Abstract
Conventional models of color vision assume that blue and yellow (along with red and green) are the fundamental building blocks of color appearance, yet how these hues are represented in the brain and whether and why they might be special are questions that remain shrouded in mystery. Many studies have explored the visual encoding of color categories, from the statistics of the environment to neural processing to perceptual experience. Blue and yellow are tied to salient features of the natural color world, and these features have likely shaped several important aspects of color vision. However, it remains less certain that these dimensions are encoded as primary or “unique” in the visual representation of color. There are also striking differences between blue and yellow percepts that may reflect high-level inferences about the world, and specifically about the colors of light and surfaces. Moreover, while the stimuli labeled as blue or yellow or other basic categories show a remarkable degree of constancy within the observer, they all vary independently of each other across observers. This pattern of variation again suggests that blue and yellow and red and green are not a primary or unitary dimension of color appearance, and instead suggest a representation in which different hues reflect qualitatively different categories rather than quantitative differences within an underlying low-dimensional “color space.”
1. Introduction
Modern color science remains strongly rooted in two foundational theories of color vision. The trichromatic theory of Young and Helmholtz provides an account of the initial absorption of light by the three classes of cone photoreceptors, and goes a long way in explaining how the visual system samples the spectrum and which spectra can be distinguished [1]. Hering’s theory of opponent processes instead focused on color appearance, and has been used to explain how signals from the cones are combined within postreceptoral mechanisms to represent the perceptual experience of color in terms of underlying red-green and blue-yellow dimensions [2]. However, as we learn increasingly more about the intricacies of the visual system both theories face challenges [3]. The genetics of the cone opsins have revealed much richer variation in the photopigments than expected, and have raised exciting new questions about how different gene variants are expressed and how these relate to the behavioral capacities and perceptions of the observer [4, 5]. An example is the ongoing research on the potential for female tetrachromacy [6, 7]. Nevertheless, the principle of univariance and the properties of color matching inspired by trichromatic theory remain solid cornerstones of color science and colorimetry [8, 9].
The opponent process theory has not fared as well. The representation of chromatic information by contrasting signals (e.g. from the cones) is a fundamental insight that has seen clear and repeated confirmation in psychophysics and physiology. Yet the specific comparisons that are made, how many are used, and how they give rise to our conscious experience of color are increasingly shrouded in mystery. In particular there is now little consensus over the nature of the Hering primaries of red-green and blue-yellow, or whether these are primary at all. For example, apart from their presumed special appearance, the unique hues do not clearly behave as if they are unique or special in many tasks (e.g. [10–14]), though there are tantalizing exceptions [15]. This debate has had the refreshing consequence of reinvigorating color science, and has given rise to many intriguing new ideas and theories about how the brain builds a representation of color. This essay is not intended to be a comprehensive review of these theories, and instead narrowly focuses on my own ideas and how they have evolved from a career chasing the elusive shades of blue and yellow.
2. Blue and yellow in the brain
Hering’s theory held that the perception of any color depended on the responses in three opponent processes signaling red vs. green, blue vs. yellow, or bright vs. dark sensations [2]. For the two chromatic processes the opponent pairs were mutually exclusive, so that a color could not be red and green at the same time. Stimuli that isolated one of the processes should give rise to the pure undiluted sensation of one of the primaries. These percepts correspond to the unique hues of red, green, blue, and yellow. For example, a unique yellow is a yellow that has neither a reddish nor greenish tinge. All other hues correspond to different combinations of the unique hues. For example, orange reflects the combined attributes of red and yellow, while purple is a combination of red and blue. A critical assumption was thus that some hues are unique because they reflect unique or special states in the brain. Measurements of the strength of red-green and blue-yellow responses at different wavelengths could map out the spectral sensitivities of the opponent chromatic response functions [16]. These could in turn be fit with the cone fundamentals to estimate how the cone signals are combined to form the opponent dimensions [17].
Color-opponent theory was revived by the classic psychophysical studies of Jameson and and Hurvich [16], but gained solid grounding when a plausible mechanism for generating an opponent-like representation was identified in physiological recordings from neurons in the fish and primate visual system [18, 19]. These showed that cells were excited by lights at some wavelengths but inhibited at others, and thus had precisely the responses that could in theory encode mutually-exclusive responses within a single opponent mechanism. However, these studies also noted important inconsistencies between the physiology and perception. In an early study comparing cell responses in the primate lateral geniculate to the color percepts reported by humans, De Valois, Jacobs, and Abramov [20] pointed to differences at wavelengths shorter than unique blue, which appear violet (i.e. reddish-blue). However, the recorded cell population did not exhibit the expected change in response polarity at these wavelengths, predicting that the shorter wavelengths should instead appear greenish-blue.
This discrepancy was brought to the fore by the seminal studies by Krauskopf and colleagues. Krauskopf, Williams, and Heeley [21] used a habituation or contrast adaptation paradigm to identify the chromatic mechanisms mediating threshold color changes and their potential cone inputs. This revealed the “cardinal mechanisms” of color opponency and showed that they corresponded to two channels comparing signals in the two longer-wavelength cones (LvsM) or to the short-wave cones compared to the longer-wave cones (SvsLM). In subsequent analyses of the LGN responses to chromatic stimuli, Derrington, Krauskopf, and Lennie [22] further showed that these were the cone inputs to the two main classes of color-opponent cells, which correspond to cells in the parvocellular (LvsM) and, as discovered later [23], the koniocellular (SvsLM) layers of the LGN, and to the midget and bistratified ganglion cell classes of the retina [24]. These studies, along with the chromaticity diagram developed earlier by MacLeod and Boynton [25] which also defined stimuli in terms of the cardinal axes, had an enormous impact on the field. It is now routine to design experiments and interpret results in terms of the cardinal or cone-opponent axes, with the advantage that these can be more directly related to the cone signals and the principal color pathways at early postreceptoral stages.
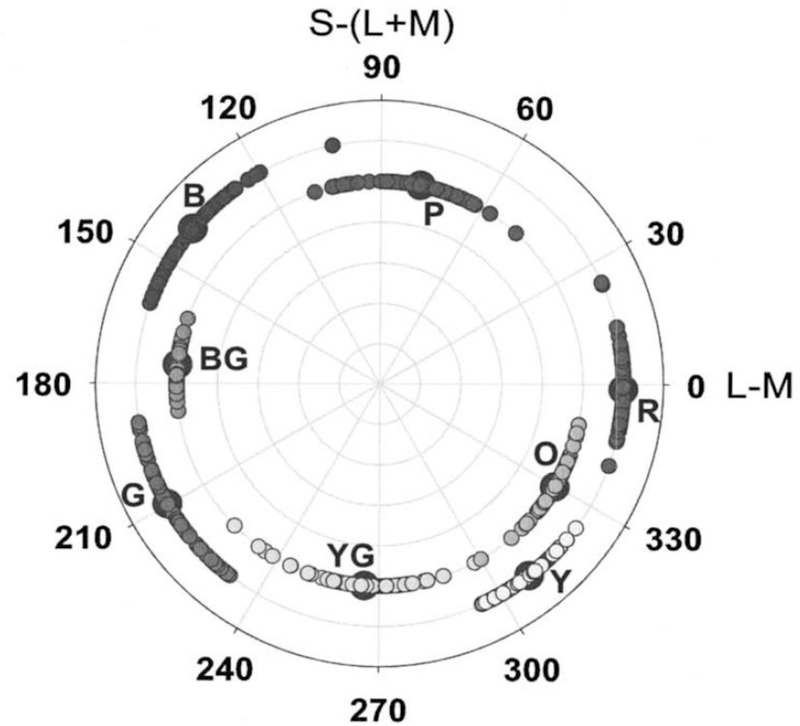
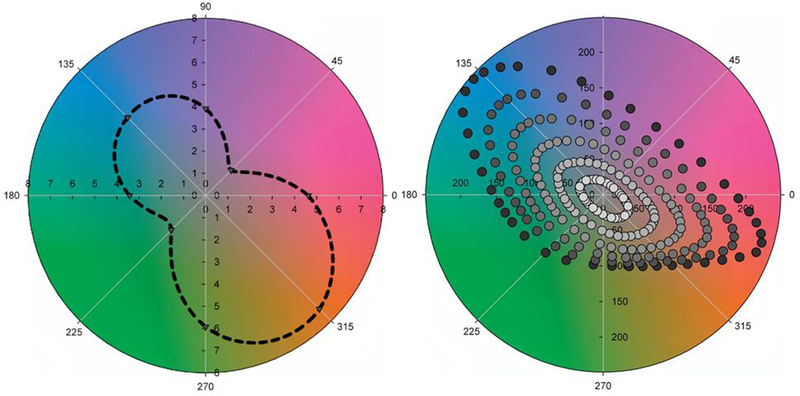
Like the work of De Valois et al. [20], one of the key findings of Krauskopf et al. [21] was that the cardinal axes are not aligned with the perceptual-opponent axes. This is illustrated in Figure 1, which plots the stimuli chosen for the unique hues or binary hues (e.g. orange or purple) for a number of color-normal observers [10] (see also [26, 27]). The SvsLM axis varies from purple to yellow-green, while the LvsM axis from red to blue-green. In contrast, unique yellow and blue lie roughly midway between the cardinal axes, and thus are not stimuli that isolate either axis. It is unfortunate that despite this, it is common in the field to refer to the LvsM and SvsLM axes as red-green and blue-yellow, just as it was once common to refer to the LMS cones as RGB. As Figure 1 shows, the colors along the SvsLM axis are in fact as red-green as they are blue-yellow, while the +M pole of the LvsM axis is close to an equal mixture of blue and green. Three of the four cardinal axis poles therefore correspond roughly to the categorical boundaries between adjacent unique hues [10]. Recent analyses have found that these axes also represent the categorical boundaries for color discrimination in infants, who might thus base their discriminations on the relative polarity of the cardinal axis mechanisms [28, 29]. However in adults the interobserver differences in the unique hues substantially exceed the range of variability in the stimuli that isolate the cardinal axes [27, 30].
