Abstract
Glucocorticoids (GCs) are involved in the regulation of an animal’s energetic state. Under stressful situations, they are part of the neuroendocrine response to cope with environmental challenges. Animals react to aversive stimuli also through behavioral responses, defined as coping styles. Both in captive and wild populations, individuals differ in their behavior along a proactive–reactive continuum. Proactive animals exhibit a bold, active-explorative and social personality, whereas reactive ones are shy, less active-explorative and less social. Here, we test the hypothesis that personality traits and physiological responses to stressors covary, with more proactive individuals having a less pronounced GC stress response. In wild populations of invasive gray squirrels Sciurus carolinensis, we measured fecal glucocorticoid metabolites (FGMs), an integrated measure of circulating GCs, and 3 personality traits (activity, sociability, and exploration) derived from open field test (OFT) and mirror image stimulation (MIS) test. Gray squirrels had higher FGMs in Autumn than in Winter and males with scrotal testes had higher FGMs than nonbreeding males. Personality varied with body mass and population density. Squirrels expressed more activity-exploration at higher than at lower density and heavier squirrels had higher scores for activity-exploration than animals that weighed less. Variation in FGM concentrations was not correlated with the expression of the 3 personality traits. Hence, our results do not support a strong association between the behavioral and physiological stress responses but show that in wild populations, where animals experience varying environmental conditions, the GC endocrine response and the expression of personality are uncorrelated traits among individuals.
Keywords: FGM concentration, glucocorticoids, HPA axis reactivity, MCMCglmm, personality-traits, Sciurus carolinensis
Wild animals interact continuously with the environment and many of these interactions are mediated in part by the action of the hypothalamic–pituitary–adrenal (HPA) axis and by the autonomic nervous system. These neuro-endocrine responses allow animals to cope with a variety of environmental stimuli through the influence of its downstream products [e.g., glucocorticoids (GCs), catecholamines] on behavior, reproduction, growth, and energy allocation (Jansen et al. 1995; Sapolsky et al. 2000; Ricklefs and Wikelski 2002; Koolhaas et al. 2010). GCs, in terms of baseline activity of the HPA axis, affect the animal’s energetic state through the regulation of circulating glucose levels related to broad changes in activity patterns (e.g., seasons and life-history stages), and by influencing circadian patterns of appetite and foraging behaviors (Sapolsky et al. 2000; Crespi et al. 2013). GCs also play a mediatory role in processes that require energetic expenditure and resource allocation trade-offs, to cope with unpredictable or predictable environmental stressors, hence in the animal’s physiological stress response (Wingfield and Sapolsky 2003; Crespi et al. 2013; Wingfield and Romero 2015).
Animals can react to environmental stimuli also through a set of behavioral responses that are consistent within individuals over time, independently from their life-history state, sex, and motivational state, referred to as coping styles (Koolhaas et al. 1999; Pfeffer et al. 2002; Réale et al. 2007). Based on previous studies of farm or laboratory animals under controlled conditions, which demonstrated how the activity of the HPA axis is associated with certain coping styles, it was proposed that behavioral reactions to stressors are mediated by hormones exerting pleiotropic actions on both the animal’s behavior and physiology (Koolhaas et al. 1999; Carere et al. 2005; Dantzer and Swanson 2017), and that these relationships are conserved throughout the vertebrate lineage (Øverli et al. 2007).
Both in captive and wild populations, individuals differ in behaviors related to the animal’s personality, hence their tendency to behave consistently over time and in different contexts (Réale et al. 2007; Carere et al. 2010), along a proactive–reactive continuum (Koolhaas et al. 1999, 2010). In mammals, proactive animals tend to exhibit a bold, active-explorative, and social personality, whereas reactive ones are shy, less active-explorative, and less social (Carere et al. 2010; Koolhaas et al. 2010). Proactive individuals are predicted to exhibit high levels of catecholamines but low levels of GCs in response to an environmental challenge, whereas reactive animals are predicted to exhibit low levels of catecholamines and high levels of GCs in response to an adverse stimulus (Carere et al. 2005; Cockrem 2007; Koolhaas et al. 2010; Pusch et al. 2018; Raulo and Dantzer 2018). Despite several studies on laboratory animals finding these associations, the causal relationship between physiological responses and behavioral reactions is still debated (Carere et al. 2010; Koolhaas et al. 2010). In fact, in their review, Koolhaas et al (2010) proposed that individual variation can have several dimensions and that the behavioral response to a challenging condition (measured as the expression of single personality traits or of coping styles), is partly independent from the physiological response (stress reactivity). This hypothesis is known as the 2-tier model. According to the 2-tier model, individuals may show stable trait-like variation on 2 independent axes, a qualitative coping style axis and a quantitative stress reactivity axis. Measurements of traits along each axis (reactive–proactive traits along the behavioral axis, and low–high reactivity along the stress axis) do not necessarily need to be correlated.
In wild mammals, the measurement of fecal glucocorticoid metabolites (FGMs; Sheriff et al. 2011; Dantzer et al. 2014), which represents a combination of both baseline and stress-induced GC levels, has been used as an integrated measure of the animal’s HPA axis activity and reactivity over a specific period of time (Touma and Palme 2005; Sheriff et al. 2011; Palme 2019). Here, we measured FGM concentrations in different wild populations of invasive Eastern gray squirrel Sciurus carolinensis in Italy, to investigate the relationship between individual personality traits and HPA axis activity. Although catecholamines, also released from the adrenals, may further influence behavioral responses to environmental stimuli, we only measured the steroid hormone response in terms of changes in GCs. FGM was determined from fresh (<3 h old) feces taken from trapped animals to ensure they reflected typical GC levels from about 12–24 h before and were not influenced by trapping and handling (Bosson et al. 2013). We characterized the personality of squirrels along the proactive–reactive continuum through the open field test (OFT) and mirror image stimulation (MIS) test (Mazzamuto et al. 2018) and measured FGMs of individuals under different intrinsic (sex, reproductive condition, and body mass) and environmental conditions (season and squirrel density). First, we determined which behavioral groups of those that we measured (activity, exploration, shyness [from OFT], sociability, avoidance, activity-exploration, and alert [from MIS]; Mazzamuto et al. 2018) were repeatable within an individual and thus could be used as personality traits to describe a proactive or reactive coping style. Based on the 2-tier model (Koolhaas et al. 2010), we predicted that the personality traits would have significant repeatability (consistent differences among individuals) and would be correlated forming coping styles. Moreover, if FGM measures are a good indicator of individual variation in the GC stress response, they should also have some degree of repeatability, as previous studies have shown (Fanson and Biro 2018; Taff et al. 2018). We further tested whether squirrels that exhibit a reactive coping style (less active, exploratory, and sociable) also have higher FGMs, whereas those with a more proactive coping style (more active, exploratory, and sociable) have lower FGMs (Raulo and Dantzer 2018).
