Abstract
Background
Animal models have demonstrated the deleterious contribution of splenic immunocytes on secondary brain injury after stroke. While previous work has demonstrated splenic contraction (SC) in patients with acute ischemic stroke (AIS) and intracranial hemorrhage (ICH), no clinical studies have examined the relationship between the systemic inflammatory response syndrome (SIRS) with SC in stroke patients.
Methods
This is a retrospective analysis of a previous prospective observational study where daily spleen sizes were evaluated in 178 acute stroke patients. Spleen contraction was based on previously established normograms of healthy volunteers from the same study. SC from the first 24 hrs of stroke onset was evaluated against criteria for SIRS for the first 5 days of admission after AIS.
Results
91patients had verified AIS without concurrent infection at admission. SIRS was not associated with SC at admission. African American patients with early SIRS had higher odds of having SC. Older patients with persistent SIRS at 72 hrs had lower odds of SC. At 48 hrs, there was significantly higher lymphocytosis and lower neutrophils present in patients with SC. Patients with SIRS at 72 hrs were more likely to have worse discharge mRS.
Conclusion
This study provides evidence for an association among SC and SIRS in African American patients suggesting that spleen changes could be a biomarker for detecting SIRS in this population. Our data also indicate a counter association between SC and a lack of SIRS in patients older than 75. Further studies are needed to ascertain how age affects this association.
Index terms: spleen, systemic inflammatory response syndrome, inflammation, ischemic stroke
Introduction
A number of preclinical studies indicate that the spleen contributes to inflammatory damage after ischemic stroke and traumatic brain injury [1–4]. After brain injury, the spleen contracts, releasing pro-inflammatory immune cells and cytokines[1–3, 5]. In these preclinical stroke models, pre or immediate post-stroke splenectomy via surgery or irradiation attenuates stroke infarct size and decrease peripheral immune cell infiltration into the infarct area[6–9].
Clinical translation of these findings is still limited by an incomplete understanding of spleen contraction (SC) in patients with acute ischemic and hemorrhagic stroke. In prior evaluations of splenic responses after stroke, SC was observed in patients with both acute ischemic stroke (AIS) and intracerebral hemorrhage (ICH) as compared with healthy controls[10–12]. SC was associated with an increase in both pro-inflammatory cytokines - interferon gamma (IFNg), interleukin (IL)-6, and IL-10 - and anti-inflammatory cytokines - IL-12. The full extent of the inflammatory response resulting from SC has yet to be characterized, and an increase in both anti- and pro-inflammatory cytokines suggests that the role of SC is nuanced and may be time-dependent.
The systemic inflammatory response syndrome (SIRS) is an inflammatory process that is detrimental to stroke recovery independent of the presence of infection. SIRS is defined by the presence of two of the following: body temperature changes, leukocytosis or leukopenia, elevated heart rate, or elevated respiratory rate[13]. The presence of non-infectious SIRS is associated with longer hospital stays and poor functional outcome at discharge[14, 15]. Leukocytosis and elevated temperature are both independently associated with poor functional outcomes in AIS patients[16–19]. An inflammatory reaction in the absence of infection results from cellular, humoral, or metabolic mechanisms and can lead to an increase in brain injury in patients with acute stroke[20–23].
How non-infectious SIRS originates in the post stroke setting is poorly understood. Given the possible role of SC in activation of cytokines and release of immune cells, we hypothesized that non-infectious SIRS is associated with SC. This correlation could uncover a new biomarker and possible target for predicting and mitigating the development of SIRS and secondary damage from peripheral immune activation. Understanding the potential associations between SC and the development of SIRS may further clarify the biological relevance of SC in patients with acute stroke. We therefore aimed to identify an association between SC and SIRS and its components in patients with AIS and characterize the patients who demonstrate this association.
Methods
Study Population
We performed analyses on our cohort of AIS patients from the Assessment of Spleen Size Reduction and Inflammatory Markers in Acute Stroke over Time (ASSIST) study [10, 11]. This patient population consisted of 178 patients with AIS or ICH whose spleens were serially evaluated by abdominal ultrasonography. The details of the protocol have been previously reported [10]. For this evaluation, we included only patients with AIS; patients with ICH or stroke mimics were excluded. Patients with evidence of infection on admission or who were on antibiotics at time of admission were also excluded from the study. Infection at admission was considered present if there was clinical suspicion for infection and evidence of a source - urinalysis profile consistent with infection, positive cultures (urine, blood, stool, or sputum), or chest x-ray demonstrating findings concerning for pneumonia. In our poststroke population, the presence of fever and/or leukocytosis without a source for infection could be secondary to onset of stroke; therefore, patients without an identifiable source were not considered infected. Data were gathered on patients enrolled between April 2010 and December 2013. All AIS patients received standard of care treatment based on American Heart Association guidelines, including acute treatment with IV tPA when appropriate.