Figure 1.
Color categories relative to the cardinal axes. Symbols show the stimuli chosen as unique (Red, Green, Blue, Yellow) and binary (Purple, Orange, Yellow-Green, Blue-Green) hues by individual color-normal observers, plotted by their angle in the LvsM and SvsLM space (after [10]).
The mismatch between the stimuli that appear perceptually pure and the stimuli that align with the cone-opponent pathways in the retina and geniculate, prompted questions about the stages of the visual system where the unique hues become more explicitly represented. One possibility is that the corresponding substrate arises further along the visual stream, because of further recombinations of the cone-opponent signals, and models of the potential transformations were subsequently proposed [31]. A number of attempts have also been made to identify downstream neural signatures of the unique hues or the perceptual organization of color [32–34]. An alternative theory is that the cardinal mechanisms reflect pathways involved in other functions of color vision, such as scene segmentation, and that color percepts are carried by a different neural circuit also arising in the retina but potentially overlooked because this pathway is smaller [35]. Several lines of evidence have been put forth in support of this model [36] including the remarkable recent achievement of stimulating single photoreceptors with adaptive optics [37]. These studies suggest that the majority of L and M cones signal achromatic percepts, with only a small proportion producing red or green sensations.
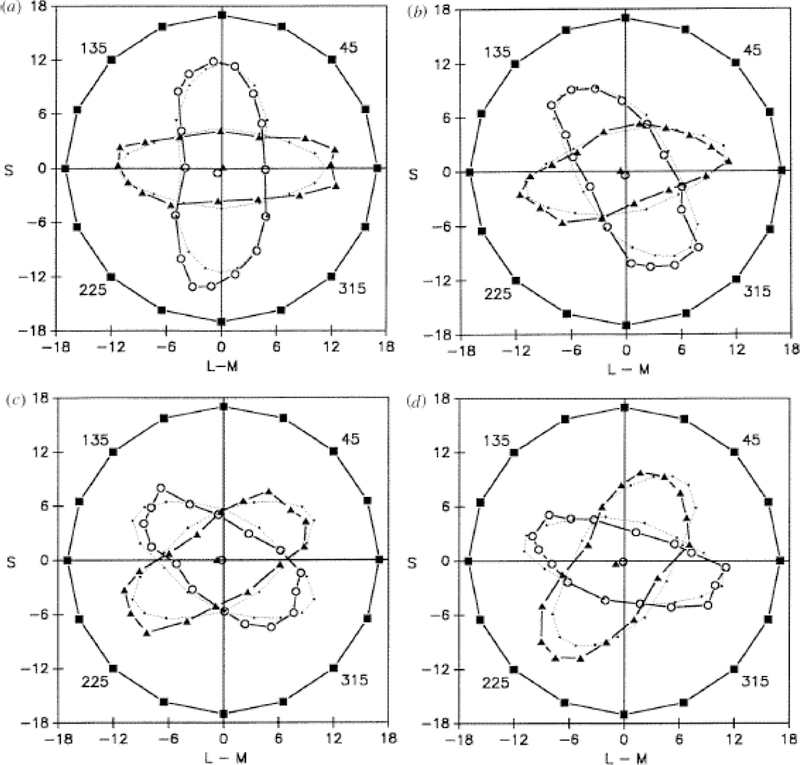
However, further work by Krauskopf et al. [38] pointed to a second fundamental problem with the Hering theory. This study used a variety of techniques to demonstrate the presence of additional “higher-order” color mechanisms tuned to directions intermediate to the cardinal axes. Webster and Mollon [39, 40] subsequently applied a similar color contrast adaptation paradigm to examine suprathreshold color appearance. Adapting to a given color axis strongly altered the perceived hue and saturation of a subsequent test stimulus, and these aftereffects were selective for any arbitrary angle in color space, implicating mechanisms tuned to these directions that contribute to color percepts (Figure 2). This could conceivably reflect tuning changes in two mechanisms rather than gain changes in multiple mechanisms [41, 42]. However paralleling these psychophysical results, recordings of chromatic sensitivity in cells at different cortical levels reveal a much broader range of preferred color directions, suggesting that the relatively discrete representation in the LGN gives way to a more uniform tiling of color space in the cortex [43, 44]. Moreover, there is abundant evidence for higher-order color mechanisms mediating visual performance in a variety of behavioral tasks that do not involve adaptation [45], though the extent to which they are manifest for specific tasks remains debated [46].
Figure 2.
Multiple chromatic channels. Asymmetric color matches (circles and triangles) to the same set of test stimuli (squares) after adaptation to chromatic modulations along different axes within the LvsM and SvsLM plane. The adaptation leads to selective losses in perceived contrast (saturation) along the adapting axis, and biases perceived hues away from the adapt axis and toward an orthogonal axis (after [39]).
The presence of these additional opponent mechanisms is difficult to reconcile with the tenet that unique hues look unique because they are the responses of the isolated opponent processes, because if the chromatic plane is represented by multiple mechanisms tuned to different color angles but with overlapping sensitivity, then there is no stimulus direction that can isolate a single mechanism [39, 40]. Instead, hue may be represented in ways analogous to spatial orientation, by the population responses of channels tuned to different directions around the (color) clock [40, 47, 48]. Such models have recently been proposed to account for color coding at higher cortical stages [49]. There are not clear signs that a discrete two-channel representation re-emerges at higher stages, and instead the chromatic tuning of cells becomes substantially narrower, further challenging the notion that a single mechanism encodes the “redness” or “greenness” of hues across different stimulus angles [43, 49]. This multiple-channel representation could yet be reconciled with opponent process theory if, for example, the representation reflected other aspects of color vision such as its role in spatial vision. However, the adaptation effects again suggest that color appearance is mediated by multiple and potentially a continuous distribution of channels, at least at the visual level affected by the adaptation.
3. Discounting the observer
Yet another obstacle in seeking an explanation for the unique hues solely from the structure of color coding in the brain is that the visual system varies widely from one individual to the next. Even in observers with normal color vision, there are large differences in spectral and neural sensitivity [50, 51]. Without correcting for these differences, very different stimuli would be required to produce the same neural states in different observers. This is also a problem within the observer, because sensitivity varies markedly over time (e.g. as we age) and over space (e.g. at different retinal locations). However, a number of mechanisms act to compensate for the optical and neural idiosyncrasies of the observer in order to maintain stable color percepts, or color constancy. Constancy is typically studied in the context of discounting the illuminant in color perception [52]. However, an equally important aspect of color constancy is discounting the observer, and this occurs for many aspects of color vision.
Compensating for white.
Variations in spectral sensitivity arise at the earliest stages of vision, from differences in the density of the lens and macular screening pigments, the optical density and spectral peaks of the cones, and in the relative number of different cone types [50, 51]. These differences have important effects on threshold sensitivity and color matching, but surprisingly little effect on color appearance. For example, white settings remain very similar in the fovea and near periphery despite the fact that the foveal cones are screened by macular pigment [53, 54]. Similarly, the lens of the eye progressively yellows with age, limiting the shorter wavelength light reaching the receptors. Yet achromatic settings again remain very stable with aging, so that what appears white is continuously adjusted to discount the sensitivity change [55, 56]. Delahunt et al. [57] tracked these adjustments in patients before and after cataract surgery. When the cataractous lens is first replaced, the world appears very blue and bright. Yet over time the stimulus that appeared white approached the settings before surgery, so that they tended to see the world in the same way yet through their different eyes. By assessing this change on a dark background (in order to remove an influence of the immediate context), the study revealed that these adjustments developed over surprisingly long timescales of weeks or months. Long-term adjustments in color appearance have also been found in a number of other studies [58].
