall score of Newcastle-Ottawa scale was 213/225 (94.6%), which is considered to represent an overall high quality. Notably, we also documented low and medium risk of bias in 19 and 6 studies respectively using the ROBINS-E approach.
Association Between BP Levels and Outcomes
Table 1, Table S6, and Table 2 present an overview on the overall unadjusted and adjusted analyses investigating the association of BP levels measured before, during, and after MT with various clinical outcomes, respectively.
Table 1.
Overview of Primary and Secondary Analyses of Pretreatment Mean BP Association With Various Outcomes
| Clinical Outcome | BP Level | Unadjusted Analyses | Adjusted Analyses* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | SMD (95% CI) | P Value | Heterogeneity (I2, P for Cochran Q) | Studies | OR (95% CI) | P Value | Heterogeneity (I2, P for Cochran Q) | ||
| FI | SBP | 7 | −0.26 (−0.34 to −0.17) | <0.001 | 0%, 0.88 | 4 | 0.86 (0.77 to 0.96) | 0.009 | 18%, 0.30 |
| DBP | 6 | −0.16 (−0.25 to −0.07) | <0.001 | 0%, 0.74 | – | – | – | – | |
| Mortality | SBP | 3 | 0.17 (0.04 to 0.29) | 0.008 | 0%, 0.42 | 2 | 1.22 (1.00 to 1.49) | 0.05 | 0%, 0.42 |
| DBP | 3 | 0.16 (0.03 to 0.28) | 0.01 | 0%, 0.64 | 2 | 1.19 (0.65 to 2.18) | 0.57 | 10%, 0.29 | |
| sICH | SBP | 3 | −0.11 (−0.31 to 0.10) | 0.30 | 19%, 0.29 | – | – | – | – |
| DBP | 3 | 0.08 (−0.09 to 0.25) | 0.37 | 0%, 0.44 | – | – | – | – | |
| Recanalization | SBP | 2 | −0.24 (−0.46 to −0.02) | 0.03 | 0%, 0.91 | – | – | – | – |
| DBP | 2 | −0.15 (−0.48 to 0.18) | 0.38 | 52%, 0.15 | – | – | – | – | |
BP indicates blood pressure; DBP, diastolic blood pressure; FI, functional independence (modified Rankin Scale score of 0–2); OR, odds ratio; SBP, systolic blood pressure; sICH, symptomatic intracranial hemorrhage; and SMD, standardized mean difference.
In the adjusted for potential confounders analyses all associations of SBP/DBP with the outcomes of interest are presented per 10 mm Hg SBP/DBP increment.
Table 2.
Overview of Primary and Secondary Analyses of Posttreatment BP Association With Various Outcomes
| Clinical Outcome | BP Level | Unadjusted Analyses | Adjusted analyses* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | SMD (95% CI) | P Value | Heterogeneity (I2, P for Cochran Q) | Studies | OR (95% CI) | P Value | Heterogeneity (I2, P for Cochran Q) | ||
| FI | Max SBP | 7 | −0.47 (−0.62 to −0.31) | <0.001 | 61%, 0.02 | 8 | 0.82 (0.73 to 0.93) | 0.001 | 72%, 0.0007 |
| Min SBP | 4 | 0.09 (−0.15 to 0.33) | 0.47 | 60%, 0.06 | 2 | 1.25 (0.79 to 1.99) | 0.34 | 64%, 0.10 | |
| Mean SBP | 9 | −0.27 (−0.42 to −0.12) | <0.001 | 65%, 0.004 | 6 | 0.80 (0.72 to 0.89) | <0.001 | 0%, 0.51 | |
| Max DBP | 5 | −0.24 (−0.39 to −0.09) | 0.002 | 51%, 0.08 | 5 | 0.83 (0.72 to 0.96) | 0.01 | 23%, 0.27 | |
| Min DBP | 3 | −0.04 (−0.21 to 0.12) | 0.61 | 0%, 0.48 | – | – | – | – | |
| Mean DBP | 8 | −0.08 (−0.20 to 0.05) | 0.23 | 43%, 0.09 | 3 | 0.80 (0.61 to 1.05) | 0.10 | 44%, 0.17 | |
| Mortality | Max SBP | 5 | 0.47 (0.12 to 0.82) | 0.009 | 75%, 0.003 | 5 | 1.19 (1.00 to 1.43) | 0.05 | 78%, 0.001 |
| Min SBP | 3 | −0.07 (−0.59 to 0.45) | 0.79 | 76%, 0.01 | – | – | – | – | |
| Mean SBP | 4 | 0.16 (−0.10 to 0.42) | 0.22 | 39%, 0.18 | 2 | 1.02 (1.00 to 1.04) | 0.06 | 0%, 0.35 | |
| Max DBP | 4 | 0.45 (0.22 to 0.69) | 0.0001 | 25%, 0.26 | 2 | 1.38 (1.09 to 1.76) | 0.008 | 3%, 0.31 | |
| Min DBP | 3 | −0.0 (−0.23 to 0.23) | 0.99 | 0%, 0.49 | – | – | – | – | |
| Mean DBP | 4 | 0.25 (0.01 to 0.49) | 0.04 | 31%, 0.23 | 2 | 1.27 (0.91 to 1.78) | 0.16 | 0%, 0.84 | |
| sICH | Max SBP | 4 | 0.17 (−0.16 to 0.50) | 0.32 | 50%, 0.11 | 7 | 1.02 (0.99 to 1.05) | 0.13 | 58%, 0.03 |
| Min SBP | 3 | 0.17 (−0.15 to 0.48) | 0.30 | 0%, 0.41 | 2 | 0.93 (0.78 to 1.12) | 0.47 | 54%, 0.14 | |
| Mean SBP | 4 | 0.16 (−0.15 to 0.46) | 0.32 | 43%, 0.16 | 4 | 1.00 (0.99 to 1.02) | 0.62 | 0%, 0.78 | |
| Max DBP | 4 | 0.18 (−0.03 to 0.38) | 0.10 | 0%, 0.73 | 2 | 1.65 (1.11 to 2.44) | 0.01 | 0%, 0.80 | |
| Min DBP | 3 | −0.08 (−0.39 to 0.24) | 0.63 | 0%, 0.74 | 2 | 0.98 (0.95 to 1.01) | 0.19 | 0%, 0.89 | |
| Mean DBP | 4 | 0.07 (−0.14 to 0.27) | 0.54 | 0%, 0.88 | 2 | 1.27 (0.91 to 1.78) | 0.16 | 0%, 0.84 | |
| mRS shift† | Max SBP | 3 | 1.25 (1.13 to 1.37) | <0.001 | 41%, 0.18 | 5 | 1.15 (1.09 to 1.21) | <0.001 | 76%, 0.002 |
| Mean SBP | 4 | 1.27 (1.15 to 1.41) | <0.001 | 27%, 0.25 | 5 | 1.28 (1.17 to 1.39) | <0.001 | 7%, 0.36 | |
| Max DBP | 3 | 1.29 (1.17 to 1.43) | <0.001 | 0%, 0.72 | 4 | 1.09 (0.98 to 1.21) | 0.13 | 0%, 0.47 | |
| Mean DBP | 4 | 1.23 (0.99 to 1.53) | 0.07 | 54%, 0.09 | 4 | 1.23 (0.99 to 1.53) | 0.07 | 54%, 0.09 | |
| Recanalization | Max SBP | 2 | −0.09 (−0.62 to 0.44) | 0.75 | 86%, 0.008 | – | – | – | – |
| Mean SBP | 2 | −0.37 (−0.96 to 0.22) | 0.22 | 75%, 0.04 | – | – | – | – | |
BP indicates blood pressure; DBP, diastolic blood pressure; FI, functional independence (modified Rankin Scale [mRS] score of 0–2); Max, maximum; Min, minimum; OR, odds ratio; SBP, systolic blood pressure; sICH, symptomatic intracranial hemorrhage; and SMD, standardized mean difference.