Materials and Methods
Study species
The Eastern gray squirrel S. carolinensis is a North American tree squirrel species which has been introduced in Britain, Ireland, and Italy (O’Teangana et al. 2000; Bertolino et al. 2014; Gurnell et al. 2015) where it negatively impacts native biodiversity, through interspecific competition for resources, disease-mediated competition, and damage to forestry (Gurnell et al. 2015; Romeo et al. 2015). Gray squirrels also increase levels of physiological stress (Santicchia et al. 2018a) and affect the expression of personality traits (Wauters et al. 2019) in co-occurring native red squirrels. Densities in natural habitats range from <1 to >5 squirrels/ha but can be much higher in parks and urban woodlands (Koprowski 1994). Home ranges overlap extensively between males and females, and home range size tends to be negatively related to squirrel density (Koprowski 1994; Shuttleworth et al. 2016). The social structure of gray squirrel populations is stable and hierarchical, principally determined by the sex, age, and relatedness, with older heavier animals dominant over smaller adults and subadults (Shuttleworth et al. 2016). This structure is mainly composed by kinship groups, which consist of mothers and their female offspring, and where agonistic behavior are minimal and amicable behaviors between related individuals are common (Koprowski et al. 1994; Gurnell et al. 2001). Alien gray squirrels in Italy have a poor macroparasite fauna (parasite-release, Romeo et al. 2014), and the probability of infection by the dominant gastro-intestinal helminth and intensity of infection (worms/infected host) are related to the squirrel’s personality (boldness-exploration tendency) and its body mass, respectively (Santicchia et al. 2019).
Study areas, trapping, and handling squirrels
We trapped gray squirrels in 5 study areas in Piedmont, Northern Italy, between November 2014 and December 2016: BER (4.9 ha); PIO (2.6 ha); RS (5.9 ha); MOR (37 ha), and COM (3.2 ha). All areas are woodlands or parks with mature broadleaf trees, mainly oaks Quercus robur, Q. petraea, hornbeam Carpinus betulus, lime Tilia cordata, and black walnut Juglans nigra, and few ornamental conifers, surrounded by agricultural landscapes. In each area, we carried out 2 (COM) to 3 (other areas) capture–mark–recapture sessions, once every 2 months between Autumn and early Spring (November/December to March/April), lasting 4–5 days. Number of traps used varied slightly between sessions and/or study areas. A trapping session involved the use of 16 (PIO), 16 (RS), 17 (BER), 30 (COM), 48 (MOR) ground-placed Tomahawk traps (model 202, Tomahawk Live Trap Co., Hazelhurst, WI, USA) evenly spaced throughout the areas, with a fine mesh added underneath traps to prevent contamination between urine and feces. Traps were checked 2–3 times/day to minimize time in trap and time since defecation (maximum 3 h). Each captured squirrel was individually marked using ear-tags, weighed (Pesola spring balance, ±5 g), measured (length of the right hind foot, ±0.5 mm), and sexed (Wauters and Dhondt 1995; Gurnell et al. 2001). Reproductive status was defined as nonbreeding (Nbr), post-estrus and pregnant (Preg), or lactating (Lact), for females, and nonbreeding with abdominal testes (Abd) or breeding with scrotal testes (Scr), for males. Details of the methods used to estimate gray squirrel population size are available in Supplementary Table S1. Trapping and handling squirrels complied with current laws on animal research in Italy and were carried out with permit of the authorities for wildlife research and management of Turin and Cuneo Provinces (Respectively, D.D. 294-34626 of 2014 and Prot. n. 0002624 of 13/01/2014) and of the Italian Institute for Environmental Protection and Research (ISPRA). All of these procedures abided by American Society of Mammalogists (ASM) guidelines (Sikes and Gannon 2011).
Feces collection, extraction of hormone metabolites, and enzyme immunoassay
Fecal samples of trapped squirrels were collected from underneath the traps and stored in vials (for details see Dantzer et al. 2010; Santicchia et al. 2018a). We only used fresh fecal samples (<3 h) from squirrels that had not previously been trapped or handled within 72 h prior to capture to minimize effects of capture stress on FGMs (Dantzer et al. 2010; Bosson et al. 2013). Each fecal sample was classified as being taken in the morning (10.00–13.00 h) or in the afternoon (15.00–18.00 h) to account for potential variation in FGMs over the 24-h cycle (Palme 2019).
We used a 5α-pregnane-3β, 11β, 21-triol-20-one enzyme immunoassay (EIA) to measure FGMs (ng/g dry feces; Touma et al. 2003; Dantzer et al. 2010; Santicchia et al. 2018a). This EIA detects GC metabolites with a 5α-3β, 11β-diol structure (for cross-reactivity see Touma et al. 2003). Assay validation in this species showed how fecal samples collected from traps represent an integrated measure of cortisol produced ∼16 h before defecation (FGMs peak between 12 and 24 h after ACTH challenge, Bosson et al. 2013). Details of the EIA procedure and its validation for Eastern gray squirrels can be found elsewhere (Bosson et al. 2013). Samples were analyzed in duplicate. We assayed 342 fecal samples of gray squirrels. Pools of gray squirrel fecal extracts were used as intra-assay controls at dilutions of 1:50 (∼30% binding) and 1:400 (∼70% binding). Average intra-assay coefficients of variation (CVs) were 8.7% and 14.8%, respectively, for pools diluted 1:50 and 1:400. Interassay CVs were estimated from standards of known concentration with a high (n = 25 plates, 12.4% binding) and low (n = 25 plates, 80.9% binding) concentration that had inter-assay CVs of 15.2% and 9.1%, respectively.
