1. Introduction
Children account for approximately 10% of TB cases worldwide, and those under five years of age are particularly susceptible to severe forms of disease [1]. Diagnosis remains a significant challenge in the pediatric population, as many children cannot expectorate and commonly present with paucibacillary form of pulmonary TB (PTB), resulting in low sensitivity of bacteriological tests (sputum smear and culture). Even in adolescents, who most often present with pulmonary TB, these tests may remain falsely negative [2,3].
In recent years, new diagnostic methods for TB have been developed, such as Xpert MTB/RIF® and, more recently, XpertUltra®, that have a higher sensitivity than smear microscopy and culture; however, in children, the sensitivity of both of these methods is lower than in adults [4,5]. The sensitivity of Xpert MTB/RIF® is estimated to range from 39% to 71.4%, according to a recent meta-analysis [6]. Additionally, the tuberculin skin test (TST) and interferon-gamma release assays (IGRAs), both used asdiagnostic methods for latent tuberculosis infection(LTBI) and active TB, are not able to reliably distinguish the two [7]. Thus, in order to improve the diagnosis of TB and to achieve the goal of the End TB strategy of the World Health Organization (WHO), the development of new point-of-care tests is necessary. These tests should be performed on samples that are easy to access and analyze, have low cost, and provide rapid results for the diagnosis of suspected TB cases, especially those with paucibacillary forms of the disease [8]. Serological tests for TB fit these criteria. However, to date, the WHO has not recommended the use of commercial serological kits because of low accuracy [9]. A review of studies on serological tests for TB in children emphasized the importance of considering certain factors in the evaluation of their sensitivity and specificity, among them: age-related immune system development in children; isotype of antibody and antigens studied; previous history of vaccination; definition of TB cases and use of in-house or commercial tests. Such differences make it difficult to compare the different studies [10].
Cellular immunity plays a key role in the pathogenesis and control of Mycobacterium tuberculosis (Mtb) infection, but research has also demonstrated the role immunoglobulins may play in regulating the immune response to intracellular bacteria [11,12]. The complex cell wall products of Mtb also play an important role in TB pathogenesis and in the induction of host immune response [13]. Among them, the Mce1A protein (mammalian cell entry) was initially shown to mediate entry of the bacterium into cells [14]. Mice infected with an Mtb strain unable to synthesize Mce1A showed a weak Th1 type response with the formation of disorganized granuloma architecture in the lungs [15]. Mce1A is a member of a putative mycolic acid importer encoded by the mce1 operon [13,16,17]. Previous research has indicated that anti-Mce1A IgG levels could be used to distinguish TB from LTBI in adults as a complementary diagnostic test [18].
In the present study, we evaluated serum levels of anti-Mce1A IgG and IgM in children and adolescents with PTB, LTBI and exposure to TB to assess the potential use of this biomarker to differentiate PTB from LTBI in the pediatric population.
2. Study population and methods
This study was approved by the Research Ethics Committee of the Universidade Federal Fluminense, in Niterói, Rio de Janeiro, Brazil (#45470115.0.0000.5243 and #26380513.3.0000.5243), as well as by all partnering health units. Parents or guardians of patients under 18 years of age provided written informed consent. Patients ages 18 years and over provided written informed consent; patients ages 7–17 years provided assent to participate.
2.1. Setting and study population
This prospective study was conducted in eight pediatric TB reference centers (five outpatient clinics and three hospitals) in five cities of Rio de Janeiro State, Brazil. From September of 2014 through June of 2017, we included children and adolescents (ages 0–19 years) with PTB (n = 43) or LTBI (n = 44), or who were exposed to TB (TBE) patients but remained TST negative (n = 20).
2.1.1. PTB group
Children and adolescents diagnosed with TB by their attending physician were invited to participate in the study. PTB was diagnosed according to signs and/or symptoms compatible with TB and one or more of the following data: chest X-ray suggestive of TB, positive tuberculin skin test (TST), positive acid-fast bacilli (AFB) sputum smear, Mtb positive sputum culture, positive GeneXpert® PCR test, and clinical improvement with the use of anti-TB drugs (MS Brazil, 2011). Patients who tested positive by culture and/or PCR were classified as confirmed TB [19].