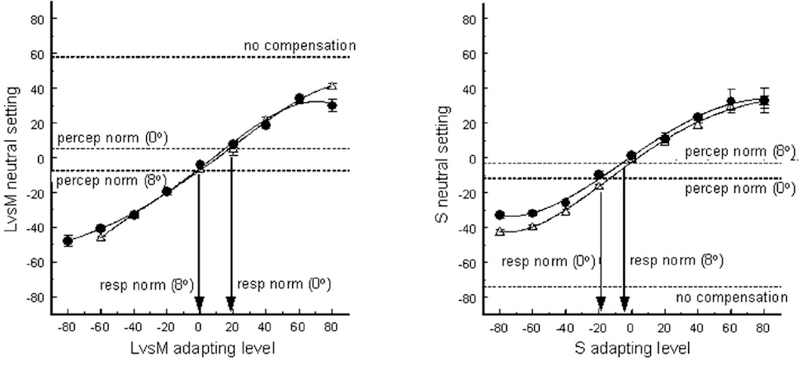
Equating the perception of white at two retinal loci could occur at many stages in the visual system, from a peripheral sensitivity calibration to a high-level learned inference that both loci should be representing the same world [53, 59]. To explore the site of the calibration, Webster and Leonard [53] compared achromatic settings in the fovea and periphery after short-term chromatic adaptation, to identify the stimulus that did not produce a color aftereffect. Chromatic adaptation adjusts sensitivity at a very early retinal level and potentially within the photoreceptors, because the gain changes are cone-specific [60] and have a spatial resolution comparable to single receptors [61] (though the afterimages have been identified with the rebound signals generated in the ganglion cells [62]). If the long-term normalization for white in the fovea and periphery also occurred at this early level, then the short-term chromatic adaptation at the two loci should be the same for the same external stimulus, but for different retinal spectra because of the differences in macular screening (i.e. the stimulus hat does not produce a color aftereffect should be the same stimulus that is perceived as achromatic). Conversely, if the normalization depended on processes later in the visual pathway, then chromatic adaptation at the two loci should be similar when the cone excitations are similar, but the stimuli differ (i.e. later compensation predicts that the neutral stimulus for chromatic adaptation may not “look” neutral). Chromatic adaptation at the two sites was indistinguishable – and for both the stimulus that was perceived as achromatic (the “perceptual norm”) was close to the adapting stimulus that did not induce an aftereffect (the “sensitivity norm”) [53] (Figure 3). Thus these results suggest that the intrinsic discounting for spectral sensitivity differences across the retina may already be present as early as the receptors, and may represent a long-term adaptation or memory for the average spectrum the cone is exposed to. The short-term fluctuations in sensitivity with light or chromatic adaptation as conventionally measured and defined thus appear to ride on top of this long-term equilibrium.
Figure 3.
Perceptual norms vs. sensitivity norms. Plots show chromatic adaptation in the fovea (circles) and 8-deg periphery (triangles). Shifts in the achromatic settings are plotted as a function of the chromaticity of the adapting stimulus along the LvsM (left) or SvsLM (right) axis. Aftereffects are similar at the two loci for the same adapting chromaticity, with a null in the aftereffect close to the stimulus level that was judged as achromatic under neutral (dark) adaptation (dashed lines labeled “percep norm”). Arrows indicate the adapting stimuli that did not alter the neutral achromatic settings (“resp norm”). Lines labeled “no compensation” show the vertical difference in the adapting curves at the two loci that would be predicted if the adaptation were instead controlled by equivalent spectra at the level of the receptors, after filtering by the macular pigment (based on estimated differences in macular pigment screening at the two loci) (after [53]).
There are two further implications of this result. First, the achromatic point is part of the family of unique hues and perhaps the most important one, for it is the equilibrium point for both opponent processes. That this percept (as measured under neutral or dark adaptation) also corresponds to the equilibrium point for chromatic adaptation reveals a close connection between “perceptual norms” – the stimuli that are experienced as neutral; and “sensitivity norms” – the stimuli that produced balanced or neutral responses in the visual system. Unlike the other unique hues, white is therefore a case where we arguably can link a special color experience to a special state in the underlying neural code [53]. The second implication is that norms are not unique to color but are common and central to the norm-based codes thought to underlie the representation of many perceptual dimensions [63]. For example, common models of face perception assume an individual is represented by how they deviate from a neutral or prototypical identity [64], analogous to the role of “gray” in color coding. Thus these models closely parallel models of color appearance, and notably face and color perception are affected by adaptation in very similar ways [65]. Similarly, in blur aftereffects, the adapting stimulus that does not bias subjective focus is the same stimulus that appears in focus to the observer, suggesting that the perceived focus reflects how spatial vision is normalized to discount the retinal image blur specific to the individual’s optical aberrations [66, 67].
Compensating for hue.
Settings for unique hues also remain stable despite changes in spectral sensitivity. For example, unique yellow is largely unaffected by the large differences in the ratio of L and M cones [68–70]. Hue percepts also remain similar between the fovea and near periphery; and while not complete, the constancy is better than predicted by adjustments only to the average color (von Kries adaptation [71]), implicating an additional correction [72, 73]. The loci of the unique hues also remain stable across the lifespan [56, 74], and again better than would be expected from adjusting only to the changes in mean spectral sensitivity [72]. These adjustments have their limits, because color in the periphery varies in ways that can be tied to the variations in retinal physiology [75, 76], and the hue percepts of color-anomalous observers show only partial compensation for their anomalous pigments [77]. However, the variations in appearance are generally less than physiological constraints alone would suggest.
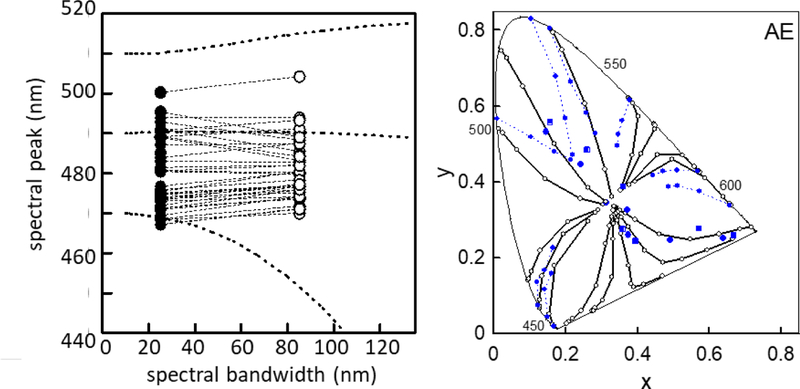
A further surprising compensation is for the effects of saturation on hue. We were led to examine this question by the suggestion of Mollon and Jordan [78] that there should be greater consensus in the unique hues for natural broadband stimuli than narrowband, since the compensations observers learn for the natural stimuli should cause the hues seen for narrowband stimuli to diverge. Mizokami et al. [79] tested this by matching the hues of Gaussian spectra with narrow or broad bandwidths. The spectral filtering of the eye should bias the cone excitations for broader spectra, requiring a shift in the stimulus peak to match the cone excitations for the narrow spectra. Yet constant hues instead corresponded to constant peaks, and thus different relative cone responses, for the different bandwidths (Figure 4). This again suggests that the visual system ties constant hues to a constant property of the physical stimulus (e.g. the estimated peak of the spectrum) rather than to constant properties of the neural response. (In a related vein, Knoblauch and Shevell showed that a single cone class need not signal a fixed hue [80].) The Gaussian prediction is only approximate, and in particular poorly fits the hue matches at longer wavelengths [81]. However, it provides a novel perspective on a classic color phenomenon – the Abney Effect – in which hue does change as saturation is varied [82]. The difference is that in this case the desaturation is produced by adding a fixed background stimulus, rather than varying the stimulus bandwidth. The Abney effect has been attributed to a nonlinearity in color mechanisms, without a functional benefit. However, the observed hue shifts roughly follow the prediction that the visual system uses the limited information from the cones to approximate the spectrum as a Gaussian and compensate for this – and in this sense applies the right correction for the wrong stimulus. Mizokami and Webster [83] also showed that this is a reasonable inference for the visual system to embody, because a Gaussian fits natural spectra as well as conventional linear models with the same number of parameters.
Figure 4.
Compensation of hue for saturation. Left: Because of filtering by the spectral sensitivity of the eye, as the bandwidth of a Gaussian spectrum is increased, the peak wavelength of the spectrum must be shifted to maintain the same relative cone excitations (dashed lines). However, the wavelength for unique blue settings for different observers remains constant for the narrow (filled symbol) or broad bandwidth (unfilled symbols). Right: Desaturating a narrowband light by instead adding a fixed background spectrum leads to a fixed filtering pattern by the visual system. In this case (mis)applying the Gaussian correction requires a shift in the dominant wavelength (unfilled lines) that roughly predicts the curved hue loci in the Abney Effect (filled dashed lines) (after [79]; Abney Effect data from [82]).
Compensating for contrast.
A final example of compensating for sensitivity limits is in the perception of color contrast. Many authors have noted that because of the overlapping sensitivities of the cones, the differences (which define chromatic contrast) are much smaller than the sums (which define luminance). Yet sensitivity to chromatic contrast is correspondingly higher, offsetting this physiological difference [84]. Moreover, the cone contrasts at which luminance and color or different chromatic axes appear equally strong are roughly consistent with the contrasts from natural scenes, suggesting that the visual system tends to balance or “sphere” the responses for natural color gamuts [85]. An intriguing extension of these ideas is to the color percepts of anomalous trichromats. These individuals retain three cone types but with a smaller separation in the peaks of their longer-wave cones (with the anomalous pigment shifted toward the peak of the normal L or M cone). The difference signals from their longwave pairs are consequently much smaller, yet in principle these observers could also amplify postreceptoral responses to compensate for the loss [86]. Studies of color salience in fact suggest that at least some anomalous observers experience reddish-greenish differences as more similar to normal than their weakened cone contrasts predict [87, 88].
4. Blue and yellow in the world
An alternative to tying the unique hues to a unique state in the brain is that they might reflect unique properties of the environment. For example, on average the stimuli that appear pure blue and yellow lie close to the daylight locus [89]. Thus these hues may appear special because they correspond to salient and potentially learned characteristics of the world, regardless of how they are represented in the neural architecture (though as discussed below, there are large individual differences in the blue and yellow loci which are difficult to attribute simply to differences in the observers’ light environments). Similarly, red has been associated with ripe fruit [90] or blood [91]. In this case the percept tends to align with the LvsM axis and is thus a unique hue that does have a potential unique neural signature, and one which has been implicated in the evolution of LvsM dimension of trichromacy [90–92]. However as noted above variability in unique red also exceeds the inter-observer variability in the angle of the LvsM axis. The unique hues have also been tied to special properties in the reflectance functions of natural spectra as sampled by the cones [93, 94], though this idea has been challenged [95]. Finally, cross-cultural studies continue to explore the role that communication may play in shaping color categories [96–100].