In the adjusted for potential confounders analyses, all associations of SBP/DBP with the outcomes of interest are presented per 10 mm Hg SBP/DBP increment.
Defined as 1-point increase in mRS scores in ordinal logistic regression analyses.
Unadjusted Analyses
BP Levels Before MT.
Lower pre-MT mean SBP (Figure S2A) and DBP levels (Figure S2B) were observed in patients with 3-month functional independence. Similarly, lower pre-MT mean SBP levels (Figure S3A) were documented in patients who achieved successful recanalization, whereas preMT mean DBP levels did not differ between patients with and without successful recanalization following MT (Figure S3B). Higher pretreatment mean SBP (Figure S4A) and DBP levels (Figure S4B) were documented in patients who were dead within 3 months from stroke onset. However, no differences in pretreatment mean SBP (Figure S5A) and DBP (Figure S5B) levels were noted in patients with and without sICH.
BP Levels During MT.
During MT procedures, lower levels of maximum SBP (Figure S6A) were observed in patients who achieved 3-month functional independence (Table S6). However, no difference was noted for minimum SBP (Figure S6B) and maximum (Figure S7A) or minimum (Figure S7B) DBP levels in patients with and without 3-month functional independence. No additional data were available for other outcomes.
BP Levels After MT.
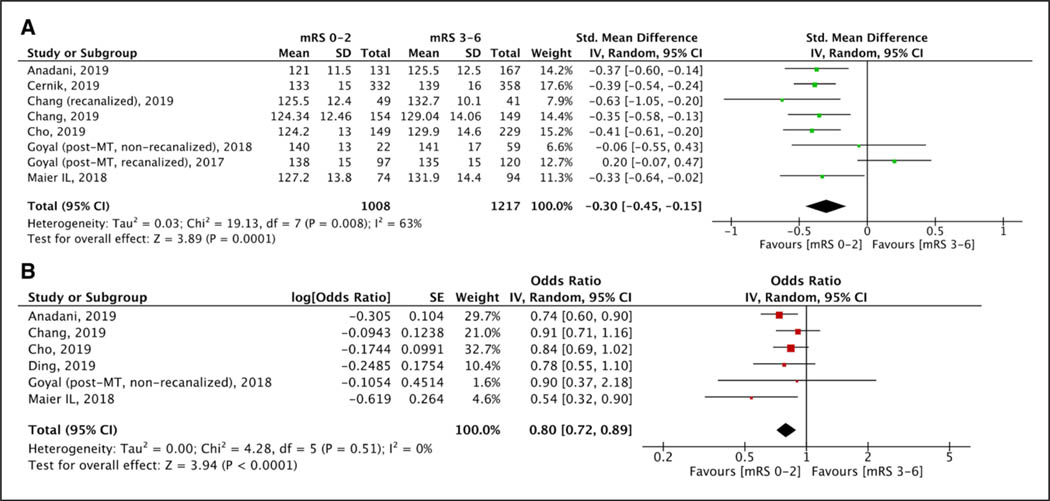
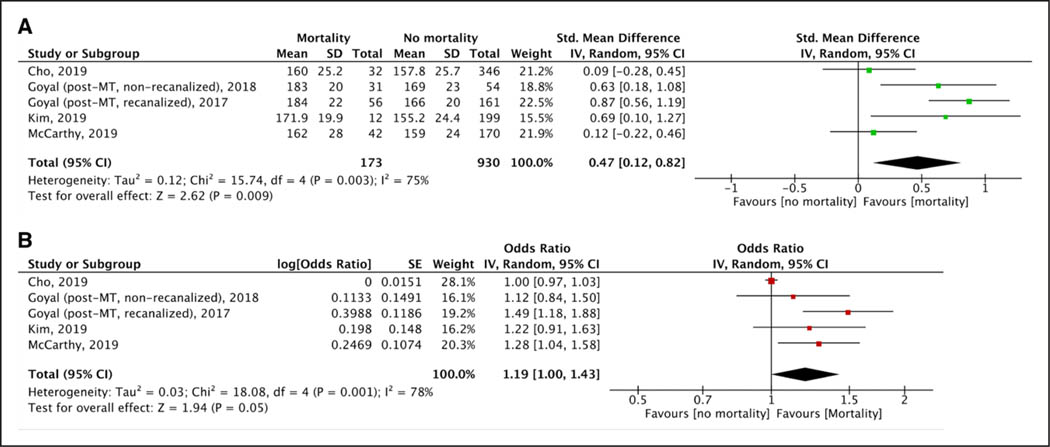
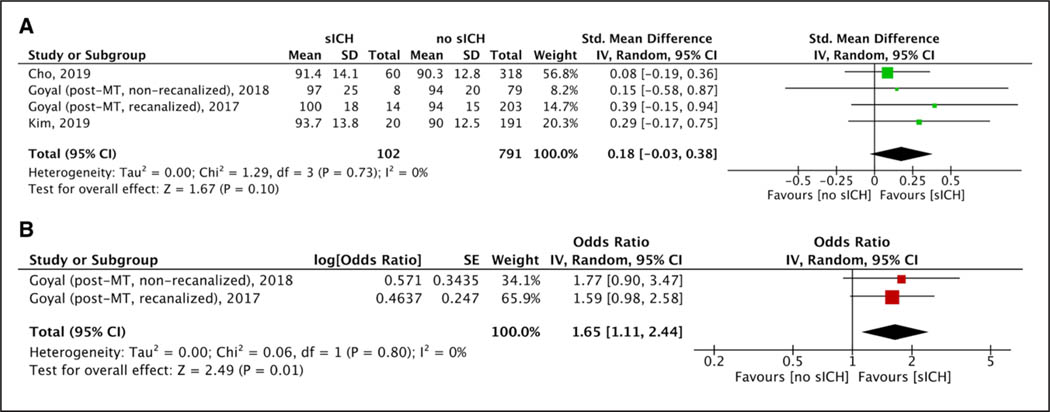
After MT procedures, lower levels of maximum SBP (Figure S8), mean SBP (Figure 1A), and maximum DBP (Figure S9A) were recorded among patients who achieved 3-month functional independence. However, mean DBP (Figure S9B) and minimum SBP (Figure S10A) or DBP (Figure S10B) levels did not differ between patients with and without functional independence. No difference was observed for both maximum (Figure S11A) and mean (Figure S11B) post-MT SBP levels in patients with and without successful recanalization. Higher maximum SBP (Figure 2A) and maximum (Figure S12A) or mean (Figure S12B) DBP levels were observed among patients who died within 3 months of stroke onset, whereas no such difference was observed for mean SBP (Figure S13), minimum SBP (Figure S14A), and minimum DBP (Figure S14B) levels. No differences in maximum SBP (Figure S15A), minimum SBP (Figure S15B), mean SBP (Figure S15C), maximum DBP (Figure 3A), minimum DBP (Figure S16A), or mean DBP (Figure S16B) levels were noted in patients with and without sICH.