2.1.2. LTBI group
Children and adolescents diagnosed with LTBI by their attending physician were also invited to participate in the present study. LTBI was diagnosed in patient who had contact with a smear-positive TB case, a reactive TST, and normal chest X-ray [20,21].
2.1.3. TBE group
We also included children and adolescents who had been exposed to a TB patient as members of a family with a case of TB included in the present study. TBE patients were defined as asymptomatic youths who had contact with a smear-positive TB case, non-reactive TST, and a normal chest X-ray.
2.1.4. Clinical diagnostic procedures
All patients were examined, diagnosed, and followed up by physicians at participating health units without interference from the research team. Completion of bacteriological tests such as sputum smear, culture, and Xpert MTB/RIF® for Mtb in patients with suspected PTB was determined by the attending physician, considering the age of the patients and their ability to expectorate, as well as the availability of these tests at each health unit.
TST was performed by intradermal injection of 2TU of PPD RT23 (Staten Serum Institute, Copenhagen, Denmark) and read after 72–96 h by a trained technician. Brazilian Ministry of Health recommendations for interpretation of TST were used. Indurations of ≥ 10 mm in BCG- vaccinated patients with less than 2 years of age and ≥ 5 mm in BCG- vaccinated children more than 2 years of age or with immunosuppression were considered positive [20]. Culture, when indicated by the attending physician, was conducted by the Ogawa-Kudoh, Lowestein-Jensen, or MGIT 90 methods, according to availability and standard procedures in participating health units.
2.2. Serum collection and ELISA
Following case definition (PTB, LTBI, or TBE) by the attending physician and informed consent procedures, 2 ml of venous blood were collected from patients in a serum separator tubes (BD Vacutainer®, SST® II Advance®, USA) by a trained health professional. Samples were sent to the Multi-user Research Support Laboratory of the Hospital Universitário Antônio Pedro of the Universidade Federal Fluminense (Laboratório Multiusúario de Apoio á Pesquisa em Nefrologia e Ciências Médicas of the Universidade Federal Fuminense - LAMAP) for processing.
Serum was obtained by centrifugation of blood (3500 rpm for 5 min) and stored at 20 C until tested. The enzyme-linked immunosorbent assay(ELISA) protocol was performed following previously published procedures (Takenami et al., 2016), with minor modifications. Mce1A protein (10 μg/ml) (provided by L.W. Riley University of California, Berkeley, CA, USA) was diluted to 1:1000 in ethanol and 50 μl of it was dried overnight in each well of polystyrene ELISA plates. Plates were blocked with 3% low fatty-acid bovine serum albumin (BSA) (US Biologicals®, USA) for 1 h at room temperature and washed twice with phosphate buffered saline (PBS) pH 7.4. Serum samples were diluted 1:100 in 3% BSA and 100 μL, were added to wells (triplicates for each participant sample) to incubate for 1 h at room temperature. After three washes with PBS, 100 μl of horseradish peroxidase (HRP) goat anti- human IgM (1: 10,000) or IgG (1: 50,000) (Sigma-Aldrich®, Germany) were added to wells and incubated at room temperature for 1 h. Subsequently, 100 μl of tetramethylbenzidine substrate (Invitrogen Life Technologies®, USA) were added, and after a 30 min incubation, the reaction was stopped with 100 μl of 1–2 M sulfuric acid (Pershy Chemicals®, Brazil). Reactions were read on a plate reader at optical density (OD) at 450 nm (SpectraMax® Molecular device, USA). Results were reported as the mean OD values of triplicate analyses; the coefficient of variation of all triplicates was less than 10%. Serum sample from patients (non-study related) with confirmed TB were used as a positive control; serum samples from patients with absence of TB signs, symptoms, or contacts of TB served as a negative control.
