Abstract
Objective:
The present study aims to describe fall-related self-efficacy as perceived by patients with chemotherapy-induced peripheral neuropathy (CIPN). The characteristics of patients associated with low perceived self-efficacy of preventing falls were investigated.
Methods:
A cross-sectional study of four hospitals in Japan. In this study, 100 outpatients who were receiving chemotherapy for cancer and experiencing CIPN were recruited. Participants completed an anonymous, self-administered questionnaire. Self-efficacy was measured with the falling self-efficacy (FSE) scale, and the severity and impact of CIPN was assessed with the Comprehensive Assessment Scale for CIPN in Survivors of Cancer (CAS-CIPN). Data about the demographic information of the patients, cancer diagnosis and treatment, pain and fatigue symptoms, and history of previous falls were collected. Logistic regression analysis was used to assess relationships between variables.
Results:
A total of 81 (81.0%) participants with CIPN completed the questionnaire. They reported experiencing fear of falling during their daily activities, which include the act of standing up, walking, and using the stairs. Small events, such as unstable postures and uncomfortable situations, affected their confidence of preventing falls. Logistic regression revealed that low FSE scores were significantly associated with female sex (P = 0.022), low body mass index (BMI; P = 0.026), and the CAS-CIPN score (P < 0.001).
Conclusions:
Female patients with CIPN and low BMI might have an increased need for enhanced fall-related self-efficacy. A comprehensive assessment of CIPN in patients at risk of low FSE scores is likely to be beneficial.
Keywords: Adverse effects, ambulatory care, chemotherapy, fall, peripheral neuropathy

Introduction
Chemotherapy, which is a systemic therapy, significantly contributes to a successful cancer treatment. The management of adverse effects is important to achieve the complete benefit of chemotherapy because severe adverse effects negatively affect the continuation of therapy and its dose intensity. In this context, the management of toxicities has achieved remarkable improvements. However, chemotherapy-induced peripheral neuropathy (CIPN) is a common dose-limiting toxicity of cancer treatment. Dose reduction and cessation of using relevant chemotherapy agents may reduce the treatment benefit, excluding the “stop and go” strategy for oxaliplatin in patients with colorectal cancer.[1]
The prevalence rate of CIPN within the 1st month after the end of chemotherapy is approximately 68.1% (95% confidence interval = 57.7–78.4). Moreover, the prevalence rates at 3 months and ≥6 months are 60.0% (36.4–81.6) and 30.0% (6.4–53.5), respectively.[2] Although the prevalence of CIPN decreases with time, it may persist after the cessation of chemotherapy. Some patients present with clinically irreversible CIPN.
Few interventions that are effective for the management of CIPN cause pain. There are no pharmacological agents recommended for the prevention of CIPN.[3] Moreover, although the recommendation to use duloxetine is moderate, only few agents are used to treat existing CIPN.[3,4] The efficacy of nonpharmacological interventions has not been established.[5,6] Recently, cryotherapy (frozen gloves and socks)[7] and low-level laser therapy, also known as photobiomodulation,[8] are considered for the prevention and treatment of CIPN, respectively. However, researchers are still obtaining more evidence about the efficacy of such treatments.
Patients with CIPN present with sensory and motor neuropathic symptoms. The typical sensory complaints include numbness, tingling, and pain. Motor difficulties are characterized by muscle weakness and impaired balance. Patients with CIPN described CIPN-related impairments in terms of functional, emotional, and social aspects.[9] Thus far, CIPN adversely affect quality of life in patients with different types of cancer.[10,11,12,13]
CIPN is closely correlated to a patient's activities of daily living, which is a risk factor of falls.[14,15,16] Falls and fall-related injuries are common problems in the older population with cancer.[17] Falls can cause injuries, particularly fractures, and result in significant limitations in activities and impairments in functional ability. Patients may restrict their activities to prevent falling even when injuries do not occur. Therefore, the fear of falling may cause inactivity and functional decline regardless of actual falls. Worse function, greater disability, and higher number of falls as well as symptoms of CIPN last for several years after chemotherapy.[16] These data indicate that patients receiving commonly used chemotherapy agents (e.g., platinum-based agents, taxanes, and vinca alkaloids) present with fear of falling in addition to the symptoms of CIPN during and after treatment.