Personality measured with arena test
We performed 128 arena tests on a restricted sample of 83 individuals, from COM (n = 35, 14 males, 21 females) and MOR (n = 48, 22 males, 26 females). In 96 cases (41 males, 55 females), we also had FGM measures. Arena tests consisted of an OFT to measure the expression of the personality traits activity, exploration, and shyness in a novel environment, followed by a MIS test to determine the animal’s degree of sociability or avoidance, aggressiveness, and being alert toward a conspecific, as well as its tendency for expressing behaviors that define a combined activity-exploration trait (Mazzamuto et al. 2018; for details see Supplementary Table S2). In this study, the OFT lasted 6 min and MIS 4 min.
Repeatability estimates of personality traits
On 37 individuals tested more than once (32, 2 times; 5, 3 times; n = 79 tests), we estimated repeatability of the expert-based personality traits (Mazzamuto et al. 2018) as the between-individual variation divided by the sum of the between-individual and residual variation, using Bayesian generalized linear mixed effects models based on a Markov Chain Monte Carlo algorithm with the R package MCMCglmm version 2.26 (Hadfield 2010). The personality-trait scores were square root transformed before analysis. Each model had a personality trait as dependent variable and study area, sex, and experiment order (1st, 2nd, or 3rd test) as fixed effects, and squirrel identity as random effect (SM3 in Supplementary Material). Posterior distributions were based on 1,050,000 iterations with a burn-in of 50,000 iterations and thinning of 40, such that 25,000 iterations were used to obtain point estimates and 95% credibility intervals (CIs). For the random effects and residual variation, an inverse-gamma prior uninformative for the model was used (Wilson et al. 2010). We found moderate repeatabilities (R > 0.20; see also Bell et al. 2009) for activity from OFT and for sociability and activity-exploration tendency (referred to trait “other” in Mazzamuto et al. 2018) from MIS (details in Supplementary Table S2 and in Section SM3 in Supplementary Material), which were further used as personality traits in the MCMCglmm model below.
Analysis of personality–stress relationships
We applied a multivariate mixed model fitted in a Bayesian framework using the package MCMCglmm in R (Hadfield 2010). The 3 retained expert-based personality traits (activity from OFT, sociability and “other” [activity-exploration] from MIS) and FGM concentrations were used as dependent variables after standardization (with 0 mean and variance equal to 1), using a Gaussian residual error distribution. As repeated observations were present, individual was added as a random effect. For both the residual and between-individual variation, an unstructured variance–covariance matrix was modelled, allowing the estimation of correlations among the response variables (covariance divided by the square root of the product of the variances). Sex, arena test order, daytime (animal sampled in morning or afternoon), season [Winter (December to March), Spring to Summer (April to August), or Autumn (September to November)] were included as fixed effects (factors) and also the standardized continuous variables body mass and population density were added as fixed effects. Reproductive condition was added as a fixed effect for males (with 2 levels: nonbreeding and breeding) and as a separate factor for females (with 3 levels: nonbreeding, pregnant, lactating). Daytime and reproductive condition were added as fixed effect to account for potential differences in FGMs due to diel rhythm and reproductive activity in males and females (Goymann 2012; Dantzer et al. 2016; Palme 2019). The effects of daytime, reproductive condition, and season were set to 0 for the dependent variables activity, sociability, and activity-exploration (hence estimating their relationship only with FGM concentrations) and the effect of arena test order was set to 0 for FGM. Posterior distributions were based on 1,050,000 iterations with a burn-in of 50,000 iterations and thinning of 40, such that 25,000 iterations were used to obtain point estimates and 95% credibility intervals. For the random effects and residual variation, a parameter-expanded prior uninformative for the model was used (Houslay and Wilson 2017). Also, we applied the Gelman–Rubin statistic (Gelman and Rubin 1992) and Geweke diagnostic (Geweke 1992) which confirmed model consistency and convergence. Details of model script and output are provided in Section SM4 in Supplementary Material. There were 3 samples with very high FGMs (66,550; 68,090; and 79,675 ng/g dry feces) but eliminating them from the dataset did not change model outputs (results not shown).
Results
Patterns of variation in FGM concentrations in gray squirrels
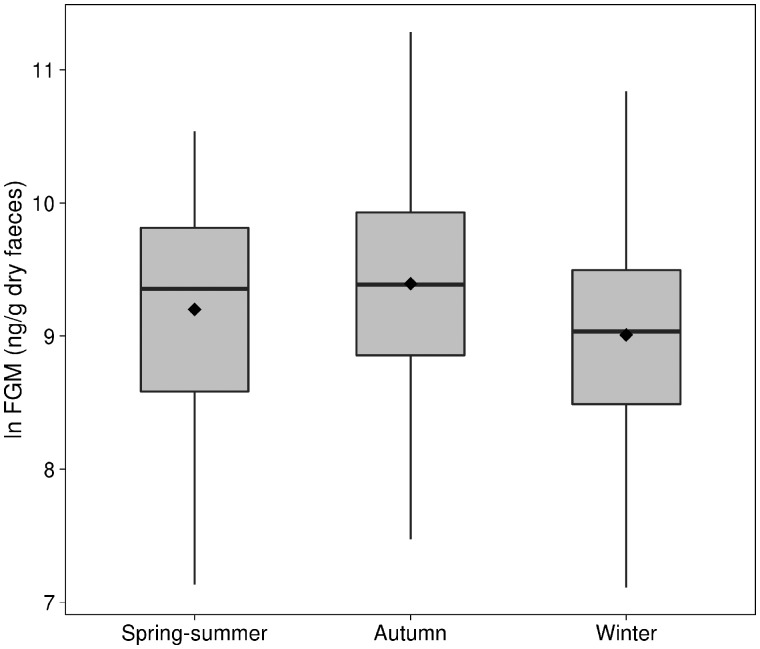
We used 340 samples of 193 different animals (mean samples/id = 1.76; range 1–4). FGM concentrations in gray squirrels were highly variable (mean ± SD = 12,610 ± 10,749; range: 1,226–79,675 ng/g feces). Samples collected during the morning had lower FGM concentrations than samples collected during the afternoon (estimate β = −0.28, 95% CI = −0.49 to −0.06, pMCMC = 0.01). FGM varied seasonally, with higher values in Autumn than in Winter (β = 0.52, 95% CI = 0.15–0.88, pMCMC = 0.005), but no difference between Autumn and Spring to Summer (Spring to Summer versus Autumn β = −0.40, 95% CI = −0.88 to 0.10, pMCMC = 0.11; Figure 1). There was no effect of gray squirrel population density on FGM (β = −0.01, 95% CI = −0.18 to 0.17, pMCMC = 0.92). Among females, changes in reproductive condition did not influence variation in FGM (see Section SM4 in Supplementary Material), whereas among males, animals with abdominal testes (nonbreeding) tended to have lower FGM concentrations than males with scrotal testes (breeding; β = −0.32, 95% CI = −0.64 to 0.00, pMCMC = 0.051; Figure 2).
