Abstract
Diverse spatio-temporal aspects of avian migration rely on relatively rigid endogenous programs. However, flexibility in migratory behavior may allow effective coping with unpredictable variation in ecological conditions that can occur during migration. We aimed at characterizing inter- and intraindividual variation of migratory behavior in a forest-dwelling wader species, the Eurasian woodcock Scolopax rusticola, focusing on spatio-temporal consistency across repeated migration episodes. By satellite-tracking birds from their wintering sites along the Italian peninsula to their breeding areas, we disclosed a remarkable variability in migration distances, with some birds flying more than 6,000 km to Central Asian breeding grounds (up to 101°E). Prebreeding migration was faster and of shorter duration than postbreeding migration. Birds moving over longer distances migrated faster during prebreeding migration, and those breeding at northernmost latitudes left their wintering areas earlier. Moreover, birds making longer migrations departed earlier from their breeding sites. Breeding site fidelity was very high, whereas fidelity to wintering areas increased with age. Migration routes were significantly consistent, both among repeated migration episodes and between pre- and postbreeding migration. Prebreeding migration departure date was not significantly repeatable, whereas arrival date to the breeding areas was highly repeatable. Hence, interindividual variation in migratory behavior of woodcocks was mostly explained by the location of the breeding areas, and spatial consistency was relatively large through the entire annual cycle. Flexibility in prebreeding migration departure date may suggest that environmental effects have a larger influence on temporal than on spatial aspects of migratory behavior.
Keywords: arrival date, breeding latitude, flexibility, repeatability, satellite tracking, wading birds
Diverse aspects of seasonal migrations are controlled by relatively rigid endogenous mechanisms, ensuring that individuals perform specific activities at the appropriate time of the annual cycle (Berthold et al. 2003). Individuals may, however, show some degree of flexibility in their scheduling of migration or in other aspects of migratory behavior, such as the direction of migration or the decision to stopover during their journeys. Behavioral flexibility, which denotes the ability of an individual to modify its behavior as a function of perceived or predicted environmental cues, may have major consequences for fitness (Dingemanse and Wolf 2013). Flexibility in migratory behavior may be advantageous as it may increase the chances of surviving unpredictable and often challenging environmental conditions encountered en route (Senner et al. 2015) and habitat alterations (Clausen and Madsen 2016).
Many previous studies of behavioral flexibility in migratory birds have focused on intraindividual variation in timing and location of breeding and nonbreeding areas. These have reported that individuals show relatively consistent migratory schedules across repeated migrations (López-López et al. 2014). Individuals can show strong breeding and nonbreeding site fidelity (Stanley et al. 2012; Hasselquist et al. 2017), although their extent may vary markedly both among- and within-species, likely as a response to spatial variation in resources (Alerstam et al. 2006; van Wijk et al. 2016). Fewer studies have addressed flexibility of migratory routes. Recent advances in individual tracking technologies have allowed the analysis of repeated migratory journeys of the same individuals using Global Positioning System dataloggers, satellite transmitters (platform transmitter terminals, hereafter PTTs) or geolocators (e.g., Stanley et al. 2012; Vardanis et al. 2016; Senner et al. 2019). Fidelity to specific stopover sites or routes may be advantageous as individuals may rely on previous experience to offset the costs of settling in novel environments, by reducing predation risk and avoiding resource-depleted or parasite-rich stopover sites (Lank et al. 2003; Lourenço et al. 2010). However, among-species variation in patterns of both spatial and temporal consistency appears very high, and the causes of such broad interspecific variation are unclear (Alerstam et al. 2006; Senner et al. 2019).
Besides within-individual variation, large differences in migratory behavior may exist among individuals. Among-individual variation may originate from age-related differences in migratory performance (Hake et al. 2003), sex differences in selective pressures (Rubolini et al. 2004) and annual cycle timing (Briedis et al. 2019). It may further originate from intraspecific variation in migratory behavior, with different populations showing different migration patterns (timing and routes) according to, for example, breeding latitude (Conklin et al. 2010) or biogeographic history (Perez-Tris et al. 2004). Differences in migratory behavior between pre- and postbreeding migration may also occur, due to fundamentally different selective pressures affecting migration to the breeding versus nonbreeding areas (Nilsson et al. 2013).