Figure 1.
Forest plot presenting the (A) unadjusted and (B) adjusted for potential confounders associations of post-mechanical thrombectomy (MT) mean systolic blood pressure levels with 3-mo functional independence. mRS indicates modified Rankin Scale.
Figure 2.
Forest plot presenting the (A) unadjusted and (B) adjusted for potential confounders associations of post-mechanical thrombectomy (MT) maximum systolic blood pressure levels with 3-mo mortality. mRS indicates modified Rankin Scale.
Figure 3.
Forest plot presenting the (A) unadjusted and (B) adjusted for potential confounders associations of post-mechanical thrombectomy (MT) maximum diastolic blood pressure levels with symptomatic intracranial hemorrhage. mRS indicates modified Rankin Scale,
After MT elevated levels of maximum SBP (Figure S17A), mean SBP (Figure S17B) and maximum DBP (Figure S18A) were observed among patients with worsening 3-month mRS (1-point increase in mRS-scores in shift analyses), whereas mean DBP just failed to reach statistical significance (Figure S18B). We conducted additional analyses to assess the associations of BPV quantified by SD, CV, and SV in BP levels with various clinical outcomes. Although lower SV for both SBPBPV and DBP-BPV were observed in patients with 3-month functional independence, no differences were observed for post-MT SBP-BPV (quantified as SD or CV; Figure S19A) and DBP-BPV (quantified as SD or CV; Figure S19B) among patients with and without functional independence. Similarly, higher levels of SV for both SBP-BPV (Figure S20A) and DBP-BPV (Figure S20B) were observed in patients who died within 3 months, whereas no differences were observed for both SBP-BPV (Figure S21A) and DBP-BPV (Figure S21B) among patients with or without sICH.
Adjusted Analyses
We performed pairwise meta-analyses using the adjusted for potential confounders associations of BP levels before, during, and after MT with various outcomes. Adjusted variables from the individual studies are listed in Table S3. All ORadj on the association of SBP and DBP increments with the respective outcomes of interest were rescaled and presented as 10 mm Hg BP increments.
BP Levels Before MT.
In adjusted analyses, increasing mean SBP levels before MT (Table 1) were associated with lower odds of 3-month functional independence (4 studies; ORadj, 0.86 [95% CI, 0.77–0.96], I2=18%, P value for Cochran Q test: 0.30; Figure S22). Elevated pre-MT mean SBP levels were associated with higher odds of 3-month mortality (2 studies; ORadj, 1.22 [95% CI, 1.00–1.49], I2=0%, P value for Cochran Q test: 0.42; Figure S23A); however, there was no independent association between pre-MT mean DBP levels and 3-month mortality (2 studies; ORadj, 1.19 [95% CI, 0.65–2.18], I2=10%, P value for Cochran Q test: 0.29; Figure S23B).
BP Levels During MT.
During MT, increasing levels of maximum SBP were independently associated with lower odds of 3-month functional independence (2 studies; ORadj, 0.93 [95% CI, 0.90–0.96], I2=0%, P value for Cochran Q test: 0.78; Figure S24; Table S6). No additional data were available for other outcomes.
BP Levels After MT.
After MT, increasing mean SBP levels (6 studies; ORadj, 0.80 [95% CI, 0.72–0.89], I2=0%, P value for Cochran Q test: 0.51; Figure 1B) and maximum DBP levels (5 studies; ORadj, 0.83 [95% CI, 0.72–0.96]; I2=23%, P value for Cochran Q test: 0.27; Figure S25A; Table 2) were independently associated with reduced likelihood of 3-month functional independence without significant evidence of heterogeneity across included studies. Increasing maximum SBP levels was also associated (8 studies; ORadj, 0.82 [95% CI, 0.73–0.93]; Figure S25B) with lower odds of 3-month functional independence, however, with considerable heterogeneity (I2=72%; P value for Cochran Q test: 0.0007). There was no independent association between minimum SBP (2 studies; ORadj, 1.25 [95% CI, 0.79–1.99]; I2=64%, P value for Cochran Q test: 0.10; Figure S26A) and mean DBP (3 studies; ORadj, 0.80 [95% CI, 0.61–1.05]; I2=44%, P value for Cochran Q test: 0.17; Figure S26B) and 3-month functional independence.
Higher maximum SBP (5 studies; ORadj, 1.19 [95% CI, 1.00–1.43], I2=78%, P value for Cochran Q test: 0.001; Figure 2B) and DBP (2 studies; ORadj, 1.38 [95% CI, 1.09–1.76], I2=3%, P value for Cochran Q test: 0.31; Figure S27A) levels were independently associated with 3-month mortality, whereas increasing levels of mean SBP levels suggested a trend toward increased 3-month mortality (2 studies; ORadj, 1.02 [95% CI, 1.00–1.04], I2=0%, P value for Cochran Q test: 0.35; Figure S27B). Higher maximum DBP levels were independently associated with sICH (2 studies; ORadj, 1.65 [95% CI, 1.11–2.44]; Figure 3B) without any evidence of heterogeneity across included studies (I2=0%; P value for Cochran Q test: 0.80). No association was noted for maximum SBP (7 studies; ORadj, 1.02 [95% CI, 0.99–1.05]; I2=58%, P value for Cochran Q test: 0.03; Figure S28A), minimum SBP (2 studies; ORadj, 0.93 [95% CI, 0.78–1.12]; I2=54%, P value for Cochran Q test: 0.14; Figure S28B), mean SBP (4 studies; ORadj, 1.00 [95% CI, 0.99–1.02]; I2=0%, P value for Cochran Q test: 0.78; Figure S28C), minimum DBP (2 studies; ORadj, 0.98 [95% CI, 0.95–1.01]; I2=0%, P value for Cochran Q test: 0.89; Figure S29A), and mean DBP (2 studies; ORadj, 1.27 [95% CI, 0.91–1.78]; I2=0%, P value for Cochran Q test: 0.84; Figure S29B) levels with the likelihood of sICH in adjusted analyses.