In this study, we investigated fall-related self-efficacy, which is the belief that individual can prevent falling during activities of daily living. Thus, this study aimed to describe fall-related self-efficacy as perceived by patients with CIPN and to explore the characteristics of patients associated with low perceived self-efficacy of preventing falls.
Methods
Study design and participants
We conducted a cross-sectional questionnaire study. The participants were recruited from four designated cancer care hospitals in Japan, which are accredited by the Ministry of Health, Labor, and Welfare. The inclusion criteria included (1) patients diagnosed with cancer, (2) those treated with chemotherapy in an outpatient setting, (3) those with CIPN symptoms (based on patients' reports), (4) those who were fluent in speaking, reading, and writing Japanese, (5) those with intact cognitive and mental function (as assessed by nurses in each hospital), and (6) those aged 20 years and over. Meanwhile, patients with missing data on measures for fall-related self-efficacy and/or CIPN were excluded from the analysis.
Data collection
Data were collected from January to April 2017. We first invited certified nurse specialists (CNSs) as research collaborators from each hospital. All the CNSs received complete explanations regarding the protocol information, and they supervised recruitment in their own hospitals. In collaboration with others (doctors, nurses, etc.), they verbally explained the study with reference to the explanation form on the scheduled visits of the candidates, who received the self-administered, anonymous questionnaire with an envelope if he/she was interested. When they provided consent to the study, they filled out and returned the questionnaires using an anonymous envelope.
Questionnaires
Fall-related self-efficacy
Fall-related self-efficacy was evaluated using the falling self-efficacy (FSE) scale,[18] which comprises 15 items that are indicators of fall events or fall-related situations during activities of daily living in elderly individuals. All items are common activities in the general populations, not limited to older individuals. Each item asks how confident the participants are in performing each action without falling. The respondents answered the degree of confidence using an 11-point Likert scale, from “no confidence” (a score of 0) to “fully confident” (score of 10). The FSE scale had a one-factor structure, and a higher total score indicates a higher fall-related self-efficacy. In this study, the Cronbach's α was 0.974. The FSE scores were significantly correlated to stride length and velocity during walking and stepping over.[18]
Chemotherapy-induced peripheral neuropathy
We assessed CIPN using the Comprehensive Assessment Scale for Chemotherapy-induced Peripheral Neuropathy in Survivors of Cancer (CAS-CIPN),[19] which is a comprehensive assessment scale developed with consideration of the lifestyle and culture of Japanese. The scale has four subscales: “Impaired fine finger movements,” “Severe anxiety about daily activity impairment due to worsening of symptoms,” “Self-confidence in selecting and continuing treatment,” and “Paresthesia (strange sensation) in the palms and soles.” The scale has a total of 15 items, and each item was answered in a 5-point Likert scale with “completely disagree” (score of 0) and “completely agree” (score of 4). The scores were summed up after invert items were allocated to the scores according to the manual. In this study, the Cronbach's α was 0.885 for the whole scale and 0.839–0.923 for the subscales.
Demographic and clinical characteristics of the participants
The following data were collected: Age, gender, height and weight, employment status, marital status, and number of household members. Collecting data regarding cancer and treatment included the primary site of cancer, types of chemotherapy agents associated with CIPN, and duration of use. Moreover, the degree of pain and fatigue was assessed on a numeric rating scale, ranging from 0 (no symptom) to 10 (worst possible symptom). A history of falling within the last month was investigated.
Data analysis
Data were summarized using descriptive statistics. The participants were then divided into two groups based on the FSE median score. We analyzed the FSE scores (high and low) and each variable using the Chi-square test, t-test, and Mann–Whitney U test. Multivariate logistic regression analysis was performed with the FSE scores as the dependent variable, and variables with P > 0.25 in the univariate analyses were excluded. None of the variables had strong linear relationship on the scatter diagrams. The variables were selected using backward elimination (likelihood ratio). All tests were two-tailed, and P < 0.05 were considered statistically significant. Data were analyzed using the Statistical Package for the Social Sciences version 22.0 (IBM Japan, Tokyo, Japan) for Windows.