SIRS criteria
Patients who developed SIRS during their hospitalization were identified via chart review. SIRS was defined by the presence of ≥2 of the criteria listed in Table 1. Relevant maximum and/or minimum values were obtained for a complete 24 hr period and assessed for whether the measurements met SIRS criteria. If a patient did not have ≥2 criteria recorded over a 24 hr period, they did not meet the SIRS definition. White blood cell counts and differentials were obtained from chart reviews and reflect daily labs collected as a part of standard of care. The cell count differential is provided as both individual cell counts and as a percentage that is calculated from the cell counts automatically by the hospital lab.
Table 1.
SIRS Criteria
| Two of more of the of the following: |
| • Body temperature <36°C or >38°C |
| • Heart rate >90 |
| • Respiratory rate >20 |
| • White blood cells <4000/mm or >12 000/mm or >10% bands over 24 hours. |
SIRS criteria based on the American College of Chest Physicians and Society of Critical Care Medicine definition form 1992[13].
Infarct volume
MRIs available for patients during the first 5 days of their hospital stay were evaluated. Imaging was available on 86 of the 91 patients evaluated for this study; all patients only had one MRI to evaluate during the specified time period. OsiriX V.9.0 imaging software was used to calculate the total infarct volume on diffusion weighted sequences. Lesions with diffusion restriction and ADC correlation were manually outlined using the OsiriX closed polygon tool creating a region of interest (ROI) and volume was calculated using the ROI volume function. Patients with multifocal infarcts had all lesions evaluated and the sum taken for total volume.
Outcomes
The primary outcomes were development of early SIRS within 48hrs of hospitalization and development of SIRS at each individual 24hr time point for the first three days. Secondary outcomes included: the individual components of SIRS at each 24 hr time point, the presence and type of infection that developed after hospital admission, white blood cell count profiles, discharge functional outcome as defined by modified Rankin Scale (mRS).
Spleen measurements and spleen contraction
The details and protocol for spleen measurement and determination of spleen contraction have been previously reported [10, 11]. Briefly, patients that were deemed eligible for the ASSIST trial had serial spleen measurements for seven days or until day of hospital discharge. The first spleen scan was obtained within 24 hrs of symptom onset followed by repeat measurement at every 24±6hr interval. Measurements were obtained at each time point for every patient in triplicate, and the final spleen volume was calculated by averaging three closet measurements, using a prolate ellipsoid formula as described previously[10]. Based on serial data from healthy volunteers, predictive normograms using body surface area (BSA) and gender were created for multiple percentiles, and a day-to-day variation was quantified. Stroke patients with observed spleen volumes smaller than expected spleen volume by a factor of at least −2 (twice) for the average day-to-day variation at the 50th percentile, were considered to have spleen contraction [10]. The term “spleen contraction” refers only to reduction in spleen size and does not refer to the mechanism of reduction. Spleen contraction has been consistently used as a term to describe changes in spleen size by our group in prior studies [10, 11].
Statistical analysis
Based on the distribution of continuous variables, their summary measures were calculated as either means with standard deviations (SDs) or medians with interquartile range (IQR). Univariate analysis was performed to determine demographic, clinical, and imaging variables that were most strongly associated with spleen volume or development of SIRS. Patients with SC were compared to those without SC using either t-tests or ANOVA when appropriate. Logistic or linear regression models were fit to determine the association of demographic, imaging and clinical factors with SC. Logistic or linear regression analyses were conducted to assess the relationship between SC and the primary and secondary outcomes as described above. We also assessed interaction between SC and either old age (>75 years) and African American race, based on our univariable demographic analyses and prior literature. Alpha was set at 0.05 for all statistical testing. Statistical analysis and graphical representation was done using STATA version 14 (StataCorp, College Station, TX), R (R Development Core Team, 2011, Vienna, Austria), and Microsoft Excel (Microsoft, 2013, Redmond, WA).
Results
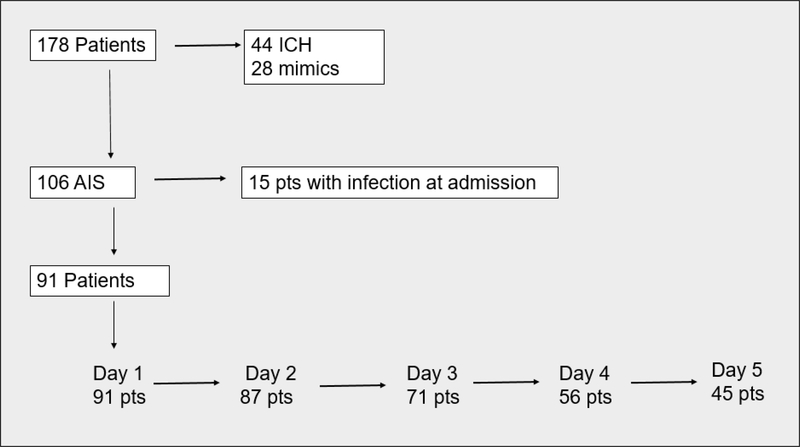
A total of 178 patients participated in the ASSIST study, of which 91 were included in this analysis. Each day of admission was defined as starting from admission with each subsequent 24 hr period. Figure 1 demonstrates the breakdown of patients per day. By day 5, only half (45 patients) of the initial study population remained inpatient.