2.3. Statistical analysis
Data were analyzed by the program GraphPad Prism® v.5.0 (GraphPad Inc., San Diego, CA). Median values and interquartile ranges (IQR) were used to describe immunoglobulin levels measured by OD. Spearman’s correlation was used to assess the linear correlation between biomarkers levels and clinical and laboratory data. Student’s t-test was used to compare the mean of biomarker levels in groups with normal data distributions. For comparison of medians between two or three study populations, the Mann-Whitney and Kruskal-Wallis test, respectively, were used followed by the Dunn’s multiple comparison test. Chi- square tests were used to assess categorical variables. The ability of immunoglobulins to discriminate PTB patients from those with LTBI and TBE was assessed by measuring the area under the receiver operating characteristic curve (AUC). As children have a developing immune system and children under five are at increased risk of developing severe forms of TB, we stratified analysis by children under five years of age and children between five and 19 years of age. Statistical significance was set at p < 0.05.
3. Results
3.1. Characteristics of the study population
We included 43 patients with PTB, 44 with LTBI, and 20 with TBE. Table 1 outlines the sociodemographic, clinical, and laboratory characteristics of patients in the PTB, LTBI, and TBE groups. The median age of the entire study population was 8 years (IQR: 3.3–12); the median age was 11 years (IQR: 1.9–15) in the PTB group, 8.5 (IQR: 4–11) in the LTBI group, and 7 (IQR: 4.3–9) in the TBE group. All study patients had BCG (Bacillus Calmette-Guerin) scar greater than 3 mm in size. The mean TST response size was 14 mm (SD = 4) in the PTB group and 13 mm (SD = 4) in the LTBI group [p = 0.289]. There was no relationship between anti- Mce1A IgM (r = 0.12, p = 0.16) oranti-Mce1AIgG levels (r = 0.13, p = 0.31) with the size of TST induration. Ten (23%) PTB cases were confirmed by culture or Xpert®.
Table 1.
Demographic, clinical, and laboratory characteristics of the study population.
| All patients (n = 107) | PTB (n = 43) | LTBI (n = 44) | TBE (n = 20) | P | |
|---|---|---|---|---|---|
| Age | |||||
| 0–4y | 37 (35%) | 18 (42%) | 14 (32%) | 5 (25%) | 0.37 |
| 5–19y | 70 (65%) | 25 (58%) | 30 (68%) | 15 (75%) | |
| Gender | |||||
| Male, n (%) | 48 (45%) | 20 (46%) | 18 (41%) | 10 (50%) | 0.76 |
| Female, n (%) | 59 (55%) | 23 (54%) | 26 (59%) | 10 (50%) | |
| Household contact | |||||
| Yes | 90 (84%) | 26 (61%) | 44 (100%) | 20 (100%) | – |
| No | 17 (16%) | 17 (39%) | 0 | 0 | _ |
| TST* | |||||
| Positive | 23 (79%) | 44 (100%) | – | – | |
| Negative | – | 6 (21%) | – | 20 (100%) | – |
| AFB* | |||||
| Positive | – | 14 (42%) | – | – | – |
| Negative | – | 19 (58%) | – | – | – |
| PCR* | |||||
| Positive | – | 4(29%) | – | – | – |
| Negative | – | 10 (71%) | – | – | – |
| Culture* | |||||
| Positive | – | 8 (38%) | – | – | – |
| Negative | – | 13 (62%) | – | – | – |
PTB = pulmonary tuberculosis; LTBI = latent tuberculosis infection; TBE = tuberculosis exposed; TST = tuberculin skin test; AFB = acid fast bacilli; PCR = polymerase chain reaction.
Percent positive and negative based on total number of performed tests.