Additionally, elevated levels of maximum (5 studies; cOR-adj, 1.15 [95% CI, 1.09–1.21]; I2=76%, P value for Cochran Q test: 0.002; Figure S30A) and mean (5 studies; cORadj, 1.28 [95% CI, 1.17–1.39]; I2=7%, P value for Cochran Q test: 0.36; Figure S30B) SBP levels were independently associated with worsening 3-month mRS (1-point increase in mRS scores in shift analyses). No association was noted for maximum (4 studies; cORadj, 1.09 [95% CI, 0.98–1.21]; I2=0%, P value for Cochran Q test: 0.47; Figure S31A) or mean (4 studies; cOR-, 1.23 [95% CI, 0.99–1.53]; I2=54%, P value for Cochran Q adj test: 0.09; Figure S31B) DBP levels in ordinal analysis.
Subgroup Analyses
We performed subgroup analyses among patients who achieved successful recanalization with MT and evaluated the association of post-MT BP with clinical outcomes. We observed that lower levels of maximum SBP (Figure S32A) and DBP (Figure S32B) were observed in patients with 3-month functional independence. Additionally, elevated levels of mean SBP (Figure S34A) were observed in patients with sICH. No difference was noted in mean SBP (Figure S33A) and DBP (Figure S33B) levels for patients with and without 3-month functional independence. Additionally, elevated levels of mean SBP (Figure S34A) were observed in patients with sICH. No differences were documented for mean DBP (Figure S34B), maximum SBP (Figure S35A), or maximum DBP (Figure S35B) levels among patients with and without sICH.
In adjusted analyses, elevated levels of mean SBP (2 studies; ORadj, 0.81 [95% CI, 0.71–0.93]; I2=0%, P value for Cochran Q test: 0.95; Figure S36A) and maximum DBP (3 studies; ORadj, 0.87 [95% CI, 0.77–0.99]; I2=0%, P value for Cochran Q test: 0.61; Figure S36B) were independently associated with reduced odds of 3-month functional independence, whereas no such association was noted for maximum SBP (3 studies; ORadj, 0.87 [95% CI, 0.73–1.03]; I2=66%, P value for Cochran Q test: 0.05; Figure S37). No association was also detected for maximum SBP and 3-month mortality (3 studies; ORadj, 1.04 [95% CI, 0.97–1.12]; I2=82%, P value for Cochran Q test: 0.004; Figure S38). Similarly, no association was documented for maximum SBP (4 studies; ORadj, 1.01 [95% CI, 0.97–1.06]; I2=58%, P value for Cochran Q test: 0.07; Figure S39A) or mean SBP (2 studies; ORadj, 1.01 [95% CI, 0.99–1.03]; I2=0%, P value for Cochran Q test: 0.66; Figure S39B) with sICH in adjusted analyses.
Publication Bias Assessment
We inspected funnel plot symmetry and Egger statistical test for outcomes involving ≥10 studies. No evidence of asymmetry or publication bias was observed in studies reporting unadjusted associations between pre-MT mean BP levels (P=0.572; Figure S40) or post-MT maximum BP levels (P=0.982; Figure S41) and 3-month functional independence. However, we detected evidence of publication bias in the adjusted associations between post-MT maximum BP parameters (P=0.034; Figure S42) and 3-month functional independence.
Discussion
Our systematic review and meta-analysis showed that increased BP levels before, during, and after MT are associated with adverse clinical outcomes including sICH, lack of successful recanalization, 3-month mortality, and 3-month functional dependence. These associations were consistent across adjusted and unadjusted analyses without substantial heterogeneity in the majority of independent associations. They also persisted in the subgroup of patients who achieved successful recanalization following MT.
Despite successful recanalization in ≈70% to 90% of patients with endovascular thrombectomy, approximately half of the recanalized patients suffer poor functional outcomes or mortality at 3 months.3,56 Previous studies have suggested that elevated baseline BP levels are associated with higher clot burden, lower likelihood of recanalization and good functional outcomes, increased infarct volumes and early ischemic stroke recurrence.12,57,58 Modifiable factors including BP levels before and after endovascular procedures are potential targets to improve clinical outcomes in these patients. The findings of the present meta-analysis argue against elevated BP levels before and after MT. These observations are in line with a recent meta-analysis by our international collaborative group reporting that elevated pre and post-MT BP levels are associated with adverse functional outcomes and increased mortality in AIS patients treated with intravenous thrombolysis.4
Additionally, our results suggest reduced rates of recanalization after MT procedures among patients with elevated BP levels before and after the procedure. This observation can be attributed to the following plausible explanations (1) acute elevation of admission BP levels, among patients with uncontrolled hypertension, may adversely affect collateral flow,59 and this may impart a stronger hemodynamic force resulting in a heavier mechanical clot impaction and impairment of mechanical clot retrieval36 and (2) elevated admission SBP isassociated with poorer collateral flow and thereby results in lower rates of recanalization and functional improvement.12 Consequently, the association between increased pretreatment BP levels and lower odds of recanalization after endovascular reperfusion could in part explain worse functional outcomes in patients with AIS.36 This hypothesis is further supported by the present meta-analysis, as patients with AIS with successful recanalization following MT had lower pretreatment BP levels compared with patients with persistent LVO. Similar findings have been reported in patients with IVT-treated AIS.4 Elevated BP levels may lead to increased baseline thrombus burden and impaired endogenous capacity for fibrinolysis.60 These findings argue against the hypothesis that higher sICH rates may represent the predominant causative link between increasing SBP levels and poor clinical outcomes.
Various hemodynamic factors including ischemic penumbra, collateral status, and clot burden play an important role in the optimization of BP in patients with AIS due to LVO.61 It is well established that the ischemic penumbra surrounding the infarct core in patients with LVO has impaired cerebral autoregulation and is sensitive to alterations in systemic blood pressure and reperfusion injury.7,61 Wide BP fluctuations, especially drop in BP levels during or within the first 24 hours after MT may result in penumbral tissue loss, exacerbation of reperfusion injury, and worse functional outcomes.62,63 Additionally, systemically elevated BP levels and increased BPV likely predispose to adverse outcomes in patients with AIS treated with MT.64 Our study results lend support to the former consideration that wide fluctuations in BP after MT may induce infarct expansion or reperfusion injury leading to lower odds of functional independence and sICH.13,61 We documented an independent association of elevated maximum DBP levels following MT with sICH only in the adjusted analysis. The other associations between BP levels and sICH failed to achieve significance in the adjusted analyses due to limited number of studies with moderate sample. Nevertheless, the direction of all associations suggested the potential detrimental effect of increasing BP levels before or after MT on the risk of sICH. Additionally, we observed greater association of clinical outcomes with post-MT SBP levels and only with maximum DBP levels, likely due to small number of studies providing available data.