Figure 1. Patient breakdown.
178 total patients with stroke were initially enrolled into ASSIST. Of these a total of 91 patients were included in our analysis. Patients with ICH. stroke mimics, and initial infection were excluded. By day 5 of admission, about half of the original patient population was left.
Factors associated with SC
Table 2 demonstrates the differences in baseline characteristics between patients who experienced SC (40.6%) and those without SC (59.4%) within the first 24hrs of symptoms onset. Patients with SC were more likely to be older than 75 years (51.3% versus 27.8%, p=0.02) and to use beta blockers prior to admission (54.1% versus 27.8%; p = 0.01). Beta blocker usage remained significantly higher in the SC group (OR = 2.88, 95% CI: 1.1–7.8) in multivariate analysis. African American race was also associated with SC after multivariate analysis (OR 3.1, 95% CI:1.1–9.0). There was no difference in SC based on insular cortex involvement, hemorrhagic transformation, right versus left sided stroke, posterior versus anterior circulation involvement or infarct volume. 17 pts (18.7%) developed infections while hospitalized, of which the vast majority were UTIs. There was no difference in the percentage of patients with infections between patients with SC and those without SC.
Table 2.
Characteristics of patients with and without SC at 24hrs after AIS.
| Variable | SC (n=37) | No SC (n=54) | OR (95% Cl) |
|---|---|---|---|
| Age [mean (sd)] | 73 ± 14.3 | 67 ±14.0 | 1.03 (1.0–1.1, p=0 04)** |
| Age >75 | 51.3% (19) | 27.3% (15) | 2.74 (1.1–6.6, p=0.02)** |
| Female | 48.6% (18) | 42.6% | 0.78 (0.34–1.82, p=0.60) |
| African American race | 35.1% (13) | 20.4% (11) | 2.68 (1.0–7.0, p = 0.04)** |
| BMI >30 | 35.1% (13) | 25.9% (14) | 1.35 (0.62–3.84, p=0.30) |
| Hypertension | 81.1% (30) | 79.6% (43) | 1.1 (0.31–3.15, p=0.90) |
| Hyperlipidemia | 32.4% (12) | 33.3% (10) | 0.96 (0.39–2.34, p=0.93) |
| Diabetes Mellitus | 32.4% (12) | 27.8% (15) | 1.25 (0.5–3.1, p=0.63) |
| Coronary Artery Disease | 16.2% (6) | 16.7% (9) | 0.97 (0.31–3.0, p=0.95) |
| Prior Stroke | 48.6% (18) | 33.3% (13) | 1.89 (0.80–4.47, p=0.14) |
| Smoking | 16.2% (6) | 31.5% (17) | 0.53 (0.22–2.34, p=0.93) |
| Hgb A1C | 6.22 ±16 | 6.45 ±1.8 | 0.90 (0.68–1.20, p=0.35) |
| LDL | 91.1 ±39 | 98.8 ±33 | 0.99 (0.98–1.00, p=0.35) |
| Length of stay | 6.1 ±5.0 | 5.5 ±4.0 | 1.03 (0.94–1.13, p = 0.5) |
| Admission BB use | 54.1% (20) | 27.3% (15) | 3.06 (1.27–7.37, p=0.01)** |
| Admission NIHSS [median (IQR)] | 8 (6–16) | 7.5 (4–13) | 1.0 (0.96–1.06, p=0.70) |
| Intubation during hospitalization | 10.8% (4) | 14.8% (8) | 0.58 (0.19–2.5, p=0.70) |
| Discharge mRS 0–3 | 62.1% (23) | 70.4% (33) | 1.61 (0.65–4.0, p=0.30) |
| tPA | 67.6% (25) | 59.3% (32) | 1.43 (0.60–3.44, p=0.40) |
| Insular cortex involvement | 37.8% (14) | 40.7% (22) | 0.39 (0.38–2.09, p=0.80) |
| Hemorrhagic transformation | 21.6% (8) | 20.4% (11) | 1.08 (0.39–3.00, p=0.90) |
| Stroke side Left | 40.5% (15) | 35.2% (19) | |
| Right | 29.7% (11) | 44.4% (24) | 0.71 (0.69–1.2, p = 0.20) |
| Multiple | 5.4% (2) | 3.7% (2) | |
| Posterior circulation stroke | 24.3% (9) | 13.0% (54) | 2.16 (0.72–6.44, p=0.20) |
| Infarct volume | 19.6 ±52 | 33.1 ±60 | 1.00 (0.99–1.00, p=0.29) |
| Infection during admission | 21.6% (8) | 13.5% (10) | 1.38 (0.40–4.98, p=0.60) |
| Etiology LVA | 13.5% | 11.1% | |
| CE | 48.6% | 48.1% | |
| SVA | 5.4% | 14.8% | 1.11 (0.75–1.63, p=0.6) |
| Cryptogenic | 24.3% | 241% | |
| Other | 0 | 5.56% |
BMI=body mass index; BB=beta blocker; mRS=modified Rankin score; tPA=tissue plasminogen activator; LVA=large vessel atherosclerosis; CE=cardioembolic; SVA=small vessel arteriopathy; CI=confidence interval
p < 0.05
Factors associated with SIRS
72 pts developed early SIRS (first 48 hrs). The percentage of patients with SIRS declined from day 1 to day 5 (Supplemental Figure 1). The median SIRS onset was hospital day 1 and average number of SIRS criteria met was 2, regardless of the presence of initial SC. There were no clear baseline differences in patients with and without early SIRS, although there was a trend toward significance for patients with early SIRS and worse mRS at discharge and discharge NIHSS/7 day NIHSS (Supplemental Table 1). When we evaluated differences in patients with and without SIRS at each individual day, we found no clear difference in baseline variables or outcome on days 1 or 2 (not shown). On day 3 after stroke, patients with SIRS had higher odds of having higher discharge NIHSS and more hemorrhagic transformation of their strokes and trended toward having larger infarct sizes (Supplemental Table 2). Tachycardia/tachypnea were the most common set of criteria that qualified patients for SIRS during the first 3 days, occurring in 31.3% of SC patients and 35.3% of non-SC patients.