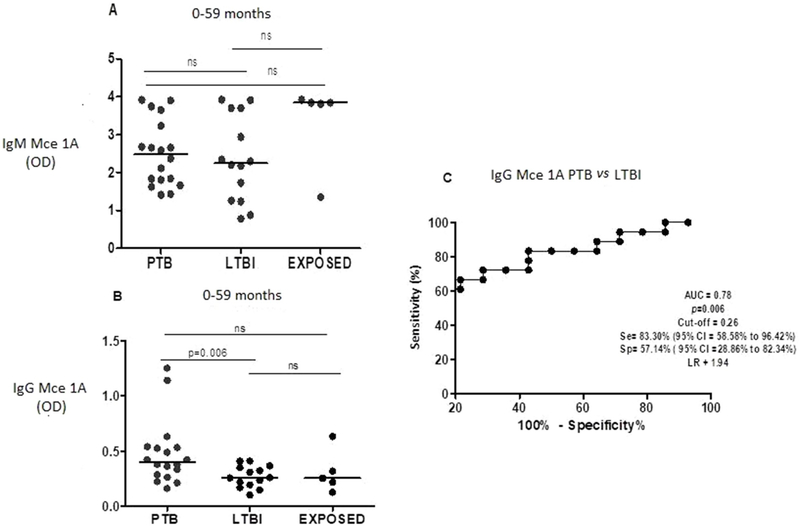
3.2. Serum anti-Mce1A IgM and IgGlevels and diagnostic value of IgG in discriminating PTB from LTBIin patients under 5 years of age
Fig. 1 shows levels of anti-Mce1A IgM (Fig. 1A) and IgG (Fig. 1B) across groups under 5 years of age. Median IgM level was 2.447 (IQR: 1.771–3.339) in the PTB group, 2.250 (IQR: 1.254–3.704) in the LTBI group, and 3.843 (IQR: 2.581–3.882) in the TBE group. There were no significant differences in IgM levels between groups in this age range. Median IgG level was 0.401 (IQR: 0.280–0.534) in the PTB group, 0.259 (IQR: 0.187–0.355) in the LTBI group, and 0.253 (IQR: 0.172–0.476) in the TBE group. IgG levels were significantly higher in the PTB than in LTBI group (p = 0.006). There was no difference in IgG levels between the PTB and TBE nor between LTBI and TBE groups. Fig. 1C shows the ROC curve comparing PTB and LTBI.
Fig. 1.
Levels of igM (A) and igG (B) anti-Mce 1A, in patients (age 0–59 months) with pulmonary tuberculosis (PTB), latent tuberculosis infection (LTBI) and exposed to TB (TBE). Receiver operater characteristic (ROC) curve (C) comparing igG anti-Mce 1A level in PTB versus (vs) LTBI. AUC = area under the curve, Se = sensitivity, sp = specificity Cl = confidence intervel, ns = not significant and LR = likehood ratio.
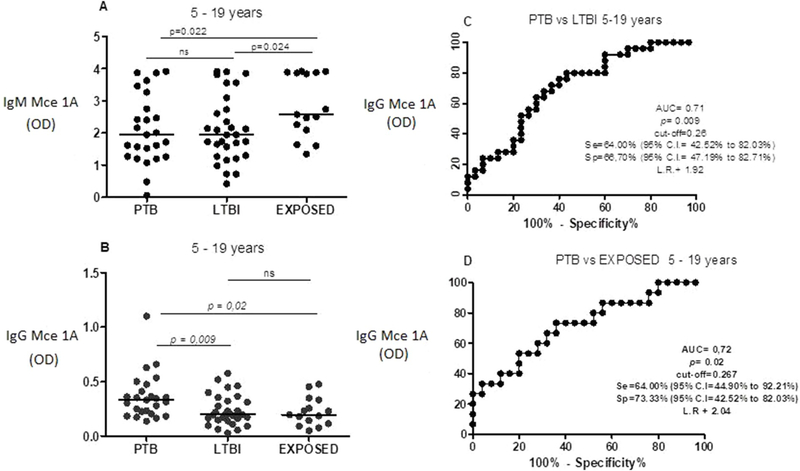
3.3. Serum anti-Mce1A IgM and IgG levels and diagnostic value of IgG in discriminating PTB from LTBI and PTB from TBE in patients between 5 and 19 years of age
Fig. 2 shows levels of anti-Mce1A IgM (Fig. 2A) and IgG (Fig. 2B) in patients ages 5–19 years. Median IgM level was 1.947 (IQR: 1.265–3.013) in the PTB group, 1.945 (IQR: 1.265–2.826) in the LTBI group, and 2.578 (IQR: 2.097–3.886) in the TBE group. There was a significant difference between the TBE and PTB groups (p = 0.022) and between TBE and LTBI (p = 0.024) groups. There was no difference between LTBI and PTB.
