Cities represent a unique ecosystem dominated by humans and high-density man-made infrastructure, associated with modifications or entire loss of local habitats. Thus, urbanization constitutes a considerable challenge for wildlife, and only a minority of species is able to persist in cities (Niemelä et al. 2011). Species that maintain populations in cities are usually characterized by significant changes in life history characteristics, population structure, and ecological relations (Niemelä et al. 2011). So far, most studies in urban ecology of vertebrates have focused on birds and mammals, whereas city-dwelling ectotherms, including reptiles, remain understudied (Niemelä et al. 2011; Hudson and Virgin 2018). However, considering that the worldwide decline of reptiles is largely associated with habitat deterioration (Gibbons et al. 2000) it is critical and urgent to assess the responses of reptilian populations toward an urbanized environment. Here, we investigated the impact of the urban environment on body size, as the primary fitness-relevant trait, in a widespread Eurasian reptile, the grass snake Natrix natrix, by comparing 2 replicate urban populations with sub- and non-urban sites. Due to the documented reduction of prey availability (amphibians) in cities including the studied metropolitan area (Budzik et al. 2013), we predict that urban grass snakes will tend to exhibit smaller body size compared with sub- and non-urban sites, similary to previous studies on snakes (Wolfe et al. 2018). In addition, a high density of roads and higher incidence of deliberate killing in cities may increase mortality mainly of larger specimens (Andrews and Gibbons 2008, Niemelä et al. 2011). Because grass snakes express clear sexual size dimorphism (SSD) with females larger than males (Madsen and Shine 1993), we expect that the anticipated mortality will result in a skewed sex ratio with underrepresentation of females in urban sites.
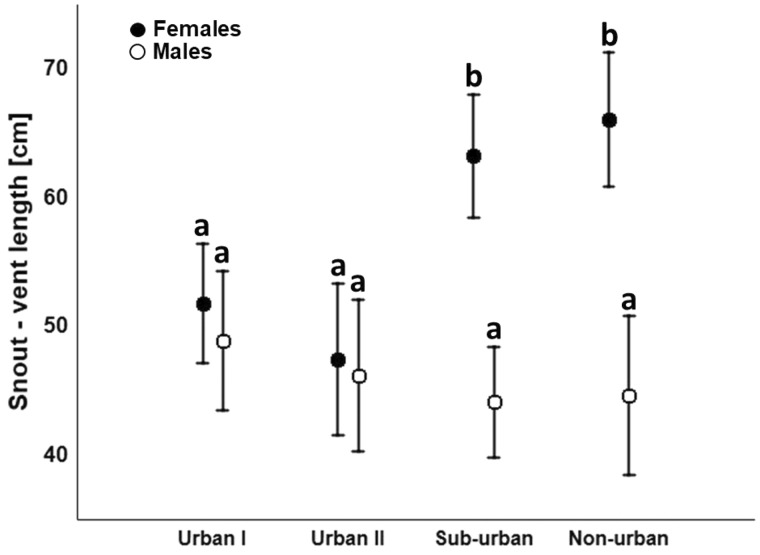
The study was conducted on 4 populations of the grass snake in southern Poland in Kraków and its vicinity. Kraków is the capital of Lesser Poland Voivodeship and is one of the largest Polish cities with a human population size exceeding 750,000 and well-developed urban infrastructure. We identified 2 urban sites with remaining patches of natural habitat occupied by the grass snake situated close to the city center and surrounded entirely (site Urban I) or in ca. 70% (site Urban II) by buildings and roads. The third population (site Sub-urban) was located in the suburbs within the administrative boundaries of Kraków in a riparian woodland along the Vistula River surrounded by allotments but not city buildings. The 4th population was located outside of Kraków (site Non-urban) entirely in a large patch of lowland forest of Puszcza Niepołomicka. Sub-adult to adult (>30 cm SVL) snakes were captured throughout the active season (April–October) in each population in parallel. For each snake, we assessed snout–vent length (SVL) and sex by examining tail morphology and probing when necessary. Snakes were marked individually with scale clipping. Overall, we collected data on 13 males and 18 females in site Urban I, 11 males and 11 females in site Urban II, 21 males and 17 females in site Sub-Urban, 10 males and 14 females in site Non-Urban. A 2-way ANOVA revealed that SVL is significantly affected by the interaction of population and sex (F3,107 = 7.71; P < 0.001; Supplementary Table S1; Figure 1). A post hoc Tukey test showed that significant differences occurred between sexes in both Sub- and Non-Urban sites (Supplementary Table S2; Figure 1), whereas no differences were detected in snakes from both urban sites (Urban I and Urban II) (Supplementary Table S2; Figure 1). Male body size did not differ between populations, whereas females were larger in Sub-urban and Non-urban sites compared with both urban (Urban I and Urban II) populations (Supplementary Table S2; Figure 1). Sex ratio did not deviate from 1:1 in Urban I (chi2 = 0.81; P = 0.37), Urban II (chi2=0.0; P = 1), Sub-urban (chi2 = 0.42; P = 0.52), or Non-urban (chi2 = 0.66; P = 0.41) populations. Descriptive statistics are provided in Supplementary Table S3.
Figure 1.
Body size (SVL) of grass snakes representing both sexes in 4 studied populations (LSM ± 95% CI). Groups for which significant differences were detected (P < 0.05) do not share a common letter.