The findings of this meta-analysis support the recent American Heart Association/American Stroke Association guidelines for BP control in patients with AIS treated with IVT and MT.5 However, these recommendations also indicate that an optimal BP target which simultaneously avoids the risk of sICH and impairment of cerebral perfusion remains unknown (Class I; Level of Evidence B). The second arm of ENCHANTED trial (Enhanced Control of Hypertension and Thrombolysis Stroke Study)65 provided randomized evidence that BP reduction in patients with AIS treated with IVT was safe but did not improve clinical outcomes despite lowering the incidence of any intracranial hemorrhage. The lack of an association between BP lowering in patients with AIS treated with systemic reperfusion therapies and improved clinical outcome may be related to the modest BP reduction (5 mm Hg) that was achieved in the active group of ENCHANTED in comparison to the control group. Although our findings guide clinicians to avoid excessive BP elevations before, during, and after MT, these findings should be cautiously interpreted as extreme BP lowering in patients with LVO before successful revascularization with MT may exacerbate cerebral hypoperfusion and predispose to ischemic injury. Also, extreme BP reductions in patients with nonrecanalized LVO after MT with potentially viable penumbra may expand the infarct core and lead to worse outcomes. Consequently, acute decision-making warrants caution as the target SBP and DBP levels need to be individualized weighing the benefits and risks to the patients, including the extent of infarct core on postprocedural imaging, degree of recanalization, collateral blood flow, type of anesthesia, and prior history of hypertension.
Certain limitations of the present report need to be acknowledged. First, our analyses were primarily based on observational studies that were not designed in a randomized fashion to evaluate the intended associations. Thus, despite the use of adjusted outcomes for potential inherent biases related to the design of the included studies, unmeasured confounders (methodology and frequency of serial BP measurements, type and dose of antihypertensive medications used to treat excessive BP levels) cannot be eliminated. We decided a priori to exclude the studies that provided median (instead of mean) SBP or DBP levels, as well as those that reported mean arterial pressure levels or % drop in BP values whereas focused our analyses on maximum, minimum, and mean BP values. This decision was based on the fact that the majority of previous studies reported independent associations between maximum or mean SBP or DBP levels and outcomes, while mean arterial pressure goals are not included in international recommendations regarding optimal blood pressure management in patients with AIS treated with systemic or endovascular reperfusion therapies. Additionally, due to limited number of studies providing association data during MT, we failed to evaluate intraprocedural BP reduction and outcomes in patients with LVO treated with MT, and these methodological shortcomings argue against the aggressive BP reduction during the procedure. Second, although we performed an adjusted pairwise meta-analysis to account for the potential confounders available from the individual studies, the adjusted variables varied among included studies in the present meta-analysis with the exception of age and admission NIHSS-score (Table S3). Different BP metrics, time points, and techniques of BP measurements across the included studies represent another source of heterogeneity that cannot be assessed using I2 and Cochran Q statistics, and this potential source of heterogeneity should be taken into account when interpreting our study findings. Third, few outcomes in adjusted analyses had substantial heterogeneity and these did not reach statistical significance, likely due to the small number of studies reporting the associations on the adjusted data for different confounding variables. Fourth, we performed no correction for multiple comparisons despite conducting a vast number of different analyses. This decision was made a priori during the preparation of our manuscript protocol. Nevertheless, it should be noted that many of the associations were highly significant (P<0.001) and reproducible in both unadjusted and adjusted analyses (Tables 1 and 2). Fifth, due to the way clinical data was presented among the included studies, it should be acknowledged that we were unable to test the cause-effect association between increased BP levels and worse clinical outcomes of patients with LVO treated with MT. We were also unable to evaluate the hypothesis of a U-shaped relationship between BP parameters and clinical outcomes of patients with AIS treated with MT. Additionally, the effect of bridging therapy (IVT followed by MT) on the association of BP levels with outcomes could not be performed. These relationships could be evaluated in the settings of an individual patient data meta-analysis pooling large datasets from numerous MT registries. Sixth, the lack of independent association of SBP levels with sICH may be related to the moderate sample of limited number of studies with available data. Last, due to limited available data, we were not able to test the impact of BP levels with admission BP-lowering medications, collateral status, final infarct volumes, choice of anesthesia that could potentially moderate the association of BP parameters with various clinical outcomes.66,67
In conclusion, the present systematic review and meta-analysis provide preliminary evidence that elevated BP levels before, during, and after MT are likely associated with detrimental effect among patients with LVO treated with endovascular reperfusion therapies because they seem to increase the likelihood of mortality and functional dependence. These associations persisted even after adjustment for potential confounders and subgroup analyses of patients achieving successful recanalization during MT. Individual patient-data meta-analysis and randomized-controlled clinical trials are needed to assess the potential beneficial effect of moderate BP control among patients with LVO treated with MT.
Perspectives
This study evaluated the association of BP levels before, during, and after mechanical thrombectomy among patients with AIS with LVO. These findings further our understanding of the interplay between BP levels and clinical outcomes among patients with AIS with LVO undergoing mechanical thrombectomy.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first systematic review and meta-analysis involving 25 studies to evaluate the association of blood pressure (BP) on clinical outcomes among acute ischemic stroke patients with large vessel occlusion treated with mechanical thrombectomy (MT).
What Is Relevant?
Higher SBP levels were noted among patients with functional dependenceand who died within 3 months.
After adjustment of potential confounders, association persisted for elevated SBP levels before and after MT with lower odds of functional independence.Similarly, elevated SBP and DBP levels after MT were associated with symptomatic intracranial hemorrhage and mortality.
Additional subgroup analyses among patients who achieved successful recanalization with MT demonstrated worse clinical outcomes with elevated BP levels.
Summary
Even after adjustment of potential confounders, elevated BP levels before and after MT are associated with worse clinical outcomes among acute ischemic stroke patients with large vessel occlusion.
Acknowledgments
Sources of Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Disclosures
E.A. Mistry reports grant funding from Society of Vascular and Interventional Neurology, University of Cincinnati Gardner Neuroscience Institute, Vanderbilt Faculty Research Scholars Program, and NIH/NINDS (K23NS113858). A.M. Spiotta reports research support from Penumbra; and consulting revenue with Penumbra, Stryker, and Cerenovus. E.C. Sandset reports speaker honoraria from Bayer and Novartis outside the submitted work. C. Krogias has received travel funding and/or speaker honoraria from Bayer, Boehrigner Ingelheim, and Daiichi Sankyo, none related to this manuscript. L. Tönges has received travel funding and/or speaker honoraria from Abbvie, Bayer, Bial, Desitin, GE, UCB, Zambon and consulted for Abbvie, Bayer, Bial, Desitin, UCB, Zambon in the last 3 years. M. Goyal reports grants from Stryker, personal fees from Stryker, personal fees from microvention, personal fees from Medtronic, personal fees from Mentice, and personal fees from Ge Healthcare outside the submitted work. A. Arthur reports consulting revenue from Balt, Johnson and Johnson, Medtronic, Microvention, Penumbra, Scientia, Siemens, and Stryker; research support from Cerenovus, Medtronic, Microvention, Penumbra, Siemens, and Stryker, and ownership in Bendit, Cerebrotech, Endostream, Magneto, Marblehead, Neurogami, Serenity, Synchron, Triad Medical, and Vascular Simulations. G. Tsivgoulis has received grant from Medtronic, Allergan, and Genesis. He additionally reports receiving personal fee from Boehringer Ingelheim, Bayer, and Pfizer. The other authors report no conflicts.
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.14230.
Contributor Information
Konark Malhotra, Department of Neurology, Allegheny Health Network, Pittsburgh, PA.
Nitin Goyal, Department of Neurology, University of Tennessee, Memphis.