Fig. 2.
Levels of igM (A) and igG (B) anti-Mce 1A, in patients (age 5–19 years) with pulmonary tuberculosis (PTB), latent tuberculosis infection (LTBI) and exposed to TB (TBE). Receiver operater characteristics (ROC) curve (C) comparing igG anti-Mce 1A level in PTB versus (vs) LTBI.(ROC) curve (D) comparing igG anti-Mce 1A level in PTB vsTBE.AUC = area under the curve, Se = sensitivity, sp = specificity Cl = confidence intervel, ns = not significant and LR = likehood ratio.
Median IgG level was 0.331 (IQR: 0.215–0.443) in the PTB group, 0.198 (IQR: 0.146–0.331) in the LTBI group, 0.196 (IQR: 0.114–0.333) in the TBE group. There was a significant difference in IgG levels between the PTB and LTBI groups (p = 0.009) and between the PTB and TBE groups (p = 0.020).
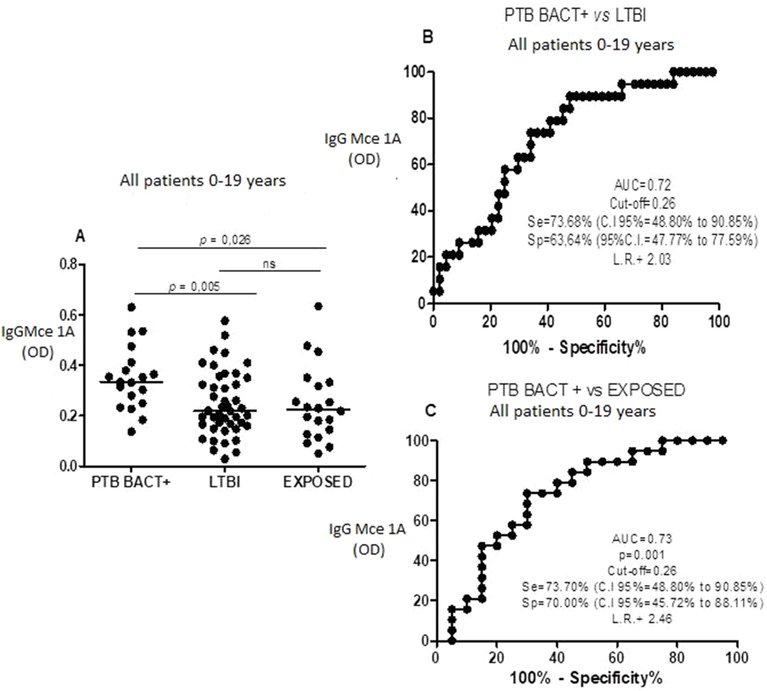
3.4. Serum anti-Mce1A IgG levels and diagnostic value of IgG in discriminating bacteriologically positive PTB (PTBBACT+) from LTBI and TBE in patients between 0 and 19 years of age
Ten patients had culture or PCR-confirmed PTB. Of these, the anti- Mce1A IgG ELISA test was positive in 7 cases (70%). When comparing the confirmed PTB group with LTBI and TBE groups, there was a significant difference in the level of the IgG (p = 0.043 and p = 0.05, respectively). As we had only 10 cases of confirmed PTB and because TB is endemic in Brazil, we extended this analysis to include the nine additional PTB patients, who had positive sputum smear, but were not confirmed by culture or PCR. This group, referred to here as bacteriologically positive PTB (PTB BACT+), included 19 patients. The median age of this group was 13 years (IQR: 8–15). The median level of anti- Mce1A IgG was 0.334 (IQR: 0.249–0.412) in the PTB + group, 0.22 (IQR: 0.161–0.344) in LTBI and 0.224 (IQR: 0.131–0.33) in TBE. We found a significant difference between the IgG levels in the PTB BACT + versus LTBI groups (p = 0.005) and the PTB BACT + versus TBE groups (p = 0.025) (Fig. 3A). In the ROC analysis, using the 0.26 OD cut-off, we found the sensitivity to be 73.7% and the specificity to be 63.6% when comparing PTB BACT+ and LTBI groups (Figs. 3B), and 73.7% and 70%, respectively, comparing the PTB BACT + vsTBE groups (Fig. 3C). In the PTB BACT + group, the IgG test was positive in 14 (73.7%) patients based on the 0.26 OD cut-off.