Association between SC and SIRS
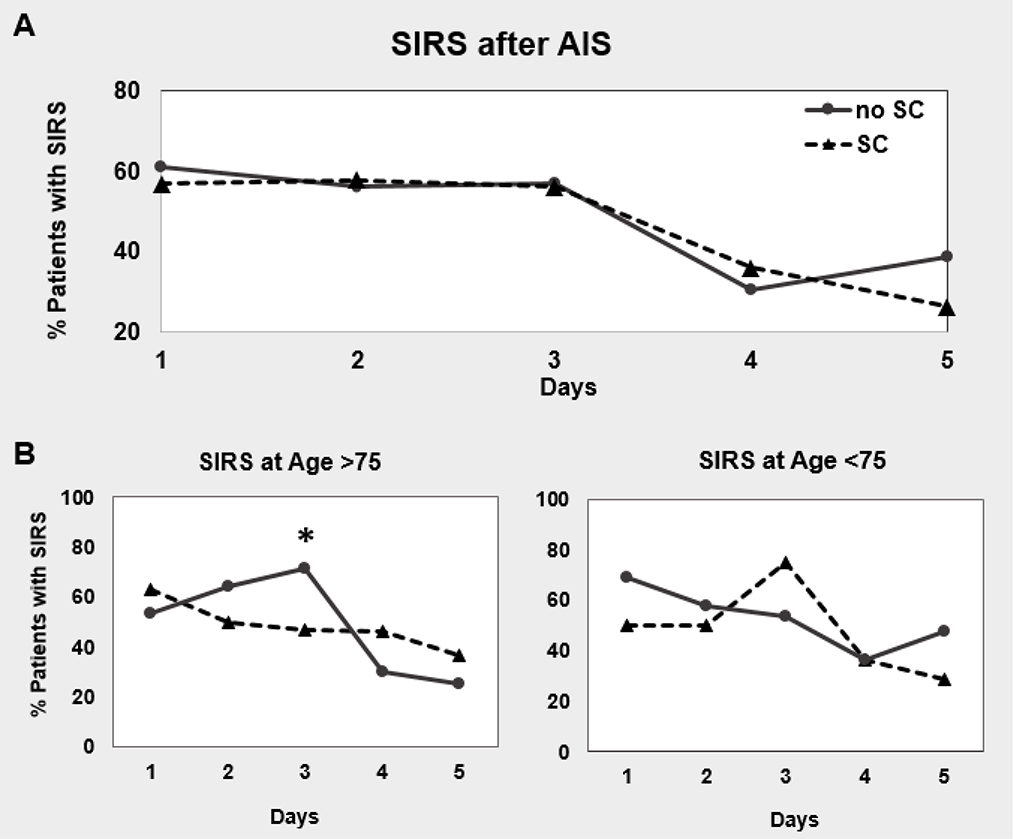
We evaluated both the presence of early SIRS and SIRS on any individual day within the first 72 hrs of stroke onset with SC. There was no association between initial SC and development of early SIRS or SIRS on individual days 1–3 (Figure 2a, Table 3) on univariate and multivariate analysis controlling for infection during admission, gender, prior stroke, and beta blocker usage in addition to age and race. We also examined potential interactions between SC and African American race and patients of older age (> 75 years). These variables were chosen based on the baseline differences between our patients with and without SC and on prior studies.
Figure 2. SIRS in patients with or without SC.