Aristeidis H. Katsanos, Department of Neurology, McMaster University/Population Health Research Institute, Hamilton, Canada
Angeliki Filippatou, Second Department of Neurology, “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece.
Eva A. Mistry, Department of Neurology, Vanderbilt University, Nashville, TN
Pooja Khatri, Department of Neurology, University of Cincinnati, OH.
Mohammad Anadani, Department of Neurology, Washington University School of Medicine, St Louis, MO; Department of Neurosurgery, Medical University of South Carolina, Charleston.
Alejandro M. Spiotta, Department of Neurosurgery, Medical University of South Carolina, Charleston
Else Charlotte Sandset, Department of Neurology, Stroke Unit, Oslo University Hospital, Norway; The Norwegian Air Ambulance Foundation, Oslo, Norway.
Amrou Sarraj, Department of Neurology, UT Houston, TX.
Georgios Magoufis, Stroke Unit, Metropolitan Hospital, Piraeus, Greece.
Christos Krogias, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Lars Tönges, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Apostolos Safouris, Stroke Unit, Metropolitan Hospital, Piraeus, Greece.
Lucas Elijovich, Department of Neurosurgery, University of Tennessee/Semmes-Murphey Clinic, Memphis.
Mayank Goyal, Departments of Radiology and Clinical Neurosciences, University of Calgary, AB, Canada.
Adam Arthur, Department of Neurosurgery, University of Tennessee/Semmes-Murphey Clinic, Memphis.
Andrei V. Alexandrov, Department of Neurology, University of Tennessee, Memphis
Georgios Tsivgoulis, Department of Neurology, University of Tennessee, Memphis; Second Department of Neurology, “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece.
References
- 1.Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8:651. doi: 10.3389/fneur.2017.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakomkin N, Dhamoon M, Carroll K, Singh IP, Tuhrim S, Lee J, Fifi JT, Mocco J. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J Neurointerv Surg. 2019;11:241–245. doi: 10.1136/neurintsurg-2018-014239 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4.Malhotra K, Ahmed N, Filippatou A, Katsanos AH, Goyal N, Tsioufis K, Manios E, Pikilidou M, Schellinger PD, Alexandrov AW, et al. Association of elevated blood pressure levels with outcomes in acute ischemic stroke patients treated with intravenous thrombolysis: a systematic review and meta-analysis. J Stroke. 2019;21:78–90. doi: 10.5853/jos.2018.02369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. ; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/S00TR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 6.Ding X, Xu C, Zhong W, Gong X, Zhou Y, Chen Z, Lou M. Association of maximal systolic blood pressure with poor outcome in patients with hyperattenuated lesions on immediate ncct after mechanical thrombectomy. J Neurointerv Surg. 2019;pii:neurintsurg-2019–014846. doi: 10.1136/neurintsurg-2019-014846 [DOI] [PubMed] [Google Scholar]
- 7.Mistry EA, Mistry AM, Nakawah MO, Khattar NK, Fortuny EM, Cruz AS, Froehler MT, Chitale RV, James RF, Fusco MR, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc. 2017;6:e006167. doi: 10.1161/JAHA.117.006167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal N, Tsivgoulis G, Pandhi A, Chang JJ, Dillard K, Ishfaq MF, Nearing K, Choudhri AF, Hoit D, Alexandrov AW, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. 2017;89:540–547. doi: 10.1212/WNL.0000000000004184 [DOI] [PubMed] [Google Scholar]
- 9.Maier B, Gory B, Taylor G, Labreuche J, Blanc R, Obadia M, Abrivard M, Smajda S, Desilles JP, Redjem H, et al. Mortality and disability according to baseline blood pressure in acute ischemic stroke patients treated by thrombectomy: a collaborative pooled analysis. J Am Heart Assoc. 2017;6:e006484. doi: 10.1161/JAHA.117.006484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho BH, Kim JT, Lee JS, Park MS, Kang KW, Choi KH, Lee SH, Choi SM, Kim BC, Kim MK, et al. Associations of various blood pressure parameters with functional outcomes after endovascular thrombectomy in acute ischaemic stroke. Eur J Neurol. 2019;26:1019–1027. doi: 10.1111/ene.13951 [DOI] [PubMed] [Google Scholar]
- 11.Chang JY, Jeon SB, Lee JH, Kwon OK, Han MK. The relationship between blood pressure variability, recanalization degree, and clinical outcome in large vessel occlusive stroke after an intra-arterial thrombectomy. Cerebrovasc Dis. 2018;46:279–286. doi: 10.1159/000495300 [DOI] [PubMed] [Google Scholar]
- 12.Goyal N, Tsivgoulis G, Iftikhar S, Khorchid Y, Fawad Ishfaq M, Doss VT, Zand R, Chang J, Alsherbini K, Choudhri A, et al. Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. J Neurointerv Surg. 2017;9:451–454. doi: 10.1136/neurintsurg-2016-012386 [DOI] [PubMed] [Google Scholar]
- 13.Goyal N, Tsivgoulis G, Pandhi A, Dillard K, Alsbrook D, Chang JJ, Krishnaiah B, Nickele C, Hoit D, Alsherbini K, et al. Blood pressure levels post mechanical thrombectomy and outcomes in non-recanalized large vessel occlusion patients. J Neurointerv Surg. 2018;10:925–931. doi: 10.1136/neurintsurg-2017-013581 [DOI] [PubMed] [Google Scholar]
- 14.Maïer B, Turc G, Taylor G, Blanc R, Obadia M, Smajda S, Desilles JP, Redjem H, Ciccio G, Boisseau W, et al. ; Endovascular Treatment in Ischemic Stroke (ETIS) Investigators. Prognostic significance of pulse pressure variability during mechanical thrombectomy in acute ischemic stroke patients. J Am Heart Assoc. 2018;7:e009378. doi: 10.1161/JAHA.118.009378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 17.Transparency and openness promotion (top) guidelines for authors pub-lishing in an american heart association journal. [Google Scholar]
- 18.Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, Guyatt G, Hooijmans C, Langendam M, Mandrioli D, et al. GRADE: assessing the quality of evidence in environmental and occupational health. Environ Int. 2016;92–93:611–616. doi: 10.1016/j.envint.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hoboken: Taylor and Francis; 1988:22. [Google Scholar]
- 21.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 22.Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Sundeman H, Reinsfelt B, Ricksten SE. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46:2678–2680. doi: 10.1161/STROKEAHA.115.009808 [DOI] [PubMed] [Google Scholar]