Compensating color perception for the sensitivity limits of the observer also emphasizes that the salient properties of color perception are set by properties of the world. One mechanism mediating these adjustments is adaptation, which regulates sensitivity according to the stimuli we are exposed to. Adaptation is a fundamental process in all sensory systems and adjusts to many of the stimulus attributes we experience [63]. Adaptation to color involves a number of distinct mechanisms, but two prominent adjustments are adaptation to the average of the stimulus (primarily retinal) and to the variance or contrast in the stimulus (primarily cortical) [101, 102]. The latter are the adaptation effects probed by Krauskopf et al. [21].
Based on these mechanisms, white or gray may simply correspond to the average spectral stimulus we are exposed to, since light adaptation in the photoreceptors will over time equate their sensitivity for the average [103]. Unique yellow has also been accounted for by an adaptation to the average spectrum that equates the relative responses in the L and M cones [77, 104]. However, what constitutes an average in both cases is unclear. Natural images can be roughly partitioned between earth and sky. Terrestrial colors are yellowish and the achromatic average thus requires some contribution from the sky, yet we presumably spend more time looking at the ground [105–107]. If adaptation is local, then this can account for the compensation for sensitivity variations across the visual field, but only if different retinal locations are exposed to the same average stimulus. It is instead possible that the upper and lower visual fields are adapted somewhat differently because of the differences in their spectral diet [105].
Adaptation to contrast can similarly explain how the visual system might compensate for the sensitivity differences for luminance and chromatic contrast. If chromatic signals are inherently weaker, then the gain of chromatic channels may adjust to offset this [107]. Again the same principle predicts that color-anomalous observers might further amplify the weakened chromatic contrasts afforded by their cones [86]. An additional sign of the general effects of adaptation on color appearance is the bias in visual sensitivity for blue and yellow (Figure 5). The cardinal axes have been postulated to represent an efficient representation of chromatic information, on the assumption that they are aligned with the principal axes of variation in natural color distributions. While this is approximately true for scenes dominated by lush foliage [108], most natural color gamuts tend to have wider variation along bluish-yellowish axes [109], a bias that is also present in paintings [110, 111]. For these distributions, color coding in the retina and geniculate therefore seems surprisingly inefficient, because the signals carried by the LvsM and SvsLM pathways are often strongly correlated [109]. As noted natural daylight also varies along the blue-yellow axis. Thus the visual system may be habitually exposed to stronger contrasts along the negative blue-yellow axis of the cardinal-axis space than for other color directions, predicting larger sensitivity losses for this axis [109]. This stimulus bias shows up as reduced sensitivity for blue-yellow contrasts in a variety of tasks [112–114]. Notably, it is also a common feature of uniform color systems or color spaces. That is, in these spaces stronger bluish-yellowish stimulus contrasts are required to produce perceptually equivalent changes [115]. The close correspondences between color in the environment and color coding – mediated by adaptation – suggest that uniform color spaces could in fact be developed by starting with natural image statistics and then asking how the visual system would adjust to these. Smet et al. [116] developed a model of color vision based on these principles. The model predicts perceptual color differences nearly as well as current uniform color metrics which are instead derived by fitting empirical measurements of color discrimination.
Figure 5.
Blue-yellow bias in the world and perception. Left: The color gamuts of natural scenes tend to show greater variation along the bluish-yellowish axis of the LvsM and SvsLM space. Right: Samples from the Munsell Color Palette projected into the cardinal axis space show that equal perceptual steps require larger chromatic contrast changes along the bluish-yellowish-orange axes (after [117]).
An important consequence of these adaptation effects is that they adjust the visual system to match the world, and this again works to discount the properties of the observer. Simple mechanisms like this can thus allow our color percepts to remain stable despite spatial or temporal variations within the individual. At the same time, they should tend to equate some aspects of color experience across observers. That is – old and young adults, and potentially normal and anomalous trichromats – may share more similar color percepts than expected from their optical and neural differences simply because they are adapted to a similar color environment [63].
However, the same processes that drive observers toward convergent percepts when they are exposed to the same environment should lead to divergent percepts when the environment changes. The color statistics of images show large changes across environments, both in the mean color and the color gamut. For example, wet environments tend to be dominated by green foliage while dry environments are more yellow, and there are corresponding swings in the dominant axis of the distributions [109]. Substantial variations also occur over time because of seasonal changes [106]. Thus individuals living in different environments should be adapted and experience color differently, and their percepts might even cycle with the seasons [118].
To explore these effects, we developed a model to simulate how adaptation should alter color appearance across different environments [86, 119, 120]. The model assumed that the photoreceptors adapt to the mean of the color gamut while cortical mechanisms selectively adapt to the contrasts, and that adaptation adjusts the gains of the mechanisms so that within each, the average response in the current environment equals the response to a reference environment. The algorithm is simple and not intended as an actual model of human vision because it does not, for example, incorporate spatial structure or the fact that scenes are not uniformly sampled. It can nevertheless provide intuitions about how colors in a scene should appear to observers immersed in a given environment. A further advantage is that the model can simulate color appearance under theoretically complete and thus potentially very long-term adaptation (Figure 6). These “adapted images” can then be used in psychophysical studies to ask what an observer can see or do within an environment that they could not do before adapting [119]. For example, one consequence of adaptation is to reduce the contrast or salience of the dominant or expected characteristics of the scene, while increasing the relative contrast or salience of more novel colors. This predicts that observers should become more efficient at searching for novel colors or statistical outliers in a color distribution if they are first adapted to the distribution, a performance improvement that has also been found in empirical measurements of adaptation and visual search [114].
Figure 6.
Swapping swatches [111]. Top: Starry Night by Munch and Man with a Hoe by Millet (digital images courtesy of Getty’s Open Content Program). Bottom: The palettes in each image have been “adapted” in a model visual system so that the average response of each color mechanism is equated for the average response of the same mechanism to the alternate original image.
5. Relationships between color categories
While these adaptation effects thus play an important role in shaping whether two observers experience color in similar or different ways, there may nevertheless be more fundamental factors contributing to individual differences in color appearance. As Figure 1 illustrates, the stimuli that color-normal observers select for the unique or binary hues shows marked variability [27, 121], to the point where one observer’s best example of orange could be close to another’s yellow or red [10]. Differences in unique hues and focal colors have also been found across different linguistic groups [122]. However the differences among individuals within each language are often far greater than the mean differences between populations [122–124].
A striking feature of these individual differences is that the focal choices for different color categories are uncorrelated [27, 123]. Thus while blue and yellow or red and green may be phenomenal opposites, how two observers differ in their unique blue choices does not predict how they will differ for yellow. Binary hues like orange and purple also vary across observers in ways that cannot be predicted from the settings for the unique hue primaries for which they are presumed to be composed [10]. This independence is inconsistent with the biases predicted by variations in the observers’ state of adaptation or their spectral sensitivity, which as noted above largely fails to account for normal variations in color appearance. The effects of adaptation and sensitivity variations also tend to be spectrally broad, and thus would predict correlated influences across different hues [27]. Thus the nature of these inter-observer variations in color categories remain enigmatic.
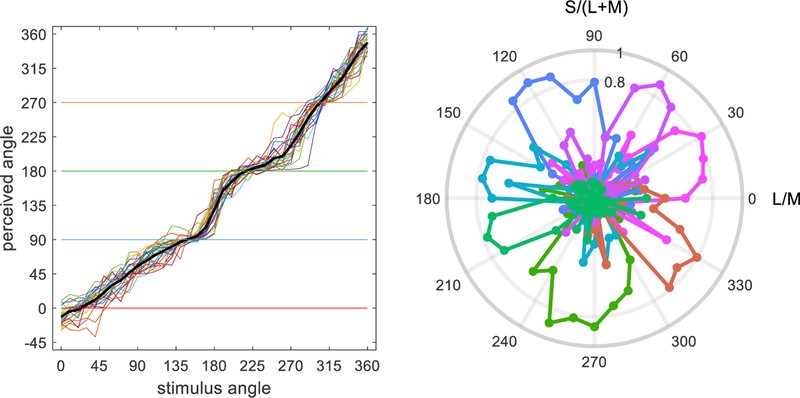
Emery et al. [125, 126] finely sampled individual differences in color appearance using a hue scaling task, in which a given hue is decomposed into the perceived proportion of the red-green or blue-yellow components [127]. For example a balanced purple can be described as 50% blue and 50% red. Settings were collected for 26 observers who scaled 36 stimuli spanning the LvsM and SvsLM plane, and factor analysis was then used to assess the underlying sources of the individual differences. A factor analysis or principal components analysis extracts the latent dimensions that best account for the pattern of correlations among the observed set of variables. These analyses are well suited to variables that reflect quantitative stimulus dimensions like wavelength or spatial frequency, and can potentially identify the precise mechanisms controlling the responses and how much they vary [50, 128].