In this study, we investigated patterns of interindividual variation and individual consistency of migratory behavior in the Eurasian woodcock (Scolopax rusticola) (hereafter, woodcock). The woodcock is a medium-sized (ca. 300 g) migratory wader breeding in Eurasian boreal and temperate forests and wintering in western Europe and the Mediterranean region (Cramp 1998). It is a mostly nocturnal species, tied to woodland habitats with open areas and clearings (Cramp 1998). Individuals were equipped with Argos PTTs on their wintering grounds along the Italian peninsula and tracked for up to 4 prebreeding migration and up to 3 postbreeding migration episodes. The species appears highly faithful to both breeding and wintering areas, suggesting a relatively high spatial consistency (Wilson 1983; Hoodless and Coulson 1994). Satellite-tracking revealed that woodcocks wintering in Spain behave as habitat generalists during their prebreeding migration (Arizaga et al. 2015; Crespo et al. 2016). Woodcocks perform a mostly straight overland migration across continental Europe (Arizaga et al. 2015; Le Rest et al. 2019) and do not select specific stopover habitats at a mesoscale range, likely because their preferred habitat (cropland-forest mosaic) occurs almost continuously along the migration route (Crespo et al. 2016).
Our objectives were to 1) describe interindividual variation in pre- and postbreeding migratory behavior; 2) investigate whether interindividual variation was explained by age, sex, migration distance, and latitude of breeding areas; 3) analyze patterns of intraindividual variation and consistency in migratory timing and routes; with regard to the latter, we expected: 1) repeatable timing of migration and fidelity to breeding and nonbreeding residence areas (e.g., Wilson 1983); 2) consistent migratory paths across repeated migrations; migrating individuals should indeed be able to consistently move along the shortest route (i.e., the orthodrome) between departure and destination because of the combined effects of low habitat selectivity during migration (Crespo et al. 2016) and lack of major ecological barriers between breeding and nonbreeding areas, leading to a straight migration (Arizaga et al. 2015; Le Rest et al. 2019).
Materials and Methods
General methods, device characteristics, Argos data filtering, and sex determination
From 2011 to 2017, during February to early March, we captured 42 woodcocks. Birds were caught while foraging at night, using a headlamp and a handheld net (1 m diameter) attached to a 9.5 m pole. Birds were weighed to the nearest 1 g using an electronic scale, and equipped with solar Argos PTTs (9.5 g, model PTT-100 by Microwave Telemetry Inc., Columbia, MD; and 12 g, model GT-12GS by GeoTrak Inc., Apex, NC) fitted on the back using an elastic nylon harness (ca. 1.5 g). The mean (standard deviation [SD]) relative load of devices (including harness) was 3.65% (0.20) (min–max: 3.18–3.92%) (PTT-100) and 4.04% (0.42) (3.33–4.35%) (GT-12GS) of body mass. The innermost secondary feather was plucked for genetic sex determination according to the sex-specific length polymorphism of the chromo-helicase-DNA binding 1 intron (Griffiths et al. 1998). Birds were classified as 1st-winter or adult (at least 2nd winter) based on plumage characteristics (Ferrand and Gossmann 2009). All birds were released within 10 min of capture. Capture, handling, and marking procedures were approved by the Istituto Superiore per la Protezione e la Ricerca Ambientale and authorized by the relevant local administration authorities, according to the prescriptions of Law 157/1992 [Art. 4 (1)].
PTTs were set on a 10 h on/48 h off duty cycle. Migration data were obtained for 25 individuals (Supplementary Table S1). For the remaining individuals either no Argos transmission was received (N = 13) or birds were shot soon after deployment (N = 4). We considered all Argos locations classified as 1–3 (highest quality; estimated error <1,500 m). Locations of lowest quality (0 = estimated error >1,500 m; A and B: accuracy estimation impossible) were used in a few cases only, provided they were compatible with the overall migration direction and travel speed. Invalid locations (Class Z) were discarded. Since woodcocks mostly frequent shaded habitats and are nocturnal, transmission rates during wintering and breeding seasons were often lower than expected (also see Arizaga et al. 2015). However, transmission rates increased during migration. Low transmission rates prevented the application of advanced filtering techniques (e.g., the “Kalman” or “Douglas” filters). We considered locations of Classes 0, A, and B only if no other data were available for a given tracking day. Moreover, if A, B, and 0 locations were available on a given day, we considered only Class 0 location(s) and discarded the others. After filtering, tracks were visually inspected and a few further locations were removed because they were clearly erroneous (i.e., unrealistic latitudes and/or longitudes). Overall, out of a total of 17,771 valid Argos locations, 7,577 (43%) (including the capture location) were retained for the analyses of migratory behavior (Supplementary Table S1). All tracking data have been uploaded on the Movebank repository (www.movebank.org).