- 23.Mulder MJHL, Ergezen S, Lingsma HF, Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lycklama À, Nijeholt G, Emmer BJ, van der Worp HB, et al. ; Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands (MR CLEAN) Investigators. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke. 2017;48:1869–1876. doi: 10.1161/STROKEAHA.116.016225 [DOI] [PubMed] [Google Scholar]
- 24.Mundiyanapurath S, Stehr A, Wolf M, Kieser M, Möhlenbruch M, Bendszus M, Hacke W, Bösel J. Pulmonary and circulatory parameter guided anesthesia in patients with ischemic stroke undergoing endovascular recanalization. J Neurointerv Surg. 2016;8:335–341. doi: 10.1136/neurintsurg-2014-011523 [DOI] [PubMed] [Google Scholar]
- 25.Pikija S, Trkulja V, Ramesmayer C, Mutzenbach JS, Killer-Oberpfalzer M, Hecker C, Bubel N, Füssel MU, Sellner J. Higher blood pressure during endovascular thrombectomy in anterior circulation stroke is associated with better outcomes. J Stroke. 2018;20:373–384. doi: 10.5853/jos.2018.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treurniet KM, Berkhemer OA, Immink RV, Lingsma HF, Ward-van der Stam VMC, Hollmann MW, Vuyk J, van Zwam WH, van der Lugt A, van Oostenbrugge RJ, et al. ; MR CLEAN investigators. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg. 2018;10:107–111. doi: 10.1136/neurintsurg-2017-012988 [DOI] [PubMed] [Google Scholar]
- 27.Eker OF, Saver JL, Goyal M, Jahan R, Levy EI, Nogueira RG, Yavagal DR, Bonafé A; SWIFT PRIME investigators. Impact of anesthetic management on safety and outcomes following mechanical thrombectomy for ischemic stroke in SWIFT PRIME cohort. Front Neurol. 2018;9:702. doi: 10.3389/fneur.2018.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcaraz G, Chui J, Schaafsma J, Manninen P, Porta-Sánchez A, Pereira VM, Venkatraghavan L. Hemodynamic management of patients during endovascular treatment of acute ischemic stroke under conscious sedation: a Retrospective Cohort Study. J Neurosurg Anesthesiol. 2019;31:299–305. doi: 10.1097/ANA.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 29.Zhang YB, Su YY, He YB, Liu YF, Liu G, Fan LL. Early neurological deterioration after recanalization treatment in patients with acute ischemic stroke: a Retrospective Study. Chin Med J (Engl). 2018;131:137–143. doi: 10.4103/0366-6999.222343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi CE, Brambrink AM, Aziz MF, Macri E, Raines J, Multani-Kohol A, Hinson HE, Lutsep HL, Clark WM, Fields JD. Association of intraprocedural blood pressure and end tidal carbon dioxide with outcome after acute stroke intervention. Neurocrit Care. 2014;20:202–208. doi: 10.1007/s12028-013-9921-3 [DOI] [PubMed] [Google Scholar]
- 31.Sivasankar C, Stiefel M, Miano TA, Kositratna G, Yandrawatthana S, Hurst R, Kofke WA. Anesthetic variation and potential impact of anesthetics used during endovascular management of acute ischemic stroke. J Neurointerv Surg. 2016;8:1101–1106. doi: 10.1136/neurintsurg-2015-011998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Athiraman U, Sultan-Qurraie A, Nair B, Tirschwell DL, Ghodke B, Havenon AD, Hallam DK, Kim LJ, Becker KJ, Sharma D. Endovascular treatment of acute ischemic stroke under general anesthesia: predictors of good outcome. J Neurosurg Anesthesiol. 2018;30:223–230. doi: 10.1097/ANA.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 33.Whalin MK, Halenda KM, Haussen DC, Rebello LC, Frankel MR, Gershon RY, Nogueira RG. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol. 2017;38:294–298. doi: 10.3174/ajnr.A4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jumaa MA, Zhang F, Ruiz-Ares G, Gelzinis T, Malik AM, Aleu A, Oakley JI, Jankowitz B, Lin R, Reddy V, et al. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41:1180–1184. doi: 10.1161/STROKEAHA.109.574194 [DOI] [PubMed] [Google Scholar]
- 35.Mattle HP, Kappeler L, Arnold M, Fischer U, Nedeltchev K, Remonda L, Jakob SM, Schroth G. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke. 2005;36:264–268. doi: 10.1161/01.STR.0000153052.59113.89 [DOI] [PubMed] [Google Scholar]
- 36.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS; MERCI; Multi MERCI Writing Committee. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431 [DOI] [PubMed] [Google Scholar]
- 37.Sweid A, Atallah E, Saad H, Bekelis K, Chalouhi N, Dang S, Li J, Kumar A, Turpin J, Barsoom R, et al. Correlation between pre-admission blood pressure and outcome in a large telestroke cohort. J Clin Neurosci. 2019;62:33–37. doi: 10.1016/j.jocn.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 38.de Havenon A, Bennett A, Stoddard GJ, Smith G, Wang H, Wold J, Chung L, Tirschwell DL, Majersik JJ. Increased blood pressure variability is associated with worse neurologic outcome in acute anterior circulation ischemic stroke. Stroke Res Treat. 2016;2016:7670161. doi: 10.1155/2016/7670161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anadani M, Orabi MY, Alawieh A, Goyal N, Alexandrov AV, Petersen N, Kodali S, Maier IL, Psychogios MN, Swisher CB, et al. Blood pressure and outcome after mechanical thrombectomy with successful revascularization. Stroke. 2019;50:2448–2454. doi: 10.1161/STROKEAHA.118.024687 [DOI] [PubMed] [Google Scholar]
- 40.Abou-Chebl A, Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Linfante I, et al. North American SOLITAIRE Stent-Retriever Acute Stroke Registry: choice of anesthesia and outcomes. Stroke. 2014;45:1396–1401. doi: 10.1161/STROKEAHA.113.003698 [DOI] [PubMed] [Google Scholar]
- 41.Anadani M, Orabi Y, Alawieh A, Chatterjee A, Lena J, Al Kasab S, Spiotta AM. Blood pressure and outcome post mechanical thrombectomy. J Clin Neurosci. 2019;62:94–99. doi: 10.1016/j.jocn.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 42.Bennett AE, Wilder MJ, McNally JS, Wold JJ, Stoddard GJ, Majersik JJ, Ansari S, de Havenon A. Increased blood pressure variability after endovascular thrombectomy for acute stroke is associated with worse clinical outcome. J Neurointerv Surg. 2018;10:823–827. doi: 10.1136/neurintsurg-2017-013473 [DOI] [PubMed] [Google Scholar]
- 43.Cernik D, Sanak D, Divisova P, Kocher M, Cihlar F, Zapletalova J, Veverka T, Prcuchova A, Ospalik D, Cerna M, et al. Impact of blood pressure levels within first 24 hours after mechanical thrombectomy on clinical outcome in acute ischemic stroke patients. J Neurointerv Surg. 2019;11:735–739. doi: 10.1136/neurintsurg-2018-014548 [DOI] [PubMed] [Google Scholar]