Figure 1.
FGM concentrations (ln transformed) in gray squirrels captured in Spring to Summer (n = 61), Autumn (n = 91), or Winter (n = 188). Boxplots show median (solid horizontal line), mean (black diamond), and 1st (25%) and 3rd (75%) quartiles.
Figure 2.
FGM concentrations (ln transformed) in female and male gray squirrels in relationship to reproductive condition, defined as nonbreeding (Nbr, n = 116), post-estrus and pregnant (Preg, n = 39) or lactating (Lact, n = 26), for females; and nonbreeding with abdominal testes (Abd, n = 70) or breeding with scrotal testes (Scr, n = 89), for males. Boxplots show median (solid horizontal line), mean (black diamond), and 1st (25%) and 3rd (75%) quartiles.
Relationship between FGM concentrations and personality
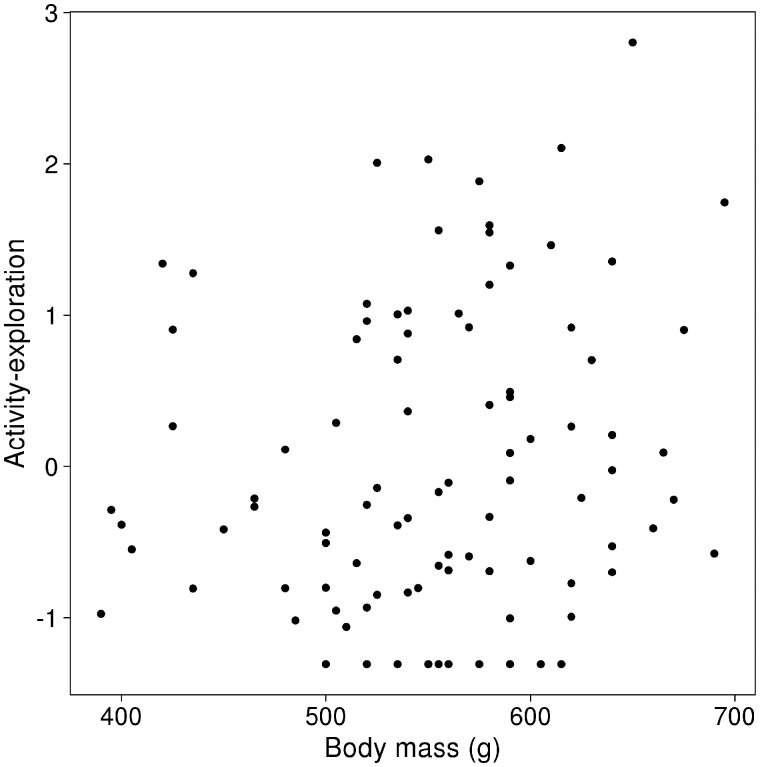
Activity measured during OFT (R = 0.43, 95% CI = 0.15–0.67) and activity-exploration tendency measured during MIS (R = 0.42, 95% CI = 0.17–0.71) were repeatable among multiple measures within the same individual. Also sociability (MIS) had moderate repeatability (R = 0.29, 95%CI = 0.004–0.54) (more details in Supplementary Table S2). Hence, we retained these 3 behavioral groups as personality traits in our MCMCglmm model (SM4). Heavier gray squirrels were more active explorers than individuals with lower body mass (β = 0.28, 95% CI = 0.07–0.47, pMCMC = 0.006; Section SM4 in Supplementary Material; Figure 3) and squirrels expressed more activity-exploration during the MIS test when densities were higher (β = 0.16, 95% CI = 0.005–0.31, pMCMC = 0.047; Section SM4 in Supplementary Material). Estimates of correlations between the 3 personality traits suggested that active individuals tended to be also more sociable and more explorative, although the credible intervals overlapped 0 (Table 1 and SM4 in Supplementary Material). The correlations of the 3 personality traits with FGMs were close to 0 (Table 1) indicating the lack of an association between the physiological and behavioral stress response in this species. Activity had the largest between-individual variance and the smallest within-individual variance, indicating that an individual squirrel is consistent in its activity in the OFT but there is broad variation in activity among individuals in the populations (Table 1). In contrast, FGMs had the smallest between-individual and the largest within-individual variance (repeatability R = 0.05, 95% CI = 0.00–0.14), suggesting it fluctuates within a limited species-specific range, but each individual’s FGM can vary strongly over most of that range (Table 1). The within-individual covariance estimates among the personality traits and FGM were small and their 95% CIs included 0 (Table 1).
Figure 3.
Association between gray squirrel body mass at the time of capture and the standardized score of the personality trait activity-exploration measured during MIS test carried out after the capture event (n = 128, for details, see results MCMCglmm model).
Table 1.