Ethical approval
This study was conducted after obtaining approval from the ethical review board of the institution to which the principal investigator belongs. The ethical review boards of each facility where data collection was conducted approved this study. All candidates were informed that participation in the study was voluntary, that there were no disadvantages even if they did not participate, that the questionnaire was anonymous and collected in an envelope, and that privacy would be protected.
Results
Total 100 questionnaires were administered, and 85 responses were collected (response rate: 85.0%). Four responses with blank items on the FSE and/or CAS-CIPN were excluded. A total of 81 responses were analyzed (ratio of valid responses: 81.0%).
Characteristics of the participants
The characteristics of the participants are shown in Table 1. Colorectal and breast cancers were the most common types of cancer, accounting for majority of cases. Taxanes were used in 53 participants and platinum-based agents in 36. Nine patients used both agents.
Table 1.
Characteristics of the participants (n=81)
| Variables | n (%) |
|---|---|
| Age (n=81), mean±SD | 63.4±10.9 |
| Gender (n=80)a | |
| Male | 40 (49.4) |
| Female | 40 (49.4) |
| BMI (kg/m2) (n=81) | |
| <18.5 | 14 (17.3) |
| ≥18.5-<25 | 54 (66.7) |
| ≥25 | 13 (16.0) |
| Employment status (n=80)a | |
| Employed | 30 (37.0) |
| Unemployed | 50 (61.7) |
| Marital status (n=81) | |
| Married | 64 (79.0) |
| Others | 17 (21.0) |
| Number of household members (n=80)a | |
| Two or more | 66 (81.5) |
| One only (alone) | 14 (17.3) |
| Primary cancer (n=81)b | |
| Colorectal | 26 (32.1) |
| Breast | 26 (32.1) |
| Gastric | 15 (18.5) |
| Pancreatic | 9 (11.1) |
| Lung | 4 (4.9) |
| Others | 6 (7.4) |
| Chemotherapy agents associated with CIPN (n=80)b | |
| Taxanes | |
| Paclitaxel | 30 (37.0) |
| Nab-paclitaxel | 13 (16.0) |
| Docetaxel | 10 (12.3) |
| Platinum-based | |
| Oxaliplatin | 28 (34.6) |
| Cisplatin | 6 (7.4) |
| Carboplatin | 2 (2.5) |
| Others | 11 (13.6) |
| Number of cycles of relevant agents (n=60), median (IQR) | 5.5 (8) |
| Pain (n=81), median (IQR) | 1 (3) |
| Fatigue (n=80), median (IQR) | 3 (4) |
aMissing data. bDuplicate. CIPN: Chemotherapy-induced peripheral neuropathy, SD: Standard deviation, IQR: Interquartile range, BMI: Body mass index
Fall-related self-efficacy
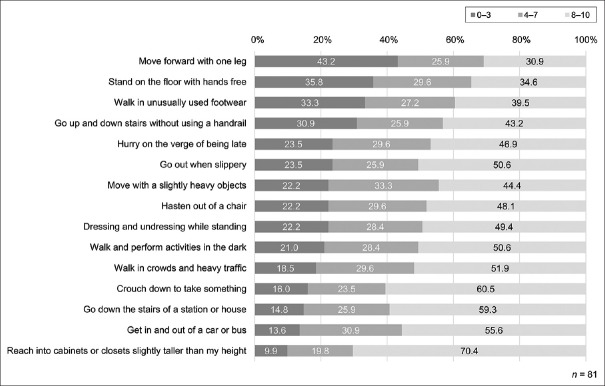
Figure 1 shows the distribution of FSE scores. In participants who obtained a score of 0–3, which indicates low self-efficacy, the items perceived by the participants as low self-efficacy were “moving forward with one leg,” “standing from a sitting position on the floor with hands free,” “walking in unusually used footwear,” and “going up and down stairs without using a handrail” in decreasing order. Approximately 30%–40% of the participants were aware of the risk of falling in these situations where they acted with unstable support. Subsequently, the patients had lower self-efficacy when “hurrying on the verge of being late” and “going out when slippery (as in rain or snow).” More than 20% of the participants recognized that they had low confidence in performing the action in these situations, which are different from the usual ones, but are commonly encountered daily.
Figure 1.