- 44.Chang JY, Jeon SB, Jung C, Gwak DS, Han MK. Postreperfusion blood pressure variability after endovascular thrombectomy affects outcomes in acute ischemic stroke patients with poor collateral circulation. Front Neurol. 2019;10:346. doi: 10.3389/fneur.2019.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagani M, Brinjikji W, Rabinstein AA, Pasternak JJ, Kallmes DF. Hemodynamics during anesthesia for intra-arterial therapy of acute ischemic stroke. J Neurointerv Surg. 2016;8:883–888. doi: 10.1136/neurintsurg-2015-011867 [DOI] [PubMed] [Google Scholar]
- 46.John S, Hazaa W, Uchino K, Hussain MS. Timeline of blood pressure changes after intra-arterial therapy for acute ischemic stroke based on recanalization status. J Neurointerv Surg. 2017;9:455–458. doi: 10.1136/neurintsurg-2016-012369 [DOI] [PubMed] [Google Scholar]
- 47.Kim TJ, Park HK, Kim JM, Lee JS, Park SH, Jeong HB, Park KY, Rha JH, Yoon BW, Ko SB. Blood pressure variability and hemorrhagic transformation in patients with successful recanalization after endovascular recanalization therapy: a retrospective observational study. Ann Neurol. 2019;85:574–581. doi: 10.1002/ana.25434 [DOI] [PubMed] [Google Scholar]
- 48.Maier IL, Tsogkas I, Behme D, Bähr M, Knauth M, Psychogios MN, Liman J. High systolic blood pressure after successful endovascular treatment affects early functional outcome in acute ischemic stroke. Cerebrovasc Dis. 2018;45:18–25. doi: 10.1159/000484720 [DOI] [PubMed] [Google Scholar]
- 49.Panni P, Gory B, Xie Y, Consoli A, Desilles JP, Mazighi M, Labreuche J, Piotin M, Turjman F, Eker OF, et al. ; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators. Acute stroke with large ischemic core treated by thrombectomy. Stroke. 2019;50:1164–1171. doi: 10.1161/STROKEAHA.118.024295 [DOI] [PubMed] [Google Scholar]
- 50.Petersen NH, Ortega-Gutierrez S, Wang A, Lopez GV, Strander S, Kodali S, Silverman A, Zheng-Lin B, Dandapat S, Sansing LH, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019;50:1797–1804. doi: 10.1161/STROKEAHA.118.024286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen M, Espelund US, Juul N, Yoo AJ, Sørensen LH, Sørensen KE, Johnsen SP, Andersen G, Simonsen CZ. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. Br J Anaesth. 2018;120:1287–1294. doi: 10.1016/j.bja.2018.01.039 [DOI] [PubMed] [Google Scholar]
- 52.Whalin MK, Lopian S, Wyatt K, Sun CH, Nogueira RG, Glenn BA, Gershon RY, Gupta R. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg. 2014;6:270–275. doi: 10.1136/neurintsurg-2013-010773 [DOI] [PubMed] [Google Scholar]
- 53.McCarthy DJ, Ayodele M, Luther E, Sheinberg D, Bryant JP, Elwardany O, Kimball J, Starke RM. Prolonged heightened blood pressure following mechanical thrombectomy for acute stroke is associated with worse outcomes. Neurocrit Care. 2019; doi: 10.1007/s12028-019-00803-7 [DOI] [PubMed] [Google Scholar]
- 54.Schönenberger S, Uhlmann L, Ungerer M, Pfaff J, Nagel S, Klose C, Bendszus M, Wick W, Ringleb PA, Kieser M, et al. Association of blood pressure with short- and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA Trial. Stroke. 2018;49:1451–1456. doi: 10.1161/STROKEAHA.117.019709 [DOI] [PubMed] [Google Scholar]
- 55.John S, Hazaa W, Uchino K, Toth G, Bain M, Thebo U, Hussain MS. Lower intraprocedural systolic blood pressure predicts good outcome in patients undergoing endovascular therapy for acute ischemic stroke. Interv Neurol. 2016;4:151–157. doi: 10.1159/000444098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsivgoulis G, Safouris A, Krogias C, Arthur AS, Alexandrov AV. Endovascular reperfusion therapies for acute ischemic stroke: dissecting the evidence. Expert Rev Neurother. 2016;16:527–534. doi: 10.1586/14737175.2016.1168297 [DOI] [PubMed] [Google Scholar]
- 57.Castillo J, Leira R, García MM, Serena J, Blanco M, Dávalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. doi: 10.1161/01.STR.0000109769.22917.B0 [DOI] [PubMed] [Google Scholar]
- 58.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA; IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66 [DOI] [PubMed] [Google Scholar]
- 59.Liebeskind DS, Starkman S, Jo KD, Ohanian AG, Sayre JW, Yun S, Kim D, Ali LK, Ovbiagele B, Towfighi A. Blood pressure in acute stroke is inversely related to the extent of collaterals. Stroke. 2008;39:538–538. [Google Scholar]
- 60.Tsivgoulis G, Saqqur M, Sharma VK, Lao AY, Hill MD, Alexandrov AV; CLOTBUST Investigators. Association of pretreatment blood pressure with tissue plasminogen activator-induced arterial recanalization in acute ischemic stroke. Stroke. 2007;38:961–966. doi: 10.1161/01.STR.0000257314.74853.2b [DOI] [PubMed] [Google Scholar]
- 61.Fischer U, Mattle HP. Blood pressure in acute stroke: still no answer for management. Stroke. 2017;48:1717–1719. doi: 10.1161/STROKEAHA.117.017228 [DOI] [PubMed] [Google Scholar]
- 62.Jeong HG, Kim BJ, Kim H, Jung C, Han MK, Liebeskind DS, Bae HJ. Blood pressure drop and penumbral tissue loss in nonrecanalized emergent large vessel occlusion. Stroke. 2019;50:2677–2684. doi: 10.1161/STROKEAHA.119.025426 [DOI] [PubMed] [Google Scholar]
- 63.Maïer B, Fahed R, Khoury N, Guenego A, Labreuche J, Taylor G, Blacher J, Zuber M, Lapergue B, Blanc R, et al. Association of blood pressure during thrombectomy for acute ischemic stroke with functional outcome: a systematic review. Stroke. 2019;50:2805–2812. doi: 10.1161/STROKEAHA.119.024915 [DOI] [PubMed] [Google Scholar]
- 64.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–199. doi: 10.1159/000353125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson CS, Huang Y, Lindley RI, Chen X, Arima H, Chen G, Li Q, Billot L, Delcourt C, Bath PM, et al. ; ENCHANTED Investigators and Coordinators. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019;393:877–888. doi: 10.1016/S0140-6736(19)30038-8 [DOI] [PubMed] [Google Scholar]
- 66.Goyal N, Malhotra K, Ishfaq MF, Tsivgoulis G, Nickele C, Hoit D, Arthur AS, Alexandrov AV, Elijovich L. Current evidence for anesthesia management during endovascular stroke therapy: updated systematic review and meta-analysis. J Neurointerv Surg. 2019;11:107–113. doi: 10.1136/neurintsurg-2018-013916 [DOI] [PubMed] [Google Scholar]
- 67.Malhotra K, Safouris A, Goyal N, Arthur A, Liebeskind DS, Katsanos AH, Sargento-Freitas J, Ribo M, Molina C, Chung JW, et al. Association of statin pretreatment with collateral circulation and final infarct volume in acute ischemic stroke patients: a meta-analysis. Atherosclerosis. 2019;282:75–79. doi: 10.1016/j.atherosclerosis.2019.01.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.