The hue scaling resulted in a pattern of 7–8 factors that each accounted for the variations only over a fairly narrow range of hues [126] (Figure 7). Moreover, none of the factors exhibited a biphasic pattern that would be expected if they represented a color-opponent dimension. These results are surprising given that the task required the observers to decompose each hue into its red-green and blue-yellow components, and moreover could only use one color of each opponent pair to describe the hue. Yet these response dimensions did not manifest as the perceptual dimensions controlling the responses. Instead, the pattern could reflect a population code for color, in which each chromatic stimulus is represented by the distribution of activity across mechanisms tuned to different directions in the chromatic plane. The variations across observers would then suggest that different hues are decoded independently, perhaps because we learn the population responses corresponding to different color categories like red and orange independently. Why there are 7–8 factors and not 4 or 16 is unclear, but it is tempting to speculate that this is because English speakers tend to describe the hue circle in terms of 8 categories corresponding to the unique and binary hues. When observers completed a second task where they not only scaled the hues but also had to label them by one of 8 terms, the analysis revealed some factors that accounted for both tasks, while others that were specific to scaling or naming [125]. This indicates that there may not be a strict correspondence between how the stimuli were perceived and verbally labeled. In any case, the fact that multiple, narrowly tuned factors emerged for both tasks suggests that the different hue categories or regions exist somewhat free-floating, rather than derived from and bound to an underlying color-opponent structure.
Figure 7.
Individual differences in hue scaling. Left: Perceived hue angle (blue-yellow / red-green) as a function of the stimulus angle in the cone-opponent space, for 26 observers. Right: Variations across the observers revealed 7 systematic factors narrowly tuned to different stimulus angles (after [129]).
6. Numbering by color
Further evidence that the unique hues or other color categories differ not only quantitatively but also conceptually comes from studies where observers are asked to infer the metrical relationships between different colors. Specifically, to what extent do we represent color in a way that we can readily perceive the coordinate relationships between colors based on the three-dimensional representation imposed by the cones? On the one hand, similarity judgments appear intuitive and can be used to recover the two-dimensional structure of hue variations. In fact this provided the initial validation of multidimensional scaling [130]. However, that color similarities can be accounted for by distances in a representational color “space” does not indicate how an individual can judge the relationships between coordinates within the space. For example, Wuerger et al. showed that such judgments are not consistent with a Euclidean representation [131]. Recently, Ennis and Zaidi [132] reported that similarities based on perceived midpoints between different color pairs could reflect an affine structure, but only if observers were explicitly instructed to judge the color differences in terms of the red-green and blue-yellow opponent axes. If left to their own devices they instead showed much less evidence for an underlying geometric representation.
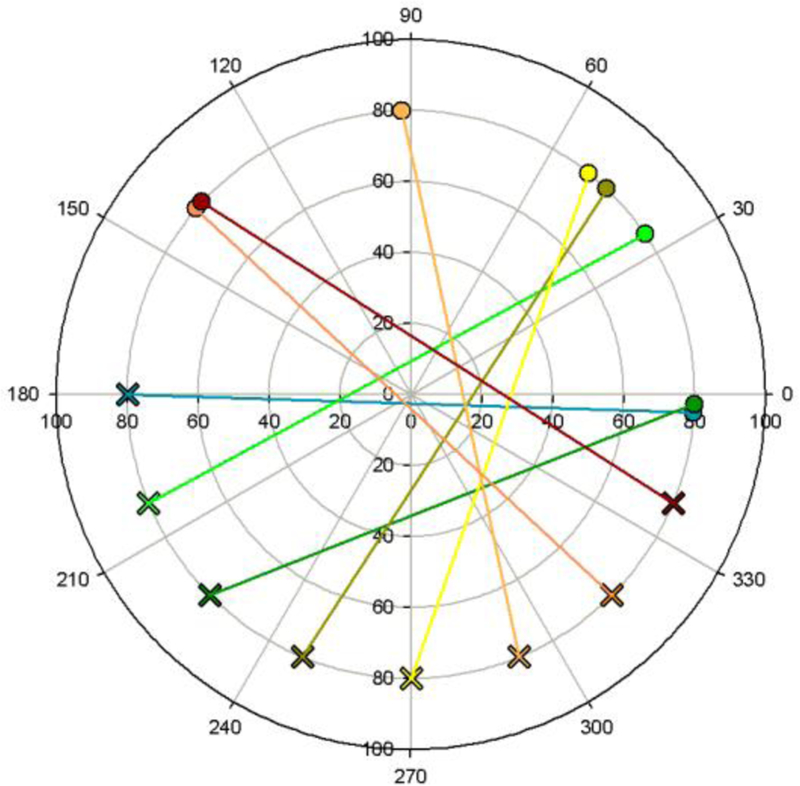
We examined the ability to infer the metrical relationships between colors in an ensemble coding task. Many studies have suggested that the visual system can reliably extract the summary statistics of a distribution of stimuli, such as their mean or variance, and that the mean estimate is often more robust than for the individual items [133]. This ensemble coding has now been demonstrated for a wide range of stimuli, including low-level features like motion or orientation and high-level features like facial expressions. Color is an example where the mean provides especially useful information, for example to the color of the illuminant. Several studies have now examined ensemble coding for color [134–137]. Sensitivity to the mean of a color distribution is high if the differences between the colors is not too large (e.g. within or between adjacent color categories), though it can be systematically biased, for example toward the more saturated elements [138, 139]. However, for very different colors the task becomes difficult. For example, Figure 8 shows results from an unpublished study where subjects were shown one hue and asked to choose the complementary hue, such that the average would be gray. We originally approached this with the goal of exploring which “space” the observer used to estimate the average, for what is complementary in one representation (e.g. the cardinal axes) is different from another (e.g. perceptual opponency). However, we instead found that observers without formal training in color vision found the task meaningless, for they had no perceptual intuition that the average of two saturated hues should be achromatic. In related work MacLeod, Pallett, and Krizay [140] found that green appeared no more different from red than a blue or yellow.
Figure 8.
Inferring color relationships. Settings for an individual observer asked to adjust the hue of a test stimulus (circles) so that it was complementary to a reference stimulus.
In a second task, we displayed a color distribution with a fixed gamut along the LvsM and SvsLM axes and asked observers to adjust the mean to be achromatic. In this case, increasing the variance along either axis reduced sensitivity along either axis [141]. For example, balancing the colors along the LvsM axis was harder when we increased the variance along the SvsLM axis or vice versa. This nonselective interference is in contrast to the strong selectivity for these axes in tasks like noise masking [142], but is consistent with the possibility that increasing the differences between the colors made it harder to judge their relationships, because the colors are encoded as qualitatively different categories rather than as points in a metrical space. Note that this may be very different from other visual attributes. For example one can readily judge the average trajectory of a field of moving dots [143], and this feels like an intuitively easy judgment, like estimating the wind direction in a snow flurry. For color it does not, and observers may instead have to indirectly infer the average (e.g. from the relative saturation of the colors). This raises important general questions about ensemble coding and which visual dimensions in fact allow an explicit representation of summary statistics, and how this depends on the nature of the visual representations for different attributes.
7. Blue-yellow asymmetries
Thus far we have considered blue and yellow as if they are – if not opposites – at least equals in the perception of color. However, a number of recent studies have pointed to important differences in how the visual system responds to blue vs. yellow hues and to possible differences in the inferences about color associated with these hues. In measures of scenes shown under different illuminants, observers are less sensitive to an illuminant change along a bluish axis than along a yellow or red or green axis [144–147]. This suggests greater constancy for blue illuminants, since their variation is less likely to visibly alter scene color.
Winkler et al. [148] compared color percepts in images shown with blue or yellow tints. The study was inspired by a comment from Lothar Spillmann to John Werner that the photographic negative of a person appeared achromatic, yet the afterimage from staring at the negative seemed strongly colored (personal communication). Since the afterimage is the complement, why should it appear more saturated? Winkler et al. found that the asymmetry was due to the yellowish skin tones appearing more colorful than the colorimetrically equivalent blue tones. However, this did not reflect knowledge about the specific object in the image, because the blue-yellow differences remained when the pixels were scrambled. The differences also persisted for uniform color patches. If the colors were shown as increments or decrements on a background, then much higher chromatic contrast had to be added in the blue than the yellow direction in order for the patch to be described as a color and not white. The asymmetries were specific to the blue-yellow axis, with no difference in the relative strength of reddish vs. greenish hues. Winkler et al. also showed that these biases occurred for many different bluish objects. For example silver coins and steel pots appear strongly golden or copper when the chromatic contrast was inverted (Figure 9). They also occur for the viral image of #thedress, which is seen by different people as either blue-black or white-gold. Inverting the image color causes the stripes to appear yellow with high consensus [148, 149].
Figure 9.
Blue light and yellow objects. When the original bluish hue of the coins is amplified the light appears bluer while the coins tend to continue to appear silver. In the complementary images the coins instead appear to become more golden while the lighting remains neutral (after [148]).
A potential explanation for these effects is that individuals do not vary in their intrinsic sensitivity to blue and yellow, but tend to attribute bluish tints to the illuminant while yellowish tints to the object [148]. Consistent with this, when the chromatic contrast of an image was amplified, in blue-tinted images the lighting appeared to be changing while in the yellow complements the object color instead seemed to change. Discounting blue as the illuminant color may decrease its effective contrast, and Retter et al. [150] showed that this reduced contrast is measureable as an asymmetry in the EEG response to an alternation in the original and inverted dress image. In fact how observers classified the dress could be predicted with more than 80% accuracy based on the topography of the EEG responses. The different attributions might also account for why yellows are more salient than blues in a visual search task [12].