Fall-related self-efficacy. Participants answered the degree of confidence in performing each action without falling using an 11-point Likert scale, from “no confidence” (score of 0) to “fully confident” (score of 10)
Characteristics associated with fall-related self-efficacy
The participants were divided into two groups based on a FSE score of 109. Results of the univariate analysis are shown in Table 2. Female sex, low body weight (body mass index, BMI < 18.5 kg/m2), moderate-to-severe pain and fatigue, and previous history of falls were significantly correlated to low FSE scores. The group with low FSE scores showed significantly higher CAS-CIPN scores. In the group with low FSE scores, the proportion of participants with normal weight (BMI ≥18.5 kg/m2 but < 25 kg/m2) and those who did not present with pain were significantly low. In the multivariate analysis, gender, BMI, and the CAS-CIPN score remained significant variables correlated to low FSE scores [Table 3]. No outlier was observed; that is, the predicted value was higher or lower than ± 3 standard deviations from the measured value.
Table 2.
Characteristics associated with fall-related self-efficacy (univariate analysis)
| Variables | Fall-related self-efficacye | P | |
|---|---|---|---|
| Low (n=40) | High (n=41) | ||
| Age, mean±SD | 63.8±10.2 | 63.1±11.7 | 0.789a |
| Gender, n (%) | |||
| Male | 15 (38.5) | 25 (61.0) | 0.044 |
| Female | 24 (61.5) | 16 (39.0) | |
| BMI (kg/m2), n (%) | |||
| <18.5 | 11 (27.5) | 3 (7.3) | 0.019 |
| ≥18.5, <25 | 21 (52.5) | 33 (80.5) | |
| ≥25 | 8 (20.0) | 5 (12.2) | |
| Employment status, n (%) | |||
| Employed | 12 (30.8) | 18 (43.9) | 0.225 |
| Unemployed | 27 (69.2) | 23 (56.1) | |
| Marital status, n (%) | |||
| Married | 32 (80.0) | 32 (78.0) | 0.829 |
| Others | 8 (20.0) | 9 (22.0) | |
| Number of household members, n (%) | |||
| Two or more | 31 (79.5) | 35 (85.4) | 0.489 |
| One only (alone) | 8 (20.5) | 6 (14.6) | |
| Painc, n (%) | |||
| No | 13 (32.5) | 24 (58.5) | 0.033 |
| Mild | 16 (40.0) | 13 (31.7) | |
| Moderate to severe | 11 (27.5) | 4 (9.8) | |
| Fatiguec, n (%) | |||
| No | 6 (15.4) | 11 (26.8) | 0.012 |
| Mild | 13 (33.3) | 22 (53.7) | |
| Moderate to severe | 20 (51.3) | 8 (19.5) | |
| History of falls within the last month, n (%) | |||
| Yes | 12 (30.0) | 5 (12.2) | 0.049 |
| No | 28 (70.0) | 36 (87.8) | |
| CIPNd, median (IQR) | 30 (15.5) | 15 (11) | <0.001b |
at-test, bMann-Whitney U test, and χ2 test, cNo: NRS 0, mild: NRS 1-3, moderate to severe: NRS ≥4, dCAS for CIPN in survivors of cancer scores, eFSE scale scores. BMI: Body mass index, CIPN: Chemotherapy-induced peripheral neuropathy, SD: Standard deviation, IQR: Interquartile range, CAS: Comprehensive assessment scale, FSE: Falling self-efficacy, NRS: Numeric rating scale. Missing data were excluded.
Table 3.