Calculation of migration-related variables
The location of putative breeding areas (hereafter, breeding areas) and of the wintering areas of each migration episode was computed as the median latitude and longitude of all stationary positions during summer and winter months, respectively. Departure/arrival dates were determined from detailed visual inspection of tracking data, and computed as the mean date between the last/first day on the stationary areas (wintering or breeding) and the first/last day on active migration. Birds were considered to have started/terminated migration when the first/last clearly directional movement of >50 km from the breeding/wintering area occurred. In 8 individuals, distinct clusters of locations, separated by 20–170 km, were observed during the breeding period. For these birds, departure/arrival dates were computed as the day of arrival/departure to/from the temporally nearest cluster. Excluding movements between these clusters, birds mostly remained stationary during both wintering and breeding: the median distance between consecutive locations was 0.95 km during wintering (25th–75th percentiles: 0.41–1.95 km, maximum: 42.8 km) and 0.94 km during breeding (25th–75th percentile: 0.40–2.18 km, maximum: 51.5 km).
We only considered departure/arrival dates whose accuracy (difference between the last/first day on migration and the first/last day on the breeding/wintering area) was <10 days (mean accuracy was 2.71 days [1.69 SD], N = 187 estimates). For each complete migration track (i.e., those tracks for which the wintering/breeding areas could be clearly identified), we computed the following variables: duration of migration (difference between arrival and departure date, in days), total migration distance (in kilometers) (sum of all distances between positions recorded on a migration track between departure and arrival), migration speed (km/day) (total migration distance/duration of migration), and track straightness (distance between the location of onset and of end of the track divided by total migration distance; Benhamou 2004).
Statistical analyses
We first analyzed variation in migratory behavior (departure, arrival, duration, total migration distance, migration speed, and track straightness) in relation to age (0 = first winter; 1 = at least 2nd winter) and sex (0 = female; 1 = male) by means of linear mixed models (LMMs) with individual identity as a random intercept effect. In birds with repeated tracking data, at the onset of their second winter, the age of birds captured as first winter was modified to at least 2nd winter. LMMs were fitted on pre- and postbreeding migration data separately. We also compared duration, total migration distance, migration speed, and track straightness between pre- and postbreeding migration by means of LMMs with individual identity as a random intercept effect. LMMs were fitted by the lmer function of the “lme4” R library (Bates et al. 2014). Significance of fixed effects was tested by the likelihood-ratio test (difference in −2 log-likelihood of the model including and the one excluding the model term of interest, which is χ2-distributed). To account for differences in transmission rates among individuals, in LMMs of total migration distance, migration speed, and straightness, we included a weight variable, that is, the daily transmission rate. This variable was computed for each migration track as the fraction of days of a given track when at least one valid location was received. Due to PTT settings, we expected at least one valid location every second-3rd day. However, actual transmission rates sometimes occurred at shorter time intervals, depending on recharge rate (e.g., Giunchi et al. 2019). The actual median daily transmission rate across all birds was 0.46, that is, at least 1 valid transmission every second day. We thus assigned weight 1 to tracks whose daily transmission rate was equal or above 0.50, whereas tracks whose daily transmission rate was < 0.50 were assigned with a proportionally smaller weight (i.e., a track with a value of 0.45 was assigned a weight of 0.90). Residuals of all LMMs were visually inspected (Zuur et al. 2010), and no evidence for deviations from normality or heteroscedasticity was found.
To test whether interindividual variation in migratory behavior (departure, arrival, duration, total migration distance, migration speed, and track straightness) was associated with the latitude of departure areas (wintering or breeding), of destination areas (breeding or wintering), or with minimum migration distance (orthodromic distance between the breeding and the wintering area, in kilometer), we relied on correlation or partial correlation tests (Pearson’s r). Because some individuals were sampled repeatedly, significance of correlation tests was conservatively assessed based on the number of individuals included in the analyses rather than the number of data points.
Spatial consistency in breeding/wintering areas was estimated by comparing the within-individual (orthodromic) distances (in kilometers) of breeding/wintering areas across repeated migration episodes to the between-individual distances. A significantly smaller within- versus between-individual distance would indicate consistency in breeding/wintering areas. These analyses were restricted to those individuals with at least 2 estimates of breeding/wintering areas. Between-individual distances were computed based on the mean geographic position of breeding/wintering areas of each individual. Similarly, whenever more than 2 estimates of breeding/wintering areas were available for each individual, within-individual distances were averaged. These procedures ensured that individuals with more than 2 repeated migration episodes were not overrepresented in the statistical analyses.