There is no difference in the development of SIRS between patients with or without SC by 24 hrs of stroke onset. After stratifying by age however, there is an increase in SIRS in patients without SC in those >75 years of age. Models are adjusted for: age, sex, race-ethnicity, infection, beta blocker usage, NIHSS at admission, prior stroke, and the interaction terms SC x Age>75 and SC x African American race.
Table 3.
Multivariate model of SC and SIRS
| Variable | SC | SCxAge >75 | SCxAfrican American race |
|---|---|---|---|
| SIRS 1 | p=0.24 | p=0.19 | p=0.05 |
| SIRS 2 | p=0.25 | p=0.63 | p=0.18 |
| SIRS 3 | p=0.82 | p=0.04** | p=0.14 |
| SIRS within 48hrs | p=0.5 | p=0.89 | p=0.02** |
Models are adjusted for: age, sex, race-ethnicity, infection, beta blocker usage, NIHSS at admission, prior stroke, and the interaction terms SC x Age>75 and SC x African American race.
p < 0.05
The presence of early SIRS within 48 hrs of stroke onset was associated with SC when the patient was of African American race (Table 3). 92.3% African American patients with SC developed SIRS compared to 62.5% of patients of other races with SC. In African Americans, there was a 5.57 increase in the odds that patients with early SIRS also have initial SC.
The presence of SIRS at 72 hrs was associated with the lack of SC in patients older than 75 years of age on both univariate and multivariate analysis (Figure 2b, Table 3). For patients >75years of age, 71.4% of patients without SC developed SIRS compared to 46.7% of patients with SC. In this older age group, there was a 0.9 decrease in the odds that patients with SIRS at 72 hrs had SC. There was no significant association between SC and SIRS in the younger age group. We excluded patients that had new onset SIRS on day 3 and examined the 37 remaining patients that had persistent SIRS through all 3 days of admission. There continued to be an association between SIRS and the lack of SC in the older patient group (p = 0.02). There was a 0.92 decrease in the odds that patients with SC would develop SIRS on day 3.
There was no association between the individual components of SIRS (leukocytosis, tachycardia, tachypnea, or temperature) and SC (Supplemental figure 2), regardless of interactions (not shown). The apparent difference in patients with leukocytosis seen in day 5 between patients with and without SC was not significant and the result of low sample size (5 versus 0 patients). The average number of SIRS criteria met in our patients was two. We evaluated combinations of SIRS components (leukocytosis-tachycardia, tachycardia-tachypnea, tachypnea-fever, fever-leukocytosis, leukocytosis-tachypnea, fever-tachycardia) and did not find a difference in patients with SC and those without (not shown). We also looked at the distribution of the number of SIRS criteria met and found that no patients with SC met all 4 criteria on days 1, 2, and 3 (Supplemental Figure 1).
Association of SC with inflammatory cells
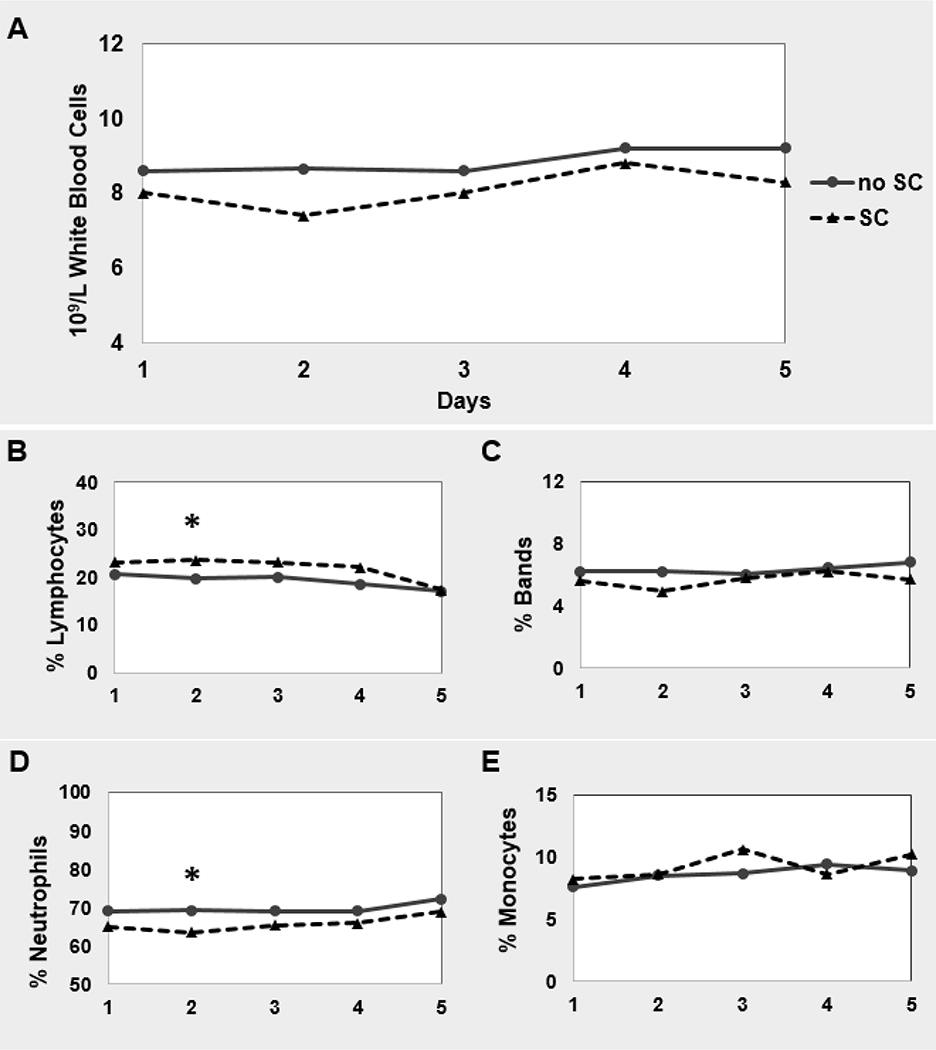
On univariate analysis, the percentage of bands and total leukocytosis were statistically lower in the patients with SC, but did not remain significantly different after multivariate regression analysis. There was a difference in white blood cell count and % monocytes in patients with or without SC on univariate analysis but not on multivariate analysis. Neutrophils were significantly lower on univariate and multivariate modeling (p = 0.004) and lymphocytes were significantly higher (p = 0.02) in patients with SC (Figure 3, Supplemental Table 3) on multivariate analysis. Mean cell counts for white blood cell count and the differentials are supplied in Supplemental Table 4. There was no association with the interaction terms SC x Age>75 or SC x African American race.
Figure 3. Components of white blood cell count in patients with and without SC.
There is no difference in leukocytosis in patients with and without SC, however on closer evaluation there are changes in the composition of the total white blood cell (WBC) count. There in multivariate modeling, there is higher lymphocyte percentage in patients with SC and a lower neutrophil percentage in patients without SC. Models are adjusted for: age, sex, race-ethnicity, infection, beta blocker usage, NIHSS at admission, prior stroke, and the interaction terms SC x Age>75 and SC x African American race.