Results of the multivariate MCMCglmm model
| Parameter | Activity | Sociability | Activity/exploration | FGM |
|---|---|---|---|---|
| Activity | 0.66 (0.29 to 1.06) | 0.40 | 0.19 | −0.15 |
| 0.30 (0.12 to 0.54) | (−0.12 to 0.88) | (−0.31 to 0.68) | (−0.88 to 0.63) | |
| Sociability | 0.18 (−0.05 to 0.45) | 0.33 (0.85−8 to 0.67) | 0.30 | −0.16 |
| 0.03 (−0.17 to 0.27) | 0.78 (0.41 to 1.18) | (−0.32 to 0.88) | (−0.88 to 0.70) | |
| Activity/exploration | 0.11 (−0.14 to 0.35) | 0.12 (−0.08 to 0.36) | 0.45 (0.36−7 to 0.79) | 0.02 |
| 0.09 (−0.09 to 0.30) | 0.12 (−0.11 to 0.37) | 0.48 (0.20 to 0.84) | (−0.74 to 0.78) | |
| FGM | −0.03 (−0.18 to 0.10) | −0.02 (−0.15 to 0.08) | 0.003 (−0.11 to 0.12) | 0.04 (0.26−9 to 0.14) |
| 0.04 (−0.19 to 0.26) | −0.14 (−0.42 to 0.13) | −0.02 (−0.27 to 0.22) | 0.91 (0.75 to 1.07) |
Between-individual and within-individual variances are listed on the diagonal (within-individual in italics), covariances below the diagonal (within-individual in italics), and correlations in bold above the diagonal (lower and upper bounds of 95% credibility intervals in brackets).
Discussion
We showed that in free-ranging gray squirrels, OFT, and MIS test in an arena returned moderate within-individual repeatability for 3 personality traits: activity (OFT), sociability (MIS), and activity-exploration (MIS) tendency. Although active squirrels also tended to be more social and more explorative, suggesting a proactive coping style, correlations from the MCMglmm model did not exclude 0 from the 95% credibility intervals. Furthermore, we found that neither of the 3 personality traits covaried with 1 measure (FGMs) of the physiological stress response in a wild mammal. This main result confirmed the findings from an earlier study on free-ranging North American red squirrels Tamiasciurus hudsonicus that variation in the behavioral response and variation in the physiological stress response are independent and not correlated (Westrick et al. 2019). Hence, our results do not fully support the 2-tier hypothesis (Koolhaas et al. 2010) but suggest that under variable natural conditions individuals can express consistent behavioral responses that are independent from their physiological stress response. In other words, whether animals exhibit a more proactive (high activity, exploration, and sociability) or a more reactive (low activity and/or exploration and less sociable) personality is not functionally related to low or high HPA axis reactivity.
General pattern of FGM concentration variation
FGM concentrations were significantly higher in Autumn than in Winter, with a nonsignificant difference between Autumn and Spring to Summer. During the Autumn gray squirrels are subject to an increase in intraspecific interactions due to competition for feeding and caching of high-energy tree seeds (Koprowski 1994). Moreover, Autumn is also the period of juvenile/subadult dispersal, which may force resident adult males and female kin group to defend their core-areas to limit immigration (Koprowski 1994; Gurnell et al. 2001). This increase in social pressure and foraging activity could explain the observed seasonal differences in FGM concentrations, that were also reported in previous studies on gray squirrels (Bosson et al. 2013) and other tree squirrel species (S. vulgaris, Dantzer et al. 2016).
Patterns of variation in FGMs with reproductive condition in gray squirrels were, albeit only partly, in agreement with findings of previous studies on sciurids (Montiglio et al. 2015; Dantzer et al. 2016). Males with scrotal testes (reproductively active) had on average higher FGMs than those with abdominal testes (no reproductive activity). Males with scrotal testes will engage in mating chases and compete intensively with other males for access to the estrus female (Koprowski 1994). This high intraspecific contact and the many aggressive interactions among the competing males may result in the observed increase in FGMs (see also Santicchia et al. 2018a for S. vulgaris). Among females, there were no marked differences in FGMs between pregnant, lactating or nonbreeding individuals, in contrast with findings on Eurasian red squirrels (Dantzer et al. 2016). Overall, differences in FGMs depending on reproductive condition could match a change in circulating hormones or, alternatively, metabolism or gut passage time modifications (Goymann 2012). Although these factors could also lead to sex differences in GC levels (Touma et al. 2003; Palme 2019), we found no difference in mean FGMs between males and females, in agreement with previous studies on tree squirrels (Dantzer et al. 2010, 2016; Bosson et al. 2013; Santicchia et al. 2018a).
FGM concentrations of gray squirrels did not covary with changes in squirrel density. This result is in contrast with findings reported in studies on other rodents (deer mice Peromyscus maniculatus, southern red-backed voles Clethrionomys gapperi: Harper and Austad 2004; root vole Microtus oeconomus: Bian et al. 2011; North American red squirrel T. hudsonicus: Dantzer et al. 2013; Algerian mice Mus spretus: Navarro-Castilla et al. 2017). We did not measure other potential sources of physiological stress that could differ among the study areas, such as anthropogenic disturbance (Wingfield 2013; Dantzer et al. 2014; Rehnus et al. 2014), differential predation pressure (Clinchy et al. 2013) or differences in parasite load (Raouf et al. 2006).
Lack of repeatability in FGM
In a recent meta-analysis on the repeatability of GC measures in vertebrates, Schoenemann and Bonier (2018) found 12 studies that used FGM as in integrated measure of GCs. Five of these were on wild mammals (4 sciurid species and 1 deer species). A previous study in our study population of Eurasian red squirrels reported high and significant repeatability for wild-caught but captive held animals on a 48-h time span (n = 17, R = 0.52, 95% CI = 0.25–0.69). However, this significant repeatability was not confirmed in a larger dataset of wild-caught animals trapped over a much longer sampling interval (n = 82, R = 0.12, 95% CI = 0–0.45; Dantzer et al. 2016). The other 4 studies from Schoenemann and Bonier (2018) reported repeatabilities of GC measures ranging from 0.12 to 0.57, with the latter value over a short sampling interval (0–7 days). Hence, the repeatability in our study (R = 0.05, 95% CI = 0–0.14) was very low and comparable with those found by Dantzer et al (2016) for wild-caught Eurasian red squirrels and reported by Schoenemann and Bonier (2018) for yellow-bellied marmots Marmota flaviventris (R = 0.12). Another meta-analysis by Fanson and Biro (2018) reported FGM repeatabilities from 16 studies on wild-caught mammals (13 species), with values ranging from 0 to 0.67.