These asymmetries may reflect general inferences about material and lighting. In natural scenes shadows tend to be blue from the diffuse skylight, and the blue chromaticity of shadows can be strongly discounted [151]. Conversely, recent analyses suggest that objects tend to be warm colors [152]. Thus the visual system may have a prior to ascribe blue to the lighting and yellow to the object. It is possible that this is why (to me) the world itself appears to change color dramatically at sunset – when the sun’s warmer rays are misattributed to the objects they illuminate (Figure 10).
Figure 10.
A tower in Tallinn Estonia, shortly after the 2019 ICVS conference in Riga. The orange hue is from the light of the setting sun. (See also [153])
Acknowledgments.
I am very grateful to G.H. Jacobs, J.D. Mollon, S.K. Shevell, and J.S. Werner for comments on the manuscript, and to the many colleagues and students who contributed to this work.
Supported by NIH EY-010834.
Footnotes
Disclosures.
The author declares no conflict of interest.
References
- 1.Stockman A and Sharpe LT, “Cone spectral sensitivities and color matching,” in Color vision: From genes to perception (1999), pp. 53–88. [Google Scholar]
- 2.Hurvich LM and Jameson D, “An opponent-process theory of color vision,” Psychological review 64, Part 1, 384–404 (1957). [DOI] [PubMed] [Google Scholar]
- 3.Webster MA, “Color vision,” in Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience 2 (2018), pp. 1–42. [Google Scholar]
- 4.Neitz J and Neitz M, “The genetics of normal and defective color vision,” Vision research 51, 633–651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs GH, “Photopigments and the dimensionality of animal color vision,” Neuroscience and biobehavioral reviews 86, 108–130 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Jordan G, Deeb SS, Bosten JM, and Mollon JD, “The dimensionality of color vision in carriers of anomalous trichromacy,” Journal of vision 10, 12 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Jameson KA, Highnote SM, and Wasserman LM, “Richer color experience in observers with multiple photopigment opsin genes,” Psychonomic bulletin & review 8, 244–261 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Stockman A, “Cone fundamentals and CIE standards,” Current Opinion in Behavioral Sciences 30, 87–93 (2019). [Google Scholar]
- 9.Brainard DH and Stockman A, “Colorimetry,” in OSA Handbook of Optics, Bass M, ed. (2010), pp. 10–11. [Google Scholar]
- 10.Malkoc G, Kay P, and Webster MA, “Variations in normal color vision. IV. Binary hues and hue scaling,” Journal of the Optical Society of America. A, Optics, image science, and vision 22, 2154–2168 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Bosten JM and Boehm AE, “Empirical evidence for unique hues?,” Journal of the Optical Society of America. A, Optics, image science, and vision 31, A385–393 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wool LE, Komban SJ, Kremkow J, Jansen M, Li X, Alonso JM, and Zaidi Q, “Salience of unique hues and implications for color theory,” Journal of vision 15(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danilova MV and Mollon JD, “Is discrimination enhanced at the boundaries of perceptual categories? A negative case,” Proceedings. Biological sciences / The Royal Society 281, 20140367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witzel C and Gegenfurtner KR, “Are red, yellow, green, and blue perceptual categories?,” Vision research 151, 152–163 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Danilova MV and Mollon JD, “Foveal color perception: minimal thresholds at a boundary between perceptual categories,” Vision research 62, 162–172 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Hurvich LM and Jameson D, “Some quantitative aspects of an opponent-colors theory. II. Brightness, saturation, and hue in normal and dichromatic vision,” Journal of the Optical Society of America 45, 602–616 (1955). [DOI] [PubMed] [Google Scholar]
- 17.Werner JS and Wooten BR, “Opponent chromatic mechanisms: relation to photopigments and hue naming,” Journal of the Optical Society of America 69, 422–434 (1979). [DOI] [PubMed] [Google Scholar]
- 18.Lee BB, “Color coding in the primate visual pathway: a historical view,” Journal of the Optical Society of America. A, Optics, image science, and vision 31, A103–112 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Baden T and Osorio D, “The retinal basis of vertebrate color vision,” Annual Review of Vision Science 5, 177–200 (2019). [DOI] [PubMed] [Google Scholar]
- 20.De Valois RL, Abramov I, and Jacobs GH, “Analysis of response patterns of LGN cells,” Journal of the Optical Society of America 56, 966–977 (1966). [DOI] [PubMed] [Google Scholar]
- 21.Krauskopf J, Williams DR, and Heeley DW, “Cardinal directions of color space,” Vision research 22, 1123–1131 (1982). [DOI] [PubMed] [Google Scholar]
- 22.Derrington AM, Krauskopf J, and Lennie P, “Chromatic mechanisms in lateral geniculate nucleus of macaque,” Journal of Physiology 357, 241–265 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin PR, White AJ, Goodchild AK, Wilder HD, and Sefton AE, “Evidence that blue-on cells are part of the third geniculocortical pathway in primates,” European Journal of Neuroscience 9, 1536–1541 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Dacey DM, “Parallel pathways for spectral coding in primate retina,” Annual review of neuroscience 23, 743–775 (2000). [DOI] [PubMed] [Google Scholar]
- 25.MacLeod DI and Boynton RM, “Chromaticity diagram showing cone excitation by stimuli of equal luminance,” Journal of the Optical Society of America 69, 1183–1186 (1979). [DOI] [PubMed] [Google Scholar]
- 26.Wuerger SM, Atkinson P, and Cropper S, “The cone inputs to the unique-hue mechanisms,” Vision research 45, 3210–3223 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Webster MA, Miyahara E, Malkoc G, and Raker VE, “Variations in normal color vision. II. Unique hues,” Journal of the Optical Society of America. A, Optics, image science, and vision 17, 1545–1555 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Kanazawa S, Yamaguchi MK, and Kuriki I, “Cortical response to categorical color perception in infants investigated by near-infrared spectroscopy,” Proceedings of the National Academy of Sciences of the United States of America 113, 2370–2375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skelton AE, Catchpole G, Abbott JT, Bosten JM, and Franklin A, “Biological origins of color categorization,” Proceedings of the National Academy of Sciences of the United States of America 114, 5545–5550 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster MA, Miyahara E, Malkoc G, and Raker VE, “Variations in normal color vision. I. Cone-opponent axes,” Journal of the Optical Society of America. A, Optics, image science, and vision 17, 1535–1544 (2000). [DOI] [PubMed] [Google Scholar]
- 31.De Valois RL and De Valois KK, “A multi-stage color model,” Vision research 33, 1053–1065 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Forder L, Bosten J, He X, and Franklin A, “A neural signature of the unique hues,” Scientific reports 7, 42364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoughton CM and Conway BR, “Neural basis for unique hues,” Current Biology 18, R698–699 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Brouwer GJ and Heeger DJ, “Decoding and reconstructing color from responses in human visual cortex,” The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 13992–14003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neitz J and Neitz M, “Evolution of the circuitry for conscious color vision in primates,” Eye (Lond) 31, 286–300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson SS, Neitz M, and Neitz J, “Reconciling Color Vision Models With Midget Ganglion Cell Receptive Fields,” Frontiers in neuroscience 13, 865 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabesan R, Schmidt BP, Tuten WS, and Roorda A, “The elementary representation of spatial and color vision in the human retina,” Science advances 2, e1600797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krauskopf J, Williams DR, Mandler MB, and Brown AM, “Higher order color mechanisms,” Vision research 26, 23–32 (1986). [DOI] [PubMed] [Google Scholar]
- 39.Webster MA and Mollon JD, “Changes in colour appearance following post-receptoral adaptation,” Nature 349, 235–238 (1991). [DOI] [PubMed] [Google Scholar]
- 40.Webster MA and Mollon JD, “The influence of contrast adaptation on color appearance,” Vision research 34, 1993–2020 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Atick JJ, Li Z, and Redlich AN, “What does post-adaptation color appearance reveal about cortical color representation?,” Vision research 33, 123–129 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Zaidi Q and Shapiro AG, “Adaptive orthogonalization of opponent-color signals,” Biological cybernetics 69, 415–428 (1993). [PubMed] [Google Scholar]
- 43.Gegenfurtner KR, “Cortical mechanisms of colour vision,” Nature reviews. Neuroscience 4, 563–572 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Lennie P, Krauskopf J, and Sclar G, “Chromatic mechanisms in striate cortex of macaque,” The Journal of neuroscience : the official journal of the Society for Neuroscience 10, 649–669 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krauskopf J, “Higher order color mechanisms,” in Color Vision: From genes to perception, Gegenfurtner K and Sharpe LT, eds. (Cambridge University Press, Cambridge, 1999), pp. 303–316. [Google Scholar]
- 46.Eskew RT Jr., “Higher order color mechanisms: a critical review,” Vision research 49, 2686–2704 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Klauke S and Wachtler T, ““Tilt” in color space: Hue changes induced by chromatic surrounds,” Journal of vision 15, 17 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Webster MA and Malkoc G, “Color-luminance relationships and the McCollough effect,” Perception & psychophysics 62, 659–672 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Zaidi Q and Conway B, “Steps towards neural decoding of colors,” Current Opinion in Behavioral Sciences 30, 169–177 (2019). [Google Scholar]