Consistency in the shape of migratory routes was estimated in 2 ways. We first compared the One-Way Distance (hereafter, OWD) (Lin and Su 2008; Ranacher and Tzavella 2014) between repeated tracks within-individual to OWD between tracks of different individuals. The OWD is an accurate and efficient function to compute the similarity between pairs of movement trajectories A and B. OWD between Track A and Track B was computed as follows:
where and are the orthodromic distances between positions of Tracks A and B or B and A, respectively, and and are the lengths of Tracks A and B, respectively. As OWD is dependent by definition on the number of locations along each track, tracks were standardized to have the same number of locations by placing on each track 20 equally spaced locations (equals to the maximum number of Argos locations we could obtain for a given track; hence, in the formula, both n and m are equal to 20). A significantly smaller within- versus between-individual OWD would indicate consistency of migration routes. In order to avoid overrepresentation of individuals with multiple tracks in the analyses, to compute between-individual OWDs the 20 locations of each repeated track were averaged and between-individual OWDs were calculated based on the resulting “average” track. Similarly, whenever more than 2 tracks were available for a given individual, within-individual OWD were averaged to obtain a single OWD value for each individual with repeated tracks. In addition, we evaluated the spatial consistency between pre- and postbreeding migration routes by comparing OWD between seasons of the same individuals versus OWD of different individuals. For this analysis, whenever more than 1 track was available for either pre- or postbreeding migration, tracks were averaged as described above to obtain a single “average” track for each individual/season, and such average tracks were used to compute OWDs both within-individuals and between-individuals. All within- versus between-individual distances were compared by a general independence permutation test (Strasser and Weber 1999) using the “coin” R package (Hothorn et al. 2008). Secondly, we compared the repeatability (R) of track straightness using a mixed model approach by means of the “rptR” R package (Stoffel et al. 2017). Significance of R was tested by a likelihood-ratio test (Stoffel et al. 2017). Analyses of spatial consistency were performed for prebreeding tracks only (repeated postbreeding migration tracks were too few) and restricted to those individuals with at least 2 prebreeding migration tracks.
Temporal consistency was assessed by estimating R of timing variables (departure, arrival, duration, and speed) for pre- and postbreeding migration separately as detailed above. All estimates of consistency and R were computed only when repeated data were available for a minimum of 5 individuals. All statistical analyses were implemented in R 3.3.3 (www.R-project.org).
Results
Variability in migratory behavior among individuals and between seasons
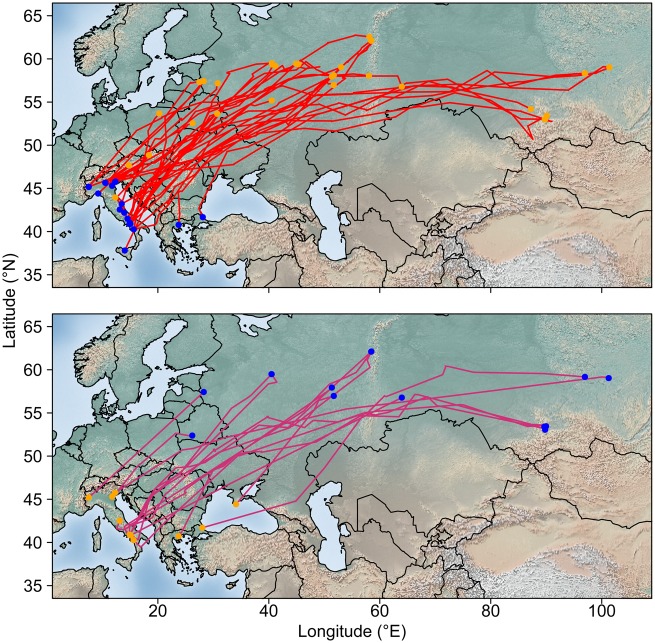
Woodcocks wintering in Italy displayed a great variability in migratory behavior, with 3 birds moving less than 800 km between the wintering and the breeding area, and others moving more than 6,000 km (Table 1 and Figure 1). Breeding areas spanned from northern Italy (1 individual wintering in southern Italy) to central-eastern Europe (Austria, Poland, Belarus, Latvia, and Slovakia; no more than 2 individuals each) and Russia (11 west and 4 east of Urals). Some individuals reached south-central Russia (Figure 1). Movements toward destination areas were relatively straight, with limited deviations from the shortest path, as gauged by the relatively high track straightness in both seasons (Table 1 and Figure 1).
Table 1.
Summary statistics of individual migration tracking data
| Variable | Prebreeding migration |
Postbreeding migration |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | Min–max | N | Mean (SD) | Min–max | N | |
| Departure | March 26 | 54–113 | 33, 34 | September 13 | 222–310 | 12, 8 |
| 84.6 (12.2) | 256.5 (26.5) | |||||
| Arrival | May 5 | 88–165 | 35, 22 | November 28 | 321–359 | 9, 6 |
| 125.0 (15.4) | 332.8 (11.6) | |||||
| Duration (days) | 41.8 (16.0) | 5–80 | 31, 22 | 70.2 (19.8) | 40–94 | 8, 6 |
| Total migration distance (km) | 3,504 (1,729) | 344–6,404 | 35, 22 | 3,958 (1,600) | 1,403–6,642 | 15, 10 |
| Migration speed (km/day) | 88.0 (37.0) | 6.5–154.2 | 31, 22 | 59.8 (18.2) | 28.6–83.7 | 8, 6 |
| Track straightness | 0.91 (0.07) | 0.64–0.99 | 35, 22 | 0.94 (0.04) | 0.87–0.99 | 15, 10 |
Departure and arrival dates are reported as days since January 1 (Day 1 = January 1); mean values are also reported as calendar dates for ease of reference. Sample size (N) shows both the number of data points and the number of individuals.