In our study, the low repeatability was likely due to a combination of relatively low among-individual and high within-individual variation in FGM. If high within-individual variance is a concern, the study should control for as many sources of environmental and life-cycle variation as possible (Schoenemann and Bonier 2018). We addressed this by adding the effects of squirrel density, daytime, season, sex, reproductive condition, and the animal’s body mass in our model. Nevertheless, we must admit that the number of samples per individual was low, which tends to increase estimates of within-individual variance.
Relationship between physiological stress and personality
We did not find significant correlations between FGMs and the 3 personality traits that describe the proactive–reactive continuum. In a recent review on a wide variety of vertebrate species, only 46% of studies that measured personality and GCs found a negative relationship between stress responsiveness or GC levels and personality as defined by the proactive and reactive profiles (Raulo and Dantzer 2018). Fifteen studies reported a lack of any correlation, as we found here. Also, other studies on sciurid species reported a similar result that certain personality traits, mainly activity and exploration, were not related to GC levels (Ferrari et al. 2013; Clary et al. 2014; Montiglio et al. 2015; Westrick et al. 2019). Also for sociability, measured as the individual’s tendency to slowly approach or sit close to its mirror image, hence its willingness to engage in social contact, there was no relationship between FGMs and the expression of sociability. This can be explained by diverse factors that can influence how the tendency for an individual to behave more socially and their levels of GCs interact with one another (Creel et al. 2013). For example, behaviors related to the acquisition and maintenance of social status (rank) are likely to affect the degree of social stress and GC levels associated with that social status (Goymann and Wingfield 2004). Moreover, environmental factors like changes in resource availability or predator pressure might have different effects on low-ranked than on high-ranked individuals, affecting the social status–GC relationships (Creel et al. 2013; Dantzer et al. 2017). The gray squirrel has a social system intermediate between solitary and social group-living species (Koprowski 1994; Gurnell et al. 2001): males are solitary but with overlapping home ranges, whereas adult females tend to form female kin-groups (philopatric daughters). Females from a kin-group do not forage together but have strongly overlapping core-areas; they rarely interact aggressively and may share dreys or dens (Gurnell et al. 2001). However, so far it is unknown to what degree differences in social status in this species are correlated with the expression of sociability measured during MIS test; a relationship that should be investigated in future studies.
In this study, more active and exploratory squirrels had a higher body mass than less active/exploring animals. A similar positive association was found between a boldness-exploration score estimated with an indirect method (PCA score derived from trappability and trap-diversity indices) and body mass of gray squirrels in our study areas (Santicchia et al. 2019). Less active-explorative animals may be less efficient foragers and/or may be less likely to find high-quality food patches than more active-explorative ones, and this could produce a fitness advantage for phenotypes with high body mass and strong active-exploration tendency, at least under certain environmental conditions (Le Coeur et al. 2015; but see Santicchia et al. 2018b). In fact, a recent study on wild great tits (Parus major) demonstrated that an individual’s morphological (body size and body condition) and behavioral traits represent an expression of an integrated phenotype and suggested that phenotypic integration can play a role in generating animal personalities (Moiron et al. 2019).
In conclusion, using FGMs as an integrated measure of physiological stress, we showed there was no significant association between the expression of personality traits and physiological stress response in wild gray squirrels that live under (spatio-temporal) variable environmental conditions. However, it should be noted that the quantification of FGM concentrations in wild mammals represents a mix of basal circulating GCs and stress-induced GCs and may not allow for direct measurements of the reactivity of the HPA axis, which may correlate more strongly with behavioral stress responses (Baugh et al. 2013; Westrick et al. 2019). Despite this caveat, our results confirm the findings of a growing number of studies that tested for covarying behavioral and physiological stress responses in natural populations of free-ranging fish, birds, and mammals (reviewed in Raulo and Dantzer 2018; and Table 1 in Westrick et al. 2019), but did not find a positive relationship between personality traits representing a reactive profile and high HPA axis reactivity. However, it must be noted that in some study species conflicting results have been found, depending on which particular personality trait was used (e.g., Clary et al. 2014) but also on the type of physiological measurement or on sample size (e.g., Baugh et al. 2013; Ferrari et al. 2013). For example, in Richardson’s ground squirrels (Urocitellus richardsonii), there was a positive association between vigilance and FGMs, but no association between exploration and FGMs (Clary et al. 2014). In alpine marmots (M. marmota), there was a positive relationship between activity/exploration and blood cortisol in a small sample (n = 28), thus in the opposite direction than predicted by the 2-tier model. However, in a larger sample (n = 146) there was no association between 3 personality traits (activity, impulsivity, and docility) and blood cortisol (Ferrari et al. 2013). In great tits, there was a positive association between exploration and true baseline blood corticosterone, but a negative association between exploration and stress-induced blood corticosterone (measured after a 90 min handling-restraint, Baugh et al. 2013). To overcome some of these problems, we suggest that studies on wild animals exploring relationships between personality/behavior and the physiological stress response should measure both FGM concentrations and the expression of several personality traits multiple times over a seasonal and/or annual time-period and determine the degree of between-individual as well as that of the within-individual variation. Combining measures of an individual’s average HPA axis reactivity and its variability over time and relating those with measures of behavioral consistency and plasticity may allow us to discover associations between personality and stress response not documented so far.
Supplementary Material
Acknowledgments
Thanks to Zainab Almusawi and Teera Losch for helping in laboratory analysis, Candice Gagnaison, Laure Vanlauwe, and Mattia Panzeri for assistance with the fieldwork. Constructive comments by David Mitchell, 2 anonymous reviewers, and the associate editor James Hare greatly helped us to improve the manuscript. We are grateful to the private land owners for access to their estates.
Authors’ Contributions
F.S., L.A.W., and N.F. designed the study and the analyses, and A.M. supervised the project. Fieldwork and data collection were done by F.S., C.R., and L.A.W. F.S. carried out laboratory analyses and B.D. supplied laboratory space, equipment, and coordinated laboratory analyses. R.P. produced and supplied reagents for lab analyses. F.S. and S.E.W. carried out statistical analyses with the contribution of C.R., D.G.P., and B.D. The manuscript was drafted by F.S. and L.A.W.; all other authors contributed to improve the manuscript and gave approval for publication.