Discussion
The contribution of the spleen to peripheral immune modulation after ischemic stroke has been addressed by many animal studies[2, 3, 8, 12, 21, 24], but the effect of SC on peripheral immune activity in humans after stroke has yet to be characterized. In this retrospective analysis, we found no difference in the development of SIRS in patients with or without SC overall. We did find that early SIRS was strongly associated with SC in African American patients, but that in older patients, there is a higher incidence of SIRS without SC at 72 hrs after stroke.
There continues to be ambiguity how non-infectious SIRS is activated post-stroke, although it is likely associated with the extent of ischemic damage since successful thrombolytic therapy attenuates the response[25]. Our findings demonstrate differences in how SC may influence the development of SIRS across race and age. The data suggest that SIRS is more likely to be associated with SC in African American patients but race was not an independent predictor for the development of SIRS as seen in other studies. Patients of African American race have been found to have higher rates of non-infectious SIRS after stroke[15, 26] and there is data demonstrating higher rates of infection and sepsis in African American patients[27–29]. African American race has also been included in a non-infectious SIRS prediction model[26]. That we did not find African American race to be an independent predictor of SIRS could represent our small sample size and our inclusion of patients who subsequently developed infections.
Age however seems to have an opposite effect – SC is associated with less persistent SIRS in older patients. There are several possible mechanisms that explain this association. In animal studies, the noradrenergic innervation of the spleen and presence of T-lymphocytes and macrophages in the white pulp both decline with age[30–32]. The association between SC and lack of SIRS may represent a subset of older patients that at baseline have smaller spleens and are less able to mount an inflammatory reaction. Alternatively, this association may represent differences in the innervation and distribution of red and white pulp with age. In animal models, older rodents had more hemosiderin deposition in the red pulp and lower percentages of lymphocytes in white pulp[33] and contraction may simply be a reflection of the change in red pulp. A lack of contraction in an older patient may not be representative of the full effect of splenic contribution to immune system activation. Both of these possibilities suggest that robust white pulp function of the spleen may be required for a SIRS response.
Our analysis reveals that patients that used βblockers prior to admission had a higher odds of developing SC. Of the 35 patients on βblockers, 30 were on selective βblockers, with no differences in distribution between patients with or without SC. We were unable to review whether βblockers continued to be used for the duration of their hospitalization. The innervation of the spleen is mostly sympathetic in origin and regulation occurs via α1- and β-adrenergic receptors with α-receptors controlling smooth muscle contraction of the splenic capsule and β-receptors modulating immune function at both a follicular and a cellular level[30, 31, 34]. Control within the spleen is achieved via high and low gradient clusters of receptors used to regulate the release of immunocytes[35, 36]. It is unclear why pre-morbid β- blocker usage would be associated with SC but this may be related to selective β1 blockade and a lack of α blockade activity. Prior animal studies have demonstrated that blockade of α1 receptors – either with prazosin or carvedilol – was necessary for attenuation of spleen contraction, although additional β blockade was also required to provide neuroprotection after stroke[37]. Blockade of β1 and β2 receptors alone – with propranolol – did not affect spleen contraction and did not have an effect on infarct volume.