Figure 1.
Migration tracks of Eurasian woodcocks equipped with Argos PTTs while wintering in Italy. Upper panel: prebreeding migration tracks (N = 35 complete migration tracks from 22 individuals); lower panel: postbreeding migration tracks (N = 15 complete migration tracks from 10 individuals). Blue dots: departure locations; orange dots: destination locations. Background image obtained from http://www.naturalearthdata.com/.
Prebreeding migration departures mainly occurred in the second half of March (mean: March 26), whereas breeding areas were reached 42 days later on average (Table 1). Individuals left their breeding areas in mid-September (mean: 13 September), reaching their wintering areas 70 days later on average (Table 1). There were no significant differences between sex or age classes in pre- or postbreeding migration characteristics (Supplementary Table S2). Total migration distance and straightness did not significantly differ between pre- and postbreeding migration (Tables 1 and 2). However, birds migrated for a significantly shorter time and at faster speed during pre- than postbreeding migration (Tables 1 and 2).
Table 2.
LMMs of the population-level differences between pre- and postbreeding migration characteristics (prebreeding = 0, postbreeding = 1)
| Trait | Estimate (SE) | χ 2 | df | P |
|---|---|---|---|---|
| Duration (days) | 25.4 (3.8) | 24.81 | 1 | <0.001 |
| Total migration distance (km) | −150 (136) | 1.21 | 1 | 0.27 |
| Migration speed (km/day) | −35.7 (9.4) | 10.30 | 1 | 0.001 |
| Track straightness | 0.015 (0.011) | 1.99 | 1 | 0.16 |
In models of total migration distance, migration speed, and straightness, we included daily transmission rate as a weight variable (see methodssections), as the daily transmission rate was significantly greater during pre- than during postbreeding migration (LMM, χ2 = 44.6, df = 1, P < 0.001).
Migration characteristics: association with latitude of departure/destination and migration distance
Migration characteristics were not significantly correlated with departure or destination latitude (Table 3), with the exception of prebreeding migration departure date, which was significantly negatively correlated with the destination latitude (Table 3). Hence, individuals that migrated from their Italian wintering sites to relatively northern latitudes departed earlier than those breeding at more southern areas (Figure 2). Minimum migration distance was significantly positively correlated to prebreeding migration duration (Table 3), with birds migrating to relatively farther distances performing longer-lasting migrations (Figure 2), and prebreeding migration speed was positively correlated with minimum migration distance (Table 3), being faster for birds whose breeding areas were farther (Figure 2). Finally, postbreeding migration departure date was strongly negatively correlated with minimum migration distance (Table 3), implying that birds that migrated farther to reach their wintering sites left their breeding areas earlier (Figure 2).
Table 3.
Correlation coefficients (Pearson’s r) between pre- and postbreeding migration characteristics and latitude of departure, latitude of destination (partial correlation controlling for minimum migration distance), and minimum migration distance
| Variable | Departure latitude | Destination latitude | Minimum migration distance |
|---|---|---|---|
| Prebreeding migration | |||
| Departure | −0.04 (24) | −0.47 (22)* | −0.10 (22) |
| Arrival | −0.17 (22) | −0.29 (22) | 0.38 (22) |
| Duration (days) | −0.10 (22) | 0.12 (22) | 0.43 (22)* |
| Total migration distance (km) | −0.25 (22) | −0.07 (22) | – |
| Migration speed (km/day) | −0.24 (22) | −0.13 (22) | 0.66 (22)** |
| Track straightness | −0.02 (22) | 0.24 (22) | 0.15 (22) |
| Postbreeding migration | |||
| Departure | −0.13 (8) | 0.12 (8) | −0.87 (8)** |
| Arrival | −0.67 (6) | 0.77 (6) | −0.41 (6) |
| Duration (days) | −0.05 (6) | 0.06 (6) | 0.80 (6) |
| Total migration distance (km) | −0.02 (10) | −0.13 (10) | – |
| Migration speed (km/day) | 0.20 (6) | −0.24 (6) | 0.64 (6) |
| Track straightness | −0.09 (10) | 0.24 (10) | −0.42 (10) |
The number of individuals is reported in brackets. Statistically significant correlation coefficients are highlighted in boldface. Significance level was conservatively determined according to the number of individuals (see “Materials and Methods” section). The correlation between total migration distance and minimum migration distance was not shown as it was redundant (r > 0.98).