References
- Baugh AT, van Oers K, Naguib M, Hau M, 2013. Initial reactivity and magnitude of the acute stress response associated with personality in wild great tits Parus major. Gen Comp Endocrinol 189:96–104. [DOI] [PubMed] [Google Scholar]
- Bell AM, Hankinson SJ, Laskowski KL, 2009. The repeatability of behaviour: ameta-analysis. Anim Behav 77:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino S, Montezemolo NC, Preatoni DG, Wauters LA, Martinoli A, 2014. A grey future for Europe: Sciurus carolinensis is replacing native red squirrels in Italy. Biol Invasions 16:53–62. [Google Scholar]
- Bian J-H, Wu Y, Getz LL, Cao Y-F, Chen F. et al. , 2011. Does maternal stress influence winter survival of offspring in root voles Microtus oeconomus? A field experiment. Oikos 120:47–56. [Google Scholar]
- Bosson CO, Palme R, Boonstra R, 2013. Assessing the impact of live-capture, confinement, and translocation on stress and fate in eastern gray squirrels. J Mammal 94:1401–1411. [Google Scholar]
- Calisi RM, Bentley GE, 2009. Lab and field experiments: are they the same animal? Horm Behav 56:1–10. [DOI] [PubMed] [Google Scholar]
- Carere C, Drent PJ, Privitera L, Koolhaas JM, Groothuis TGG, 2005. Personalities in great tits, Parus major: stability and consistency. Anim Behav 70:795–805. [Google Scholar]
- Carere C, Caramaschi D, Fawcett TW, 2010. Covariation between personalities and individual differences in coping with stress: converging evidence and hypotheses. Curr Zool 56:728–740. [Google Scholar]
- Clary D, Skyner LJ, Ryan CP, Gardiner LE, Anderson WG. et al. , 2014. Shyness-boldness, but not exploration, predicts glucocorticoid stress response in Richardson’s ground squirrels Urocitellus richardsonii. Ethology 120:1101–1109. [Google Scholar]
- Clinchy M, Sheriff MJ, Zanette LY, 2013. Predator-induced stress and the ecology of fear. Funct Ecol 27:56–65. [Google Scholar]
- Cockrem JF, 2007. Stress, corticosterone responses and avian personalities. J Ornithol 148:169–178. [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR, 2013. The ecology of stress: effects of the social environment. Funct Ecol 27:66–80. [Google Scholar]
- Crespi EJ, Williams TD, Jessop TS, Delehanty B, 2013. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct Ecol 27:93–106. [Google Scholar]
- Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S. et al. , 2010. Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167:279–286. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S. et al. , 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340:1215–1217. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ, 2014. Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol 2:cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer B, Santicchia F, van Kesteren F, Palme R, Martinoli A. et al. , 2016. Measurement of fecal glucocorticoid metabolite levels in Eurasian red squirrels Sciurus vulgaris: effects of captivity, sex, reproductive condition, and season. J Mammal 97:1385–1398. [Google Scholar]
- Dantzer B, Bennet NC, Clutton-Brock TH, 2017. Social conflict and costs of cooperation in meerkats are reflected in measures of stress hormones. Behav Ecol 28:1131–1141. [Google Scholar]
- Dantzer B, Swanson EM, 2017. Does hormonal pleiotropy shape the evolution of performance and life history traits? Integr Comp Biol 57:372–384. [DOI] [PubMed] [Google Scholar]
- Fanson KV, Biro PA, 2018. Meta-analytic insights into factors influencing the repeatability of hormone levels in agricultural, ecological, and medical fields. Am J Physiol Regul Integr Comp Physiol 316:R101–R109. [DOI] [PubMed] [Google Scholar]
- Ferrari C, Pasquaretta C, Carere C, Cavallone E, von Hardenberg A. et al. , 2013. Testing for the presence of coping styles in a wild mammal. Anim Behav 85:1385–1396. [Google Scholar]
- Gelman A, Rubin DB, 1992. Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472. [Google Scholar]
- Geweke J, 1992. Evaluating the accuracy of sampling-based approaches to the calculation of posterior moments In: Bernardo JM, Berger AP, Dawid AP, Smith AFM, editors. Bayesian Statistics 4. Oxford: Oxford University Press; 169–193. [Google Scholar]
- Goymann W, 2012. On the use of non‐invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3:757–765. [Google Scholar]
- Goymann W, Wingfield JC, 2004. Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav 67:591–602. [Google Scholar]
- Gurnell J, Wauters LA, Preatoni D, Tosi G, 2001. Spacing behaviour, kinship and dynamics of the grey squirrel in a newly colonised deciduous woodland in north Italy. Can J Zool 79:1533–1543. [Google Scholar]
- Gurnell J, Lurz PWW, Wauters LA, 2015. Years of interactions and conflict in Europe: competition between Eurasian red squirrels and North American grey squirrel In: Shuttleworth CM, Lurz PWW, Hayward MW, editors. Red Squirrels: Ecology, Conservation & Management in Europe. England: European Squirrel Initiative, Warwickshire. 19–37. [Google Scholar]
- Hadfield JD, 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Soft 33:1–22. http://www.jstatsoft.org/v33/i02/. [Google Scholar]
- Harper JM, Austad SN, 2004. Fecal corticosteroid levels in free-living populations of deer mice Peromyscus maniculatus and southern redbacked voles Clethrionomys gapperi. Am Midl Nat 152:400–409. [Google Scholar]
- Houslay TM, Wilson AJ, 2017. Avoiding the misuse of BLUP in behavioural ecology. Behav Ecol 28:948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen ASP, Van Nguyen X, Karpitskiy V, Mettenleiter TC, Loewy AD, 1995. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270:644–646. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Dervegt BJ, Van Reenen CG. et al. , 1999. Coping style in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B, 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31:307–321. [DOI] [PubMed] [Google Scholar]
- Koprowski JL, 1994. Sciurus carolinensis. Mamm Species 480:1–9. [Google Scholar]
- Le Coeur C, Thibault M, Pisanu B, Thibault S, Chapuis J-L. et al. , 2015. Temporally fluctuating selection on a personality trait in a wild rodent population. Behav Ecol 26:1285–1291. [Google Scholar]