- 50.Webster MA and MacLeod DI, “Factors underlying individual differences in the color matches of normal observers,” Journal of the Optical Society of America. A, Optics and image science 5, 1722–1735 (1988). [DOI] [PubMed] [Google Scholar]
- 51.Asano Y, Fairchild MD, and Blonde L, “Individual Colorimetric Observer Model,” PloS one 11, e0145671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster DH, “Color constancy,” Vision research 51, 674–700 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Webster MA and Leonard D, “Adaptation and perceptual norms in color vision,” Journal of the Optical Society of America A 25, 2817–2825 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beer D, Wortman J, Horwitz G, and MacLeod D, “Compensation of white for macular filtering [Abstract],” Journal of vision 5, 282a (2005). [Google Scholar]
- 55.Werner JS and Schefrin BE, “Loci of achromatic points throughout the life span,” Journal of the Optical Society of America A 10, 1509–1516 (1993). [DOI] [PubMed] [Google Scholar]
- 56.Wuerger SM, “Colour constancy across the life span: Evidence for compensatory mechanisms,” PloS one 8(5), e63921 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delahunt PB, Webster MA, Ma L, and Werner JS, “Long-term renormalization of chromatic mechanisms following cataract surgery,” Visual neuroscience 21, 301–307 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tregillus KE and Engel SA, “ Long-term adaptation to color,” Current Opinion in Behavioral Sciences 30, 116–121 (2019). [Google Scholar]
- 59.O’Regan JK and Noe A, “A sensorimotor account of vision and visual consciousness,” The Behavioral and brain sciences 24, 939–973; discussion 973–1031 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Chichilnisky EJ and Wandell BA, “Photoreceptor sensitivity changes explain color appearance shifts induced by large uniform backgrounds in dichoptic matching,” Vision research 35, 239–254 (1995). [DOI] [PubMed] [Google Scholar]
- 61.MacLeod DI, Williams DR, and Makous W, “A visual nonlinearity fed by single cones,” Vision research 32, 347–363 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Zaidi Q, Ennis R, Cao D, and Lee B, “Neural locus of color afterimages,” Current biology : CB 22, 220–224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webster MA, “Visual adaptation,” Annual Review of Vision Science 1, 547–567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valentine T, Lewis MB, and Hills PJ, “Face-space: A unifying concept in face recognition research,” Q J Exp Psychol (Hove), 1–24 (2015). [DOI] [PubMed]
- 65.Webster MA and MacLeod DIA, “Visual adaptation and face perception,” Philosophical transactions of the Royal Society of London. Series B, Biological sciences 366, 1702–1725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radhakrishnan A, Dorronsoro C, Sawides L, Webster MA, and Marcos S, “A cyclopean neural mechanism compensating for optical differences between the eyes,” Current biology : CB 25, R188–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawides L, de Gracia P, Dorronsoro C, Webster MA, and Marcos S, “Vision is adapted to the natural level of blur present in the retinal image,” PloS one 6, e27031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brainard DH, Roorda A, Yamauchi Y, Calderone JB, Metha A, Neitz M, Neitz J, Williams DR, and Jacobs GH, “Functional consequences of the relative numbers of L and M cones,” Journal of the Optical Society of America. A, Optics, image science, and vision 17, 607–614 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Miyahara E, Pokorny J, Smith VC, Baron R, and Baron E, “Color vision in two observers with highly biased LWS/MWS cone ratios,” Vision research 38, 601–612 (1998). [DOI] [PubMed] [Google Scholar]
- 70.Jordan G and Mollon JD, “Unique hues in heterozygotes for protan and deutan deficiencies,” in Colour Vision Deficiencies XIII, Cavonius CR, ed. (Kluwer, Dordrecht, 1997), pp. 67–76. [Google Scholar]
- 71.Worthey JA and Brill MH, “Heuristic analysis of von Kries color constancy,” Journal of the Optical Society of America. A, Optics and image science 3, 1708–1712 (1986). [DOI] [PubMed] [Google Scholar]
- 72.Webster MA, Halen K, Meyers AJ, Winkler P, and Werner JS, “Colour appearance and compensation in the near periphery,” Proceedings of the Royal Society B-Biological Sciences 277, 1817–1825 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bompas A, Powell G, and Sumner P, “Systematic biases in adult color perception persist despite lifelong information sufficient to calibrate them,” Journal of vision 13(2013). [DOI] [PubMed] [Google Scholar]
- 74.Schefrin BE and Werner JS, “Loci of spectral unique hues throughout the life span,” Journal of the Optical Society of America. A, Optics and image science 7, 305–311 (1990). [DOI] [PubMed] [Google Scholar]
- 75.Mullen KT and Kingdom FA, “Losses in peripheral colour sensitivity predicted from “hit and miss” post-receptoral cone connections,” Vision research 36, 1995–2000 (1996). [DOI] [PubMed] [Google Scholar]
- 76.Murray IJ, Parry NRA, and McKeefry DJ, “Cone opponency in the near peripheral retina,” Visual neuroscience 23, 503–507 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Neitz J, Carroll J, Yamauchi Y, Neitz M, and Williams DR, “Color perception is mediated by a plastic neural mechanism that is adjustable in adults,” Neuron 35, 783–792 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Mollon JD and Jordan G, “On the nature of unique hues,” in John Dalton’s Colour Vision Legacy, Dickenson C, Maurray I, and Carden D, eds. (Taylor and Francis, London, 1997). [Google Scholar]
- 79.Mizokami Y, Werner JS, Crognale MA, and Webster MA, “Nonlinearities in color coding: compensating color appearance for the eye’s spectral sensitivity,” Journal of vision 6, 996–1007 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knoblauch K and Shevell SK, “Relating cone signals to color appearance: failure of monotonicity in yellow/blue,” Visual neuroscience 18, 901–906 (2001). [DOI] [PubMed] [Google Scholar]
- 81.O’Neil SF, McDermott KC, Mizokami Y, Werner JS, Crognale MA, and Webster MA, “Tests of a functional account of the Abney effect,” Journal of the Optical Society of America. A, Optics, image science, and vision 29, A165–173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burns SA, Elsner AE, Pokorny J, and Smith VC, “The Abney effect: chromaticity coordinates of unique and other constant hues,” Vision research 24, 479–489 (1984). [DOI] [PubMed] [Google Scholar]
- 83.Mizokami Y and Webster MA, “Are Gaussian spectra a viable perceptual assumption in color appearance?,” Journal of the Optical Society of America. A, Optics, image science, and vision 29, A10–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MacLeod DIA, “Colour discrimination, colour constancy, and natural scene statistics (The Verriest Lecture),” in Normal and Defective Colour Vision, Mollon JD, Pokorny J, and Knoblauch K, eds. (Oxford University Press, London, 2003). [Google Scholar]
- 85.McDermott KC and Webster MA, “The perceptual balance of color,” Journal of the Optical Society of America. A, Optics, image science, and vision 29, A108–117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webster MA, Juricevic I, and McDermott KC, “Simulations of adaptation and color appearance in observers with varying spectral sensitivity,” Ophthalmic Physiol Opt 30, 602–610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Regan BC and Mollon JD, “The relative salience of the cardinal axes of colour space in normal and anomalous trichromats” in Colour Vision Deficiencies Cavonius CR, ed. (Dordrecht, Kluwer, 1997), pp. 261–270. [Google Scholar]
- 88.Boehm AE, MacLeod DI, and Bosten JM, “Compensation for red-green contrast loss in anomalous trichromats,” Journal of vision 14(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mollon JD, “Monge (The Verriest Lecture),” Visual neuroscience 23, 297–309 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Regan BC, Julliot C, Simmen B, Vienot F, Charles-Dominique P, and Mollon JD, “Frugivory and colour vision in Alouatta seniculus, a trichromatic platyrrhine monkey,” Vision research 38, 3321–3327 (1998). [DOI] [PubMed] [Google Scholar]
- 91.Changizi MA, Zhang Q, and Shimojo S, “Bare skin, blood and the evolution of primate colour vision,” Biology Letters 2, 217–221 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Osorio D and Vorobyev M, “Colour vision as an adaptation to frugivory in primates,” Proceedings. Biological sciences / The Royal Society 263, 593–599 (1996). [DOI] [PubMed] [Google Scholar]
- 93.Philipona DL and O’Regan JK, “Color naming, unique hues, and hue cancellation predicted from singularities in reflection properties,” Visual neuroscience 23, 331–339 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Witzel C, Cinotti F, and O’Regan JK, “What determines the relationship between color naming, unique hues, and sensory singularities: Illuminations, surfaces, or photoreceptors?,” Journal of vision 15, 19 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Johnson K and Wright W, “Reply to Philipona and O’Regan,” Visual neuroscience 25, 221–224 (2008). [DOI] [PubMed] [Google Scholar]
- 96.Lindsey DT, Brown AM, Brainard DH, and Apicella CL, “Hunter-Gatherer Color Naming Provides New Insight into the Evolution of Color Terms,” Current biology : CB 25, 2441–2446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gibson E, Futrell R, Jara-Ettinger J, Mahowald K, Bergen L, Ratnasingam S, Gibson M, Piantadosi ST, and Conway BR, “Color naming across languages reflects color use,” Proceedings of the National Academy of Sciences of the United States of America 114, 10785–10790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kay P and Regier T, “Language, thought and color: recent developments,” Trends in cognitive sciences 10, 51–54 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Abbott JT, Griffiths TL, and Regier T, “Focal colors across languages are representative members of color categories,” Proceedings of the National Academy of Sciences of the United States of America 113, 11178–11183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park J, Tauber S, Jameson KA, and Narens L, “The Evolution of Shared Concepts in Changing Populations. ,” Review of Philosophy and Psychology, 1–20 (2018).30881528