Characteristics associated with fall-related self-efficacy (multivariate analysis) (n=78)
| Characteristics | B | P | OR (95% CI) |
|---|---|---|---|
| Sex (1: Female, 0: Male) | 1.405 | 0.022 | 4.075 (1.229-13.514) |
| BMI (kg/m2) (1: <18.5,0: ≥18.5) | 2.059 | 0.026 | 7.834 (1.284-47.800) |
| History of falls within the last month (1: Yes, 0: No) | 1.275 | 0.093 | 3.579 (0.808-15.862) |
| CIPNa | 0.115 | <0.001 | 1.122 (1.057-1.192) |
aCAS for CIPN in survivors of cancer (CAS-CIPN) scores. Multivariate logistic regression using backward elimination (likelihood ratio). Independent variables: sex, BMI, employment status, pain, fatigue, history of falls in a past month, and CAS-CIPN score. Hosmer and Lemeshow: 0.344, model Chi-square test: P<0.001, coefficient of determination: 75.6%. CIPN: Chemotherapy-induced peripheral neuropathy, CI: Confidence interval, CAS: Comprehensive assessment scale, OR: Odds ratio, BMI: Body mass index
Discussion
Fall-related self-efficacy perceived by patients with chemotherapy-induced peripheral neuropathy
Our results indicated that patients with CIPN were conscious about falling during highly common activities, which include standing up, walking, and using the stairs. In addition, their perception of fall-related self-efficacy can be susceptible to changes in situation and environment, such as time and weather. Among patients with CIPN, a higher number of falls occurred in flat areas than in high-risk areas.[15,20] The existing data may indicate that falls occur without continuous caution of falling. A recent study has reported that CIPN was associated with functional impairments, but not with the incidence of falls.[20] Patients with CIPN are at risk of giving up various activities to prevent falls. The interference of CIPN symptoms with the several aspects of daily living leads to negative feelings, such as frustration, depression, and loss of purpose, which results from having to give up enjoyable activities.[21] Similarly, low perceived self-efficacy of preventing falling would repeatedly make patients with CIPN feel the fear of falling and would result in psychological distress.
Characteristics associated with low self-efficacy of preventing falls
Low FSE scores were significantly associated with female sex, low BMI, and higher CAS-CIPN scores. Female sex and leanness were more likely to cause a decrease in bone density leading to fracture; therefore, patients with low self-efficacy of avoiding falls are estimated to be easily damaged from falls. Female sex and leanness are the common risk factors of falling in community-living older individuals,[22] although the results of the relationship between these variables and falls is not consistent in patients with cancer.[23] Moreover, the CAS-CIPN score reflects the psychological impact of CIPN and self-confidence regarding cancer treatment as well as strange sensation and impaired movement caused by CIPN.[19] The relationship between the CAS-CIPN score and fall-related self-efficacy indicates the importance of comprehensive assessment to understand a patient's experience, thereby not limiting the degree of physical symptoms and functional impairments.
Limitations
Our final sample had the sufficient ratio of valid responses but not from a consecutive recruitment. The results of this study should be considered carefully because of selection bias. It should also be noted that our sample of this exploratory study had a limited number of participants. This study used a cross-sectional design, and the results did not identify the causal relationship between variables. Moreover, not all risk factors of CIPN and falls were assessed, which is considered another limitation of the study. For example, the number of treatment cycles was investigated, with consideration of cumulative toxicity, but was excluded from the statistical analysis due to missing data (21 missing data, 25.9%). Further studies with larger samples from probability sampling must be conducted to validate the risk factors that lower fall-related self-efficacy based on the study results.
Implications for practice
In this study, we focused on fall-related self-efficacy, which is an important element affecting the activity and functional ability of patients. Based on the results, cancer patients with CIPN were confirmed to live with fear of falling. Actions with unstable support and frequent changes in the environment were common settings where patients cared about falling. Nurses should consider the potential risk of low self-efficacy regarding falls in patients treated with chemotherapy agent causing CIPN. The pre-assessment of the FSE scale before treatment is an available method to reveal the change following the occurrence and/or deterioration of CIPN. Interventions that improve the stability of actions and living safety in changing environment may be helpful for patients with CIPN. The interventions focusing on individual cognitive processing can be effective because fall-related self-efficacy has been developed based on self-efficacy, which is a theoretical framework of cognitive mechanism.[24] To evaluate the need for enhancing fall-related self-efficacy, a comprehensive assessment of CIPN is likely to be beneficial. Female patients with CIPN and a low BMI may have an increased need for treatment.
Conclusion
Patients with CIPN may be at risk of experiencing fear of falling during their daily activities, which include standing up, walking, and using the stairs. The participants' confidence in preventing falls is susceptible to waver even with the occurrence of small events, such as unstable postures and uncomfortable situations. Female sex, low BMI, and a comprehensive assessment of CIPN may be useful in assessing the need of enhanced fall-related self-efficacy.