- Mazzamuto MV, Cremonesi G, Santicchia F, Preatoni D, Martinoli A. et al. , 2018. Rodents in the arena: a critical evaluation of methods measuring personality traits. Ethol Ecol Evol 31:38–58. [Google Scholar]
- Moiron M, Araya-Ajoy YG, Mathot KJ, Mouchet A, Dingemanse NJ, 2019. Functional relations between body mass and risk-taking behaviour in wild great tits. Behav Ecol 30:617–623. [Google Scholar]
- Montiglio P-O, Garant D, Pelletier F, Réale D, 2015. Intra-individual variability in fecal cortisol metabolites varies with lifetime exploration and reproductive life history in eastern chipmunks Tamias striatus. Behav Ecol Sociobiol 69:1–11. [Google Scholar]
- Navarro-Castilla Á, Diaz M, Barja I, 2017. Does ungulate disturbance mediate behavioural and physiological stress responses in Algerian mice Mus spretus? A wild exclosure experiment. Hystrix 28:165–172. [Google Scholar]
- O’Teangana D, Reilly S, Montgomery WI, Rotchford J, 2000. Distribution and status of the red squirrel Sciurus vulgaris and grey squirrel Sciurus carolinensis in Ireland. Mamm Rev 30:45–56. [Google Scholar]
- Øverli Ø, Sørensen C, Pulman KG, Pottinger TG, Korzan W. et al. , 2007. Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev 31:396–412. [DOI] [PubMed] [Google Scholar]
- Palme R, 2019. Non-invasive measurement of glucocorticoids: advances and problems. Physiol Behav 199:229–243. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Fritz J, Kotrschal K, 2002. Hormonal correlates of being an innovative greylag goose Anser anser. Anim Behav 63:687–695. [Google Scholar]
- Pusch EA, Bentz AB, Becker DJ, Navara KJ, 2018. Behavioral phenotype predicts physiological responses to chronic stress in proactive and reactive birds. Gen Comp Endocrinol 255:71–77. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available from: https://www.R-project.org/ [Google Scholar]
- Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR, 2006. Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim Behav 71:39–48. [Google Scholar]
- Raulo A, Dantzer B, 2018. Associations between glucocorticoids and sociality across a continuum of vertebrate social behavior. Ecol Evol 8:7697–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ, 2007. Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. [DOI] [PubMed] [Google Scholar]
- Rehnus M, Wehrle M, Palme R, 2014. Mountain hares Lepus timidus and tourism: stress events and reactions. J Appl Ecol 51:6–12. [Google Scholar]
- Ricklefs R, Wikelski M, 2002. The physiology/life-history nexus. Trends Ecol Evol 17:462–468. [Google Scholar]
- Romeo C, Wauters LA, Ferrari N, Lanfgranchi P, Martinoli A. et al. , 2014. Macroparasite fauna of alien grey squirrels Sciurus carolinensis: composition, variablity and implications for native species. PLoS ONE 9:e88002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo C, Ferrari N, Lanfranchi P, Saino N, Santicchia F. et al. , 2015. Biodiversity threats from outside to inside: effects of alien grey squirrel Sciurus carolinensis on helminth community of native red squirrel Sciurus vulgaris. Parasitol Res 114:2621–2628. [DOI] [PubMed] [Google Scholar]
- Romero LM, 2004. Physiological stress in ecology: lessons from biomedical research Trends Ecol Evol 19:249–255. [DOI] [PubMed] [Google Scholar]
- Santicchia F, Dantzer B, van Kesteren F, Palme R, Martinoli A. et al. , 2018a. Stress in biological invasions: introduced invasive grey squirrels increase physiological stress in native Eurasian red squirrels. J Anim Ecol 87:1342–1352. [DOI] [PubMed] [Google Scholar]
- Santicchia F, Gagnaison C, Bisi F, Martinoli A, Matthysen E. et al. , 2018b. Habitat-dependent effects of personality on survival and reproduction in red squirrels. Behav Ecol Socbiol 72:134. [Google Scholar]
- Santicchia F, Romeo C, Ferrari N, Matthysen E, Vanlauwe L. et al. , 2019. The price of being bold? Relationships between personality and endoparasitic infection in a tree squirrel. Mamm Bio 97:1–8. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Schoenemann KL, Bonier F, 2018. Repeatability of glucocorticoid hormones in vertebrates: a meta-analysis. PeerJ 6:e4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R, 2011. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CM, Lurz PWW, Gurnell J, 2016. The Grey Squirrel: Ecology and Management of an Invasive Species in Europe European Squirrel Initiative. England: Warwickshire. [Google Scholar]
- Sikes RS, Gannon WL, 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff CC, Schoenle LA, Vitousek MN, 2018. The repeatability of glucocorticoids: a review and meta-analysis. Gen Comp Endocrinol 260:136–145. [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R, 2005. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046:54–74. [DOI] [PubMed] [Google Scholar]
- Touma C, Sachser N, Möstl E, Palme R, 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278. [DOI] [PubMed] [Google Scholar]
- Wauters LA, Dhondt AA, 1995. Lifetime reproductive success and its correlates in female Eurasian red squirrels. Oikos 72:402–410. [Google Scholar]
- Wauters LA, Mazzamuto MV, Santicchia F, Van Dongen S, Preatoni DG. et al. , 2019. Interspecific competition affects the expression of personality-traits in natural populations. Sci Rep 9:11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrick SE, van Kesteren F, Palme R, Boonstra R, Lane JE. et al. , 2019. Stress activity is not predictive of coping style in North American red squirrels. Behav Ecol Socbiol 73:113. [Google Scholar]
- Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E. et al. , 2010. An ecologist’s guide to the animal model. J Anim Ecol 79:13–26. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM, 2003. Reproduction and resistance to stress: when and how. J Neuroendocrinol 15:711–724. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, 2013. Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct Ecol 27:37–44. [Google Scholar]
- Wingfield JC, Romero ML, 2015. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford series in behavioural neuroendocrinology. New York: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.