Fig. 3.
Levels of igG anti-Mce 1A (A) in patients (age 0–19 years) with pulmonary TB positive bacteriogically (PTB BACT+), latent tuberculosis infection (LTBI) and exposed to TB (TBE). Receiver operator characteristic (ROC) curve (B) Comparing igG anti-Mce 1A level in PTB BACT + versus (vs) ILTB and (C) ROC curve comparing igG anti-Mce 1A level in PTB BACT + versus (vs) TBE. AUC = area under the curve, Se = sensitivity, sp = specificity Cl = confidence intervel, ns = not significant and LR = likehood ratio.
4. Discussion
In this study, whose main objective was to evaluate whether serum levels of anti-Mce1A IgM and IgG were able to differentiate PTB and LTBI in children and adolescents, significantly higher levels of anti-Mce1A IgG were detected in patients with PTB. In the overall group, the best result for anti-Mce1A IgG was obtained with the ELISA cut-off set at 0.26 OD for comparison of both PTB vs LTBI and PTB vs TBE groups.
Previous research evaluating the potential use of anti-Mce1A IgG biomarker for the diagnosis of active TB in adults found higher levels of this antibody in patients with PTB compared to LTBI as well as PTB compared to TBE [18]. In children and adolescents, we show here that anti-Mce1A IgG is able to distinguish PTB BACT + vs TBE with 74% sensitivity and 70% specificity and PTB BACT + vs LTBI with 74% sensitivity and 64% specificity. These results demonstrate that anti-Mce1A IgG has similar or better test accuracy than previously evaluated serological tests assessing various Mtb antigens [10,22].
In children, whose immune system is under development, IgM, IgG and IgA levels similar to those of adults are reached on average at two, six and ten years of age, respectively [23]. In our age-stratified analysis, anti-Mce1A IgG was able to discriminate PTB from LTBI in children under 5 years of age. In patients ages 5–19, anti-Mce1A IgG also discriminated between PTB and LTBI, as well as PTB from TBE. Children, especially those under 10, often develop paucibacillary form of PTB and also have difficulty expectorating [2]. In the present study, we found a small number of cases of confirmed PTB (23%), according to criteria of Graham et al., 2015 [19]. AFB sputum smear and Xpert® MTB/RIF, which offer results more quickly, were used for the diagnosis of 40% of patients, who had positive test results. In the remaining 60%, the initial diagnosis was based on the Brazilian scoring system for diagnosing pediatric TB [20]. In the overall group, based on the 0.26 OD cut-off, the anti-Mce1A IgG ELISA tested positive in 31 (72%) of all cases of PTB, indicating that there was a 32% increase in case-detection using the ELISA test compared to smear microscopy and Xpert® MTB/RIF. When we added PTB cases with positive AFB sputum test to those with confirmed PTB (PTB BACT + group), the ELISA also discriminated PTB BACT+ from LTBI and TBE, a finding also found by Takenami et al. (2016) in adults [18].
In Brazil, the BCG (Mycobacterium bovis) vaccination is recommended in the first month of life, with coverage higher than 90% of the target population] [24]. The Mce1A protein is present in both the cell wall of Mycobacterium bovis and Mtb. Although some studies have found higher levels of immunoglobulins for certain antigens when compared to vaccinated and non-vaccinated children [25], others have generally shown that prior BCG vaccination did not influence antibody levels against Mtb proteins. In our study population, 100% of the children and adolescents were vaccinated, so it was not possible to compare anti-Mce1A IgM and IgG levels between vaccinated and unvaccinated patients. However, we did not find a significant association between anti-Mce1A IgM or IgG levels and participant age, indicating the time period from of vaccination did not impact biomarker levels.