P < 0.05;
P < 0.01.
Figure 2.
Statistically significant associations between migration characteristics and destination latitude or migration distance (see Table 3). Upper left: prebreeding migration departure date versus destination latitude (breeding area); upper right: prebreeding migration duration versus minimum migration distance; lower left: prebreeding migration speed versus minimum migration distance; lower right: postbreeding migration departure date versus minimum migration distance. Open circles: prebreeding migration; filled circles: postbreeding migration.
Spatial consistency, intra- and interindividual variation, and repeatability
Birds were generally faithful to their wintering site (Table 4), but 3 out of 11 individuals changed their wintering area in successive winters, moving at least once at a distance >10 km among successive winters. One individual even shifted its wintering site from northern Italy to the Crimean peninsula (1,971 km eastward) in the subsequent winter. The tendency to change wintering site was significantly associated with age at capture, first-winter birds being more likely to change their wintering site (3 out of 4 birds) than adult ones (0 out of 7 birds) (Fisher’s exact test, P = 0.024). No significant sex differences in winter site fidelity were found (P = 0.55).
Table 4.
Consistency in the location of wintering areas, breeding areas, and migration routes
| Variable | Within-individual | Between-individuals | Z | P |
|---|---|---|---|---|
| Distance among wintering areas (km) | 1.0 (0.5–122.8; 11) | 563.3 (286.2–675.3; 55) | 1.99 | 0.046 |
| Distance among breeding areas (km) | 0.8 (0.4–11.3; 8) | 2102.7 (773.5–2930.1; 28) | 3.62 | <0.001 |
| OWD among prebreeding tracks | 0.52 (0.50–0.71; 8) | 2.48 (1.96–4.11; 28) | 3.35 | <0.001 |
| OWD between pre- and postbreeding tracks | 0.63 (0.54–0.85; 10) | 3.28 (1.93–5.41; 90) | 3.02 | 0.003 |
Values are median distances, with 25th and 75th percentiles and sample size (within-individual: number of individuals; between-individuals: number of between-individual distances) in brackets.
Breeding site fidelity was very high (Table 4). Out of 8 birds with repeated locations of breeding areas and 22 breeding events, 2 individuals (both 1st-winter females) dispersed to 48 and 92 km between consecutive breeding seasons, whereas all the other 6 stayed within 2 km of the previous year breeding area.
Migration routes were highly consistent within-individuals, both among consecutive prebreeding migration tracks and between pre- and postbreeding migration tracks (Table 5). Prebreeding track straightness also showed statistically significant repeatability, the mean intraindividual variation being relatively small compared with the interindividual variation (Table 5).
Table 5.
Intra-/interindividual variation (SD, with sample size in square brackets) and repeatability (R) estimates of prebreeding migration spatial and temporal characteristics
| Variable | Intraindividual variation | Interindividual variation | R | P | N obs | N ind |
|---|---|---|---|---|---|---|
| Spatial variation | ||||||
| Straightness | 0.03 (0.02) [13] | 0.20 (0.11) [5] | 0.53 | 0.017 | 21 | 8 |
| Temporal variation | ||||||
| Departure date (days) | 9.44 (5.36) [9] | 19.40 (13.30) [5] | 0.13 | 0.45 | 14 | 5 |
| Arrival date (days) | 5.63 (4.29) [12] | 39.20 (16.23) [5] | 0.89 | <0.001 | 21 | 8 |
| Duration (days) | 7.11 (5.75) [9] | 36.00 (15.92) [5] | 0.66 | 0.019 | 14 | 5 |
| Migration speed (km/day) | 15.04 (18.65) [9] | 101.15 (27.51) [5] | 0.53 | 0.051 | 14 | 5 |
The number of observations (N obs) and individuals (N ind) contributing to the repeatability estimates is shown (individuals with at least 2 repeated measurements only). The intraindividual variation is computed as the difference between consecutive observations of the same individual (absolute value), averaged across all individuals with repeated observations, whereas the interindividual variation was computed as the difference between the individual with the highest and the lowest value for each year of tracking, averaged across all years (for departure and arrival dates, the measurement units is days) (see Senner et al. 2019).
Repeatability of the prebreeding migration timing variables was significant only for arrival date and duration (Table 5), whereas it was nonsignificant for departure date and speed (Table 5). The intraindividual variation was 9.44 days for departure date (interindividual variation: 19 days) and 5.63 days for arrival date (interindividual variation: 39 days) (Table 5).