There was no difference in the rates of infection in patients with or without SC, taking into account Age >75 and race. Preclinical models have suggested that the immune response after stroke is a biphasic phenomenon[38]. There is an initial activation of local and peripheral immune responses in the initial hours followed by stroke-induced immune depression (SID) leading to increased odds of developing infection[38–40]. The association of SC with early SIRS in African Americans was significant, and some population studies have demonstrated higher rates of infection and sepsis in African Americans after stroke[27, 28]. These results may hint at a relationship between SC, early SIRS, and the development of infection from SID.
There was no difference in discharge mRS when comparing patients with SC and without SC in our AIS subset while our larger database of ischemic and hemorrhagic strokes – from which these patients were derived – demonstrated that SC is associated with a higher mRS at discharge. This difference may reflect the smaller sample size when focusing just on patients with AIS without infection. We confirmed with our dataset that patients with SIRS at 72 hrs had worse NIHSS at discharge/7 day NIHSS (Supplemental Table 2) compared with those without SIRS. Patients who develop non-infectious SIRS are more prone to poor outcomes[15, 26].
Consistent with other studies on stressful states, we did find there was a significant increase in lymphocytosis at 48 hrs in patients with SC compared with those without SC that occurs in other stressful states[12, 41]. There was no increase in the rate of leukocytosis or infection in the first 72hrs of admission in patients with SC. We also found a decrease in neutrophils in patients with SC, 69.1% in patients without SC compared to 64.9% in patients with SC (Figure 3, Supplemental Table 3). This decrease in neutrophil content in the serum of patients with SC may represent neutrophil distribution to other body compartments, namely the brain. Neutrophil infiltration into the brain occurs early after stroke, in the first several hours. Decreased serum neutrophil concentrations at day 2 after stroke may represent increased extravasation from blood to brain after SC[42–44].
Our study is limited by its retrospective nature. Some of the components of SIRS require measurements of vital signs and lab-work that could have been inconsistently measured among patients. During the course of evaluating patients for SIRS, we noted a large number of our patients had elevated respiratory rates, many of whom were designated as having SIRS because tachypnea was present. While sometimes indicating a true pathology, tachypnea may be improperly recorded or indicative of other stressful states (anxiety, pain, exertion after therapy) unrelated to actual lung injury. Furthermore, very few patients had leukocytosis or leukopenia that qualified them for SIRS criteria (not shown). Finally, this study involved a small sample size at one academic center which limits the generalizability of the results. Prospective studies that include the systematic collection of SIRS data and the measurement of inflammatory markers and outcomes to determine the associations between SC and inflammatory status in post-AIS are needed to further clarify the role of SC in post-stroke peripheral immune activation.
Conclusion
This study found that associations between SC and SIRS appear to depend on age and ethnicity. Our data indicate the need for additional prospective work in appropriately selected patients in order to understand how SC contributes to peripheral immune activation in post-stroke humans.
Supplementary Material
Acknowledgments
We would like to acknowledge the staff of UT Ultrasonology lab
Sources of Funding
This study was supported by NIH grant, 5 T32 NS007412.
Footnotes
Conflict(s)-of-Interest/Disclosure(s)
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
For this type of study formal consent is not required.