Financial support and sponsorship
The study was supported by a grant from the JSPS KAKENHI (Grant number: 15K15828).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer – A GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 2.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–70. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 4.Hou S, Huh B, Kim HK, Kim KH, Abdi S. Treatment of chemotherapy-induced peripheral neuropathy: Systematic review and recommendations. Pain Physician. 2018;21:571–92. [PubMed] [Google Scholar]
- 5.Visovsky C, Collins M, Abbott L, Aschenbrenner J, Hart C. Putting evidence into practice: Evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2007;11:901–13. doi: 10.1188/07.CJON.901-913. [DOI] [PubMed] [Google Scholar]
- 6.Oncology Nursing Society. PEP topics, Peripheral Neuropathy. Oncology Nursing Society; 2017. May 10, [Last accessed on 2019 Jul 20]. Available from: https://www.ons.org/pep/peripheral-neuropathy . [Google Scholar]
- 7.Hanai A, Ishiguro H, Sozu T, Tsuda M, Yano I, Nakagawa T, et al. Effects of cryotherapy on objective and subjective symptoms of paclitaxel-induced neuropathy: Prospective self-controlled trial. J Natl Cancer Inst. 2018;110:141–8. doi: 10.1093/jnci/djx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argenta PA, Ballman KV, Geller MA, Carson LF, Ghebre R, Mullany SA, et al. The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: A randomized, sham-controlled clinical trial. Gynecol Oncol. 2017;144:159–66. doi: 10.1016/j.ygyno.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Bakitas MA. Background noise: The experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56:323–31. doi: 10.1097/01.NNR.0000289503.22414.79. [DOI] [PubMed] [Google Scholar]
- 10.Hong JS, Tian J, Wu LH. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Curr Oncol. 2014;21:174–80. doi: 10.3747/co.21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckhoff L, Knoop A, Jensen MB, Ewertz M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer. 2015;51:292–300. doi: 10.1016/j.ejca.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Simon NB, Danso MA, Alberico TA, Basch E, Bennett AV. The prevalence and pattern of chemotherapy-induced peripheral neuropathy among women with breast cancer receiving care in a large community oncology practice. Qual Life Res. 2017;26:2763–72. doi: 10.1007/s11136-017-1635-0. [DOI] [PubMed] [Google Scholar]
- 13.Tofthagen C, Donovan KA, Morgan MA, Shibata D, Yeh Y. Oxaliplatin-induced peripheral neuropathy's effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21:3307–13. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20:583–9. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016;73:860–6. doi: 10.1001/jamaneurol.2016.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35:2604–12. doi: 10.1200/JCO.2016.71.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattar S, Alibhai SM, Spoelstra SL, Fazelzad R, Puts MT. Falls in older adults with cancer: A systematic review of prevalence, injurious falls, and impact on cancer treatment. Support Care Cancer. 2016;24:4459–69. doi: 10.1007/s00520-016-3342-8. [DOI] [PubMed] [Google Scholar]
- 18.Takenaka K, Chikagawa M, Honda G, Matsuzaki C. Development of falling self-efficacy scale for elderly people: The reliability and validity. Japan J Phys Educ Heallth Sport Sci. 2002;47:1–13. [Google Scholar]
- 19.Kanda K, Fujimoto K, Mochizuki R, Ishida K, Lee B. Development of the comprehensive assessment scale for cipn in survivors of cancer. Cancer Nurs. 2016;39:S71–2. doi: 10.1186/s12885-019-6113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu H, Yagasaki K, Komatsu Y, Yamauchi H, Yamauchi T, Shimokawa T, et al. Falls and functional impairments in breast cancer patients with chemotherapy-induced peripheral neuropathy. Asia Pac J Oncol Nurs. 2019;6:253–60. doi: 10.4103/apjon.apjon_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tofthagen C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2010;14:E22–8. doi: 10.1188/10.CJON.E22-E28. [DOI] [PubMed] [Google Scholar]
- 22.Tinetti ME, Kumar C. The patient who falls: “It's always a trade-off”. JAMA. 2010;303:258–66. doi: 10.1001/jama.2009.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wildes TM, Dua P, Fowler SA, Miller JP, Carpenter CR, Avidan MS, et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6:70–83. doi: 10.1016/j.jgo.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]