Discussion
Interindividual variation in breeding areas and effects on migratory behavior
Woodcocks wintering in Italy displayed a remarkable geographic variability of breeding areas, spanning more than 90° longitude. Ring recoveries suggest that most Italian wintering woodcocks originate from north and eastern Europe, including Russia west of Urals (Spina and Volponi 2008). Hence, recoveries clearly overestimate the importance of the Baltic as a source of the Italian wintering populations (also see Arizaga et al. 2015). Notably, birds wintering in Italy migrated to breeding areas that were located even farther east (up to 101° E) than those of satellite-tracked woodcocks wintering in Spain and France (Arizaga et al. 2015; Le Rest et al. 2019).
Such a broad variation in breeding origin had some effects on migratory behavior. Birds migrating to more northern latitudes departed earlier from their wintering grounds, independently of their migration distance, and birds that migrated to farther wintering areas showed an earlier departure date from their breeding areas. These associations are likely ultimately shaped by variation in timing of seasonal events across such a broad breeding range (Conklin et al. 2010). For instance, earlier prebreeding migration departures of birds breeding farther north may allow them to better tune arrival date to spring progression en route. The earlier postbreeding migration departure of birds migrating farther may reflect either selective pressures to avoid delayed arrival at optimal wintering areas, or an earlier onset of autumn in continental regions of central Russia compared with those of north-eastern Europe or the Baltic, forcing birds to leave earlier.
Birds migrating to farther breeding areas migrated at a faster rate. This association may reflect differences in flight efficiency between birds from different breeding populations. It is likely that the observed huge interindividual differences in migration distance are associated to variation in the morphology of the flight apparatus (e.g., wing shape) which affects flight efficiency (Lockwood et al. 1998). Alternatively, birds migrating over longer distances may have accumulated relatively larger fuel stores before migration (Vincze et al. 2019), leading to shorter stopovers and faster migration. This would be in line with short-distance migrating individuals adopting an energy-minimization prebreeding migration strategy, whereas long-distance migrating ones may rather minimize time spent on migration (Klaassen et al. 2012).
Patterns of spatial consistency
Philopatry arises whenever the benefits of site fidelity outweigh those of dispersing to novel areas (Piper 2011). However, among long-lived species, individuals can vary their nonbreeding area with age, showing a tendency to approach their breeding site among successive winters (e.g., Marques et al. 2010; Lok et al. 2011). Our data agree with the broader picture, as 1st-winter woodcocks were significantly more likely to change their wintering site than adults. Moreover, those 1st-winter individuals that changed wintering site did so by getting closer to their breeding areas, by 504 km on average (5–1,382 km closer). Age-related changes in nonbreeding site may reflect different processes (Marques et al. 2010). In the case of woodcocks, these might involve age-related improvement of migratory performance, whereby birds may decide to stop at suitable stopover sites along the migration path instead of migrating farther. The observed high fidelity to breeding areas is in line with previous studies (Hoodless and Coulson 1994).
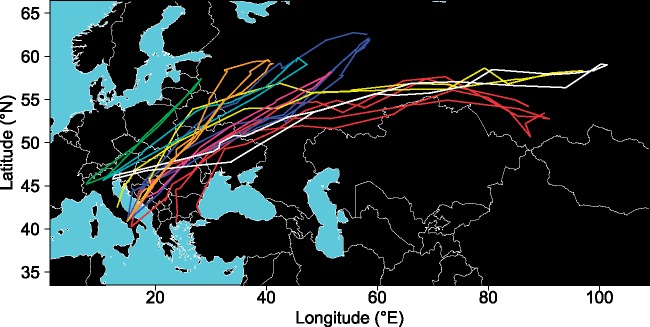
Philopatric behavior co-occurred with consistency of migratory routes. The latter was expected because of low stopover habitat selectivity during migration (Crespo et al. 2016) and because woodcocks mainly migrate over mainland (Arizaga et al. 2015; Le Rest et al. 2019). Although route consistency was partly related to the huge interindividual differences in breeding origin, repeated migration routes of the same individuals were largely overlapping (Figure 3). Moreover, birds appeared to follow similar routes during both pre- and postbreeding migration. These patterns are different from other landbird migrants, which showed variable and nonrepeatable routes (e.g., Stanley et al. 2012; López-López et al. 2014). Differences among species in route fidelity are largely due to temporally variable impacts of en route wind conditions on migratory flights (e.g., Vidal-Mateo et al. 2016), and may be partly related to differences among species in the susceptibility to these conditions (Vardanis et al. 2016). At the proximate level, consistency of migratory routes—both among repeated prebreeding migrations and between pre- and postbreeding migration—could arise because of route recapitulation, whereby individuals performing a successful migratory movement tend to repeat this same movement over successive migration episodes by relying on visual landmark recognition along their migratory path (Meade et al. 2005).
Figure 3.
Repeated prebreeding migration tracks from 8 individuals (N = 21 complete tracks). Tracks from different individuals are plotted with different colors.