References
- 1.Liu ZJ, et al. , Splenic responses in ischemic stroke: new insights into stroke pathology. CNS Neurosci Ther, 2015. 21(4): p. 320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Offner H, et al. , Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab, 2006. 26(5): p. 654–65. [DOI] [PubMed] [Google Scholar]
- 3.Rasouli J, et al. , Brain-Spleen Inflammatory Coupling: A Literature Review. Einstein J Biol Med, 2011. 27(2): p. 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennypacker KR, Targeting the peripheral inflammatory response to stroke: role of the spleen. Transl Stroke Res, 2014. 5(6): p. 635–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seifert HA, et al. , A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol, 2012. 7(4): p. 1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrowski RP, et al. , Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res, 2012. 3(4): p. 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang BJ, et al. , Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl), 2013. 126(12): p. 2354–60. [PubMed] [Google Scholar]
- 8.Ajmo CT Jr., et al. , The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res, 2008. 86(10): p. 2227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fathali N, et al. , Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl Stroke Res, 2013. 4(2): p. 208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahidy FS, et al. , Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J Cereb Blood Flow Metab, 2016. 36(6): p. 1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahota P, et al. , Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke, 2013. 8(2): p. 60–7. [DOI] [PubMed] [Google Scholar]
- 12.Chiu NL, et al. , The Volume of the Spleen and Its Correlates after Acute Stroke. J Stroke Cerebrovasc Dis, 2016. 25(12): p. 2958–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med, 1992. 20(6): p. 864–74. [PubMed] [Google Scholar]
- 14.Boehme AK, et al. , Systemic Inflammatory Response Syndrome and Outcomes in Intracerebral Hemorrhage. Neurocrit Care, 2016. 25(1): p. 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehme AK, et al. , Systemic inflammatory response syndrome in tissue-type plasminogen activator-treated patients is associated with worse short-term functional outcome. Stroke, 2013. 44(8): p. 2321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phipps MS, et al. , Epidemiology and outcomes of fever burden among patients with acute ischemic stroke. Stroke, 2011. 42(12): p. 3357–62. [DOI] [PubMed] [Google Scholar]
- 17.Saini M, et al. , Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke, 2009. 40(9): p. 3051–9. [DOI] [PubMed] [Google Scholar]
- 18.Kumar AD, et al. , Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis, 2013. 22(7): p. e111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nardi K, et al. , Admission leukocytosis in acute cerebral ischemia: influence on early outcome. J Stroke Cerebrovasc Dis, 2012. 21(8): p. 819–24. [DOI] [PubMed] [Google Scholar]
- 20.Bowen KK, Naylor M, and Vemuganti R, Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int, 2006. 49(2): p. 127–35. [DOI] [PubMed] [Google Scholar]
- 21.Seifert HA, et al. , The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis, 2012. 27(2): p. 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarting S, et al. , Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke, 2008. 39(10): p. 2867–75. [DOI] [PubMed] [Google Scholar]
- 23.Lakhan SE, Kirchgessner A, and Hofer M, Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med, 2009. 7: p. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogelgesang A, Becker KJ, and Dressel A, Immunological consequences of ischemic stroke. Acta Neurol Scand, 2014. 129(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- 25.Audebert HJ, et al. , Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke, 2004. 35(9): p. 2128–33. [DOI] [PubMed] [Google Scholar]
- 26.Boehme AK, et al. , Predictors of systemic inflammatory response syndrome in ischemic stroke undergoing systemic thrombolysis with intravenous tissue plasminogen activator. J Stroke Cerebrovasc Dis, 2014. 23(4): p. e271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore JX, et al. , Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Crit Care, 2015. 19: p. 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colbert JF, et al. , Sex-Related Differences in the Risk of Hospital-Acquired Sepsis and Pneumonia Post Acute Ischemic Stroke. J Stroke Cerebrovasc Dis, 2016. 25(10): p. 2399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehme AK, et al. , Infections present on admission compared with hospital-acquired infections in acute ischemic stroke patients. J Stroke Cerebrovasc Dis, 2013. 22(8): p. e582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellinger DL, et al. , Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol, 1992. 14(3): p. 329–44. [DOI] [PubMed] [Google Scholar]
- 31.Madden KS, Thyagarajan S, and Felten DL, Alterations in sympathetic noradrenergic innervation in lymphoid organs with age. Ann N Y Acad Sci, 1998. 840: p. 262–8. [DOI] [PubMed] [Google Scholar]
- 32.ThyagaRajan S, et al. , Age-associated alterations in sympathetic noradrenergic innervation of primary and secondary lymphoid organs in female Fischer 344 rats. J Neuroimmunol, 2011. 233(1–2): p. 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cesta MF, Normal structure, function, and histology of the spleen. Toxicol Pathol, 2006. 34(5): p. 455–65. [DOI] [PubMed] [Google Scholar]
- 34.Nance DM and Sanders VM, Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun, 2007. 21(6): p. 736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronte V and Pittet MJ, The spleen in local and systemic regulation of immunity. Immunity, 2013. 39(5): p. 806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mina-Osorio P, et al. , Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol Med, 2012. 18: p. 618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajmo CT Jr., et al. , Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol, 2009. 218(1): p. 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Famakin BM, The Immune Response to Acute Focal Cerebral Ischemia and Associated Poststroke Immunodepression: A Focused Review. Aging Dis, 2014. 5(5): p. 307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harms H, et al. , Influence of stroke localization on autonomic activation, immunodepression, and post-stroke infection. Cerebrovasc Dis, 2011. 32(6): p. 552–60. [DOI] [PubMed] [Google Scholar]
- 40.Walter U, et al. , Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol, 2013. 20(1): p. 153–9. [DOI] [PubMed] [Google Scholar]
- 41.Bakovic D, et al. , Effect of human splenic contraction on variation in circulating blood cell counts. Clin Exp Pharmacol Physiol, 2005. 32(11): p. 944–51. [DOI] [PubMed] [Google Scholar]
- 42.Ruhnau J, et al. , Thrombosis, Neuroinflammation, and Poststroke Infection: The Multifaceted Role of Neutrophils in Stroke. J Immunol Res, 2017 2017: p. 5140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-de-Puig I, et al. , Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol, 2015. 129(2): p. 239–57. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad M and Graham SH, Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res, 2010. 1(2): p. 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.