Our study represents the first report of decrease in size of 1 of sexes leading to loss of sexual size dimorphism in native species imposed by the urban environment (Hudson and Virgin 2018). As predicted, urban snakes tended to exhibit smaller body sizes compared with sub- and non-urban populations. However, this pattern was visible only for females, whereas for males we did not detect any differences among populations. In consequence, the magnitude of sexual size dimorphism of grass snakes in the city is reduced compared with both sub- and non-urban populations. The observed pattern clearly shows that the environmental pressure of the city acts asymmetrically on both sexes. Reduced female size in urban populations may be explained by resource scarcity in the city (Kozłowski 1992) or mortality biased toward larger specimens (Andrews and Gibbons 2008, Niemelä et al. 2011). However, size-biased mortality affecting larger females would eventually lead to a reduced fraction of females in the population visible as male-biased sex ratio (Kurek et al. 2019). This is not what we observed, as the sex ratio of urban grass snakes did not deviate from 1:1, suggesting that mortality is not the main factor underlying the detected variation in the body size. Therefore, we propose that smaller female size in the city represents a result of reduced resource availability in Kraków (Budzik et al. 2013). A similar response in female size was in fact documented for insular grass snakes where females also attain smaller sizes due to limited prey availability compared with mainland, food-rich sites (Madsen and Shine 1993). However, disentangling the factors that underlie the impact of the urban environment on the body size of snakes, including heritable effects and phenotypic plasticity, requires further studies, for example, assessment of age structure and common environment experiments.
Sexual size dimorphism, with females growing larger than males, is commonly observed in snakes and is discussed in terms of positive selection toward high fecundity (Shine 1994). Indeed, a positive association between female size and clutch size or offspring number is well documented in snakes (Shine 1994), including grass snakes. Therefore, reduced female size in urban sites may have a direct effect on population dynamics as it may reduce reproductive potential and population growth. This, in turn, reduces population size and, simultaneously, increases the risk of extinction of urban populations. Nevertheless, despite an obvious alteration in body size, no apparent deviations in population structure were observed, at least at the level of sex ratio. Thus, we suggest that optimization of body size to prevailing conditions may serve as a mechanism that buffers the negative impact of resource scarcity on survival. Therefore, we suggest that future studies should pay more attention to the mechanisms underlying body size variation in ectotherms under urban pressure. Such data would provide substantial understanding of the individual-based processes related to population-level responses toward increasing urbanization.
Supplementary Material
Acknowledgments
The study was approved by the Regional Directorate for Environmental Protection in Kraków (no. OP-I.6401.154.2014.MMr, OP-I.6401.21.2015.PKw, OP-I.6401.368.2016.PKw, OP-I.6401.112.2018.PKw). The authors thank 2 anonymous reviewers for their valuable comments that helped to improve the manuscript. The authors thank Agata Bury and Maciej Pabijan of Jagiellonian University for comments and linguistic corrections on the manuscript.
Funding
This study was financially supported by a grant from the National Science Centre in Poland awarded to S.B. (grant no. UMO-2016/21/N/NZ8/00959) and grant from Jagiellonian University in Krakwarded to B.Z. (grant no. DS/MND/WBiNoZ/INoŚ/24/2014).
References
- Andrews KM, Gibbons JW, 2008. Roads as catalysts of urbanization: snakes on roads face differential impacts due to inter- and intraspecific ecological attributes. In: Mitchell JC, Jung Brown RE, Bartholomew B, editors. Urban Herpetology. Salt Lake City (UT: ): Society for the Study of Amphibians and Reptiles. 145–153. [Google Scholar]
- Budzik KA, Budzik KM, Żuwała K, 2013. Amphibian situation in urban environment - History of the common toad Bufo bufo in Kraków (Poland). Ecol Quest 18:73–77. [Google Scholar]
- Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD. et al. , 2000. The global decline of reptiles, Déjà Vu amphibians. Bioscience 50:653–666. [Google Scholar]
- Hudson SB, Virgin EE, 2018. Town and country reptiles: a review of reptilian responses to urbanization. Integr Comp Biol 58:948–966. [DOI] [PubMed] [Google Scholar]
- Kozłowski J, 1992. Optimal allocation of resources to growth and reproduction: implications for age and size at maturity. Trends Ecol Evol 7:15–19. [DOI] [PubMed] [Google Scholar]
- Kurek K, Ćmiel A, Bury S, Zając B, Najberek K. et al. , 2019. What has happened to the females? Population trends in the Aesculapian snake at its northern range limit. Glob Ecol Conserv 17:e00550. [Google Scholar]
- Madsen T, Shine R, 1993. Phenotypic plasticity in body sizes and sexual size dimorphism in European grass snakes. Evolution 47:321–325. [DOI] [PubMed] [Google Scholar]
- Niemelä J, Breuste JH, Elmqvist T, Guntenspergen G, James P. et al. , 2011. Urban Ecology: Patterns, Processes and Applications. New York: Oxford University Press. [Google Scholar]
- Shine R, 1994. Sexual size dimorphism in snakes revisited. Copeia 1994:326–346. [Google Scholar]
- Wolfe AK, Bateman PW, Fleming PA, 2018. Does urbanization influence the diet of a large snake? Curr Zool 64:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.