Woodcocks migrating over Eurasian land masses likely experienced only minor wind displacement, as gauged by the very high-track straightness. We suggest that low stopover habitat selectivity (Crespo et al. 2016) and high-weather sensitivity of stopover decisions (Le Rest et al. 2019) may both promote straight and repeatable migration routes, with individuals stopping over whenever and wherever unfavorable weather increases the cost of onward movements. Alternatively, consistency of migratory routes might be associated with low interannual/interseasonal variability of environmental conditions (e.g., dominant winds) encountered during migration. Future studies should evaluate the importance of the location of stopover sites and the duration of stopover periods on the spatial consistency of migratory routes. Unfortunately, the relatively coarse temporal resolution of our Argos locations data prevented a detailed analysis of stopover behavior.
Patterns of temporal consistency and seasonal differences in migratory behavior
Prebreeding migration arrival date was significantly repeatable, whereas departure date was not. Although the low repeatability of departure date should be taken cautiously because of the small sample size, these findings suggest that local environmental conditions strongly affected departure decisions. Migrating woodcocks can indeed adjust migration speed according to weather en route, high temperatures and northward winds (tailwinds) significantly increasing the probability of performing onward flights during prebreeding migration (Le Rest et al. 2019). During the optimal prebreeding migration departure “window,” birds may thus flexibly choose to leave as soon as local conditions are favorable, then progressively adjusting timing of migration during the migratory flight according to contingent weather (Le Rest et al. 2019). Highly repeatable arrival dates may result from a combination of fine-tuning of migratory behavior en route coupled with seasonal advancement and progressive improvement of weather conditions along the migratory trip.
Finally, we observed significantly faster migration movements in spring than in autumn, in accordance with the idea that birds minimize time during prebreeding (but not postbreeding) migration, following selective pressures for earlier arrival to the breeding sites (Nilsson et al. 2013).
Concluding Remarks
Our results expand current knowledge about the breeding origin of woodcocks wintering in the Mediterranean basin, a major wintering region for the Eurasian populations of this important quarry species, where it is subjected to strong hunting pressure (Duriez et al. 2005). After accounting for age effects, woodcocks showed a relatively large spatial consistency through the entire annual cycle (wintering, migration, and breeding), whereas temporal consistency was variable, being significant only for arrival date to breeding areas. Flexibility in timing of prebreeding migration departure may reflect a relatively low environmental canalization (Pulido and Widmer 2005). Unraveling drivers of interindividual and interpopulation variation in flexibility would improve our understanding of the evolutionary potential of migratory species in response to environmental and climatic changes.
Supplementary Material
Acknowledgments
The authors wish to express their gratitude to all those people that helped them during field activities and to B. Crestanello for assistance with molecular sexing. Constructive comments by R. Ambrosini and 3 anonymous reviewers helped them improving a previous draft of the manuscript. They acknowledge support by Parco Nazionale della Majella, Parco Naturale La Mandria, Parco Regionale dei Colli Euganei, and Parco Regionale dei Monti Picentini. Partial financial support was provided by Regione Veneto, ATC Salerno 2, ATC Avellino, ATC Benevento, ATC Caserta, ATC Napoli, ATC Macerata 2, ATC Frosinone 1, ATC Cosenza 1, ATC Vastese, ATC Foggia, ATC Genova, ATC La Spezia, Comprensorio Alpino Torino 4, Provincia di Udine, M. Gemin, local FIDC sections (Roma, Umbria, Lombardia), and Ente Nazionale della Cinofilia.
Authors’ Contributions
A.T., M.So., M. Sp., and L.G. conceived the study; A.T., M.B., I.T., and R.I. conducted fieldwork and collected the data; D.R., F.D.P., and N.T. analyzed the data; D.R. and F.D.P. wrote the article, with inputs from M.So. and A.T.
References
- Alerstam T, Hake M, Kjellén N, 2006. Temporal and spatial patterns of repeated migratory journeys by ospreys. Anim Behav 71:555–566. [Google Scholar]
- Arizaga J, Crespo A, Telletxea I, Ibáñez R, Díez F. et al. 2015. Solar/Argos PTTs contradict ring-recovery analyses: woodcocks wintering in Spain are found to breed further east than previously stated. J Ornithol 156:515–523. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2014. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Benhamou S, 2004. How to reliably estimate the tortuosity of an animal’s path: straightness, sinuosity, or fractal dimension? J Theor Biol 229:209–220. [DOI] [PubMed] [Google Scholar]
- Berthold P, Gwinner E, Sonnenschein E, 2003. Avian Migration. Berlin: Springer. [Google Scholar]
- Briedis M, Bauer S, Adamík P, Alves JA, Costa JS. et al. 2019. A full annual perspective on sex-biased migration timing in long-distance migratory birds. Proc R Soc B 