After infection, the Mtb bacillus can be eliminated by the immune system, remain in the latent state, or proliferate to cause active disease. The bacillus may be eradicated at all stages [26]. In our study, 6 (30%) TBE and 17 (39%) LTBI patients had positive ELISA tests for anti-Mce1A IgG. It is possible that these individuals were newly infected, had a higher bacillary load, or had a higher risk of progression to subclinical or TB. Future studies in which LTBI and PTB patients are followed over time are required to determine if their higher anti-Mce1A IgG levels are associated with progression to active disease.
This study has several limitations, including a small sample size and the small number of confirmed PTB. Therefore, further studies with larger sample sizes are needed. Additionally, we were unable to assess the association of ELISA results and clinical progression outcomes, although we intend to follow the patients to further evaluate this question. Finally, we were not able to include and compare antibody levels in serum from patients infected with non-tuberculous mycobacteria. Such infections, are rare in our geographic region.
The WHO recommends that large-scale diagnostic tests for TB should have 90% sensitivity and 70% specificity [22]. Although anti-Mce1A IgG did not meet these targets, it has achieved a level of sensitivity comparable to or better than that reported by Xpert, which requires sputum samples or extrapulmonary samples that are not readily obtained from children. Thus, the anti-Mce1A IgG ELISA could potentially be used to screen suspected cases of pediatric and adolescent PTB during the initial evaluation phase.
Acknowledgements
The authors would like to thank Dr. Iukary Takenami for the support in implementing the ELISA protocol and Estela M. Cosme, Thaís R.D. Oliveira, Fernanda P. Barra, and Halana S.A.T. Sias for assistance with data collection and organization.
Funding
The present study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) #001, the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil. CAAC was supported by the CAPES (grant number 17862/12-4) and the FAPERJ (grant number E-26.202.777.2015]. KLL was supported by the National Institutes of Health Fogarty International Center [grant number R25 TW009338] and by a Fulbright Postdoctoral Scholarship and CAPES. CCS was supported by the CNpq [grant number 305044/2012-5].
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tube.2019.101893.
References
- [1].WHO. Global tuberculosis report 2018, vol. 2018 Geneva: World Health Organization; 2018. WHO/HTM/TB/2017.23, http://apps.who.int/medicinedocs/documents/s23553en/s23553en.pdf. [Google Scholar]
- [2].Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8:392–402. [PubMed] [Google Scholar]
- [3].Sant’Anna CC, Schmidt CM, March M de FBP, Pereira SM, Barreto ML. Tuberculose em adolescentes em duas capitais brasileiras. Cad Saúde Pública 2013;29(1): 111–6. . [DOI] [PubMed] [Google Scholar]
- [4].Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis 2011;11(11):819–24. 10.1016/S1473-3099(11)70167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sabi I, Rachow A, Mapamba D, Clowes P, Ntinginya NE, Sasamalo M, et al. Xpert MTB/RIF Ultra assay for the diagnosis of pulmonary tuberculosis in children: a multicentre comparative accuracy study. J Infect 2018;77(4):321–7. 10.1016/j.jinf.2018.07.002. [DOI] [PubMed] [Google Scholar]
- [6].Ioos V, Cordel H, Bonnet M. Alternative sputum collection methods for diagnosis of childhood intrathoracic tuberculosis: a systematic literature review. Arch Dis Child 2018;104(7):629–35. 10.1136/archdischild-2018-315453. [DOI] [PubMed] [Google Scholar]
- [7].Starke JR, Committee on Infectious Disease ACP. Interferon- release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics 2014;134(6): e1763–73. 10.1542/peds.2014-2983. [DOI] [PubMed] [Google Scholar]