- 101.Webster MA, “Human colour perception and its adaptation,” Network: Computation in Neural Systems 7, 587–634 (1996). [Google Scholar]
- 102.Webster MA and Mollon JD, “Colour constancy influenced by contrast adaptation,” Nature 373, 694–698 (1995). [DOI] [PubMed] [Google Scholar]
- 103.Brainard DH and Wandell BA, “Asymmetric color matching: how color appearance depends on the illuminant,” Journal of the Optical Society of America. A, Optics and image science 9, 1433–1448 (1992). [DOI] [PubMed] [Google Scholar]
- 104.Pokorny J and Smith VC, “L/M cone ratios and the null point of the perceptual red/green opponent system,” Farbe 34, 53–57 (1987). [Google Scholar]
- 105.Juricevic I and Webster MA, “Variations in normal color vision. V. Simulations of adaptation to natural color environments,” Visual neuroscience 26, 133–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Webster MA, Mizokami Y, and Webster SM, “Seasonal variations in the color statistics of natural images,” Network 18, 213–233 (2007). [DOI] [PubMed] [Google Scholar]
- 107.von der Twer T and MacLeod DI, “Optimal nonlinear codes for the perception of natural colours,” Network 12, 395–407 (2001). [PubMed] [Google Scholar]
- 108.Ruderman DL, Cronin TW, and Chiao CC, “Statistics of cone responses to natural images: implications for visual coding,” Journal of the Optical Society of America A 15, 2036–2045 (1998). [Google Scholar]
- 109.Webster MA and Mollon JD, “Adaptation and the color statistics of natural images,” Vision research 37, 3283–3298 (1997). [DOI] [PubMed] [Google Scholar]
- 110.Montagner C, Linhares JM, Vilarigues M, and Nascimento SM, “Statistics of colors in paintings and natural scenes,” Journal of the Optical Society of America. A, Optics, image science, and vision 33, A170–177 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Tregillus KE and Webster MA, “Swapping swatches: Adapting to and from an artist’s palette,” Electronic Imaging 16, 1–8 (2016). [Google Scholar]
- 112.Juricevic I, Land L, Wilkins A, and Webster MA, “Visual discomfort and natural image statistics,” Perception 39, 884–899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goddard E, Mannion DJ, McDonald JS, Solomon SG, and Clifford CW, “Combination of subcortical color channels in human visual cortex,” Journal of vision 10, 25 (2010). [DOI] [PubMed] [Google Scholar]
- 114.McDermott KC, Malkoc G, Mulligan JB, and Webster MA, “Adaptation and visual salience,” Journal of vision 10, 17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McDermott KC and Webster MA, “Uniform color spaces and natural image statistics,” Journal of the Optical Society of America. A, Optics, image science, and vision 29, A182–187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smets K, Whitehead L, and Webster MA, “A simple principled approach for understanding uniform color metrics,” Journal of the Optical Society of America A 33, A319–A3311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee KR and Webster MA, “Environmental influences on color vision,” in Encyclopedia of Color Science and Technology (in press). [Google Scholar]
- 118.Welbourne LE, Morland AB, and Wade AR, “Human colour perception changes between seasons,” Current biology : CB 25, R646–647 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Webster MA, “Probing the functions of contextual modulation by adapting images rather than observers,” Vision research (2014). [DOI] [PMC free article] [PubMed]
- 120.Webster MA and Tregillus KE, “Visualizing Visual Adaptation,” JoVE (Journal of Visualized Experiments) 122, e54038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuehni RG, “Variability in unique hue selection: a surprising phenomenon,” Color research and application 29, 158–162 (2004). [Google Scholar]
- 122.Webster MA, Webster SM, Bharadwaj S, Verma R, Jaikumar J, Madan G, and Vaithilingham E, “Variations in normal color vision. III. Unique hues in Indian and United States observers,” Journal of the Optical Society of America. A, Optics, image science, and vision 19, 1951–1962 (2002). [DOI] [PubMed] [Google Scholar]
- 123.Webster MA and Kay P, “Individual and population differences in focal colors,” in Anthropology of Color, MacLaury RE, Paramei GV, and Dedrick D, eds. (John Benjamins, Amsterdam, 2007), pp. 29–53. [Google Scholar]
- 124.Lindsey DT and Brown AM, “World Color Survey color naming reveals universal motifs and their within-language diversity,” Proceedings of the National Academy of Sciences of the United States of America 106, 19785–19790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Emery KJ, Volbrecht VJ, Peterzell DH, and Webster MA, “Variations in normal color vision. VII. Relationships between color naming and hue scaling,” Vision research 141, 66–75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Emery KJ, Volbrecht VJ, Peterzell DH, and Webster MA, “Variations in normal color vision. VI. Factors underlying individual differences in hue scaling and their implications for models of color appearance,” Vision research 141, 51–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gordon J, Abramov I, and Chan H, “Describing color appearance: hue and saturation scaling,” Perception & psychophysics 56, 27–41 (1994). [DOI] [PubMed] [Google Scholar]
- 128.Peterzell DH, “Discovering sensory processes using individual differences: A review and factor analytic manifesto,” Electronic Imaging 2016, 1–11 (2016). [Google Scholar]
- 129.Emery KJ and Webster MA, “Individual differences and their implications for color perception,” Current Opinion in Behavioral Sciences 30, 28–33 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shepard RN, “The analysis of proximities: Multidimensional scaling with an unknown distance function. II. ,” Psychometrika 27, 219–246 (1962). [Google Scholar]
- 131.Wuerger SM, Maloney LT, and Krauskopf J, “Proximity judgments in color space: tests of a Euclidean color geometry,” Vision research 35, 827–835 (1995). [DOI] [PubMed] [Google Scholar]
- 132.Ennis RJ and Zaidi Q, “Geometrical structure of perceptual color space: Mental representations and adaptation invariance,” Journal of vision 19, 1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whitney D and Leib A. Yamanashi, “Ensemble Perception,” Annual review of psychology 69, 105–129 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Maule J, Witzel C, and Franklin A, “Getting the gist of multiple hues: metric and categorical effects on ensemble perception of hue,” Journal of the Optical Society of America. A, Optics, image science, and vision 31, A93–102 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Maule J and Franklin A, “Effects of ensemble complexity and perceptual similarity on rapid averaging of hue,” Journal of vision 15, 6 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Webster J, Kay P, and Webster MA, “Perceiving the average hue of color arrays,” Journal of the Optical Society of America. A, Optics, image science, and vision 31, A283–292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chetverikov A, Campana G, and Kristjansson A, “Representing Color Ensembles,” Psychological science 28, 1510–1517 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Kimura E, “Averaging colors of multicolor mosaics,” Journal of the Optical Society of America. A, Optics, image science, and vision 35, B43–B54 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Kuriki I, “Testing the Possibility of Average-Color Perception from Multi-Colored Patterns,” Optical Review 11, 249–257 (2004). [Google Scholar]
- 140.MacLeod D, Pallett P, and Krizay E, “Are there phenomenal complementaries?,” Journal of vision 8, 1097–1097 (2008). [Google Scholar]
- 141.Rajendran SS and Webster MA, “Color variance and achromatic settings,” Journal of the Optical Society of America A (submitted). [DOI] [PMC free article] [PubMed]
- 142.Sankeralli MJ and Mullen KT, “Postreceptoral chromatic detection mechanisms revealed by noise masking in three-dimensional cone contrast space,” Journal of the Optical Society of America. A, Optics, image science, and vision 14, 2633–2646 (1997). [DOI] [PubMed] [Google Scholar]
- 143.Williams DW and Sekuler R, “Coherent global motion percepts from stochastic local motions,” Vision research 24, 55–62 (1984). [DOI] [PubMed] [Google Scholar]
- 144.Krieger A, Dubin H, Pearce B, Aston S, Hurlbert A, Brainard D, and Radonjic A, “Illumination discrimination depends on scene surface ensemble,” Journal of vision 15, 403 (2015). [Google Scholar]
- 145.Pearce B, Crichton S, Mackiewicz M, Finlayson GD, and Hurlbert A, “Chromatic illumination discrimination ability reveals that human colour constancy is optimised for blue daylight illuminations,” PloS one 9, e87989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weiss D, Witzel C, and Gegenfurtner K, “Determinants of Colour Constancy and the Blue Bias,” i-Perception 8, 2041669517739635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hurlbert A, “Challenges to color constancy in a contemporary light,” Current Opinion in Behavioral Sciences 30, 186–193 (2019). [Google Scholar]
- 148.Winkler AD, Spillmann L, Werner JS, and Webster MA, “Asymmetries in blue-yellow color perception and in the color of ‘the dress’,” Current biology : CB 25, R547–548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gegenfurtner KR, Bloj M, and Toscani M, “The many colours of ‘the dress’,” Current biology : CB 25, R543–544 (2015). [DOI] [PubMed] [Google Scholar]
- 150.Retter TL, Gwinn OS, O’Neil SF, Jiang F, and Webster MA, “Neural correlates of perceptual color inferences as revealed by #thedress,” Journal of vision (in press). [DOI] [PMC free article] [PubMed]
- 151.Churma ME, “Blue shadows: physical, physiological, and psychological causes,” Applied optics 33, 4719–4722 (1994). [DOI] [PubMed] [Google Scholar]
- 152.Rosenthal I, Ratnasingam S, Haile T, Eastman S, Fuller-Deets J, and Conway BR, “Color statistics of objects, and color tuning of object cortex in macaque monkey,” Journal of vision 18, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shevell SK and Kingdom FA, “Color in complex scenes,” Annual review of psychology 59, 143–166 (2008). [DOI] [PubMed] [Google Scholar]