- [8].BronnerMurrison L, Ananthakrishnan R, Sukumar S, Augustine S, Krishnan N, Pai M, et al. Use of rapid, point-of-care assays by private practitioners in Chennai, India: priorities for tuberculosis diagnostic testing. PLoS One 2016;15(6): e0155775 10.1371/journal.pone.0155775.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Steingart KR, Flores LL, Denderkuri N, Schiller I, Laal S, Ramsay A, Hopewell PC, et al. Commercial Serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and Meta-Analysis. PLoS Med 2011;8(8):e100106 10.1371/journal.pmed.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Achkar JM, Ziegenbalg A. Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value. Clin Vaccine Immunol 2012;19(12):1898–906. 10.1128/cvi.00501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chan J, Mehta S, Bharrhan S, Chen Y, Achkar JM, Casadevall A, et al. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Semin Immunol 2014;26(6):588–600. 10.1016/j.smim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jacobs AJ, Mongkolsapaya J, Screaton GR, McShane H, Wilkinson RJ. Antibodies and tuberculosis. Tuberculosis 2016;101:102–13. 10.1016/j.tube.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forrellad MA, Klepp LI, Gioffre A, García JS, Morbidoni HR, de la Paz Santangelo M, et al. Virulence factors of the mycobacterium tuberculosis complex. Virulence 2013;1(1):3–66. 10.4161/viru.22329.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley LW. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. 261 Science 1993;10(5127):1454–7. 10.1126/science.8367727.1993. [DOI] [PubMed] [Google Scholar]
- [15].Shimono N, Morici L, Casali N, Cantrell S, Sidders B, Ehrt S, et al. Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon. Proc Natl Acad Sci 2003;23(26):15918–23. 10.1073/pnas.2433882100.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Casali N, Riley LW. A phylogenomic analysis of the Actinomycetalesmce operons. BMC Genomics 2007;26(8):60 10.1186/1471-2164-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dunphy KY, Senaratne RH, Masuzawa M, Kendall LV, Riley LW. Attenuation of Mycobacterium tuberculosis functionally disrupted in a fatty acyl–coenzyme A synthetase gene fadD5. J Infect Dis 2010;15(8):1232–9. 10.1086/651452.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takenami I, de Oliveira CC, Lima FR, Soares J, Machado A, Riley LW, et al. Immunoglobulin G response to mammalian cell entry 1A (Mce1A) protein as biomarker of active tuberculosis. Tuberculosis 2016;100:82–8. 10.1016/j.tube.2016.07.012. [DOI] [PubMed] [Google Scholar]
- [19].Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015;15:S179–87. 10.1093/cid/civ581.61Suppl3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brasil. Manual de recomendaçoes para o controle da tuberculose no Brasil. Ministerio da Saúde; 2011. http://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil.pdf.
- [21].WHO. Guidelines on the management of latent tuberculosis infection. WHO/HTM/ TB/2015.01, https://apps.who.int/iris/bitstream/handle/10665/136471/9789241548908_eng.pdf;jsessionid=1C3C48B26BDE69A661E53457DE4F1054?sequence=1; 2015. [PubMed]
- [22].Nonyane BAS, Nicol MP, Andreas NJ, Rimmele S, Schineiderhan-Marra N, Workman LJ, et al. Serologic responses in childhood pulmonary tuberculosis. Pediatr Infect Dis J 2018;37(1):1–9. 10.1097/INF.0000000000001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schroeder Jr HW, et al. Developmental regulation of the human antibody repertoire. Ann N Y Acad Sci 1995;764:242–60. 10.1111/j.1749-6632.1995.tb55834.x. [DOI] [PubMed] [Google Scholar]
- [24].Domingues CMAS, Teixeira AM da S. Coberturas vacinais e doenças imunopreveníveis no Brasil no período 1982–2012: avanços e desafios do Programa Nacional de Imunizaçoes. Epidemiol. Serv. Saúde, Brasília 2013;22(1):9–27. 10.5123/S1679-49742013000100002. [DOI] [Google Scholar]
- [25].Beyazova U, Rota S, Cevheroǧlu C, Karsligi̊l T Humoral immune response in infants after BCG vaccination. Tuber Lung Dis 1995;76(3):248–53. 10.1016/S0962-8479(05)80013-9.Jun. [DOI] [PubMed] [Google Scholar]
- [26].Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018;(4):31 10.1128/cmr.00021-18.Jul18. [DOI] [PMC free article] [PubMed] [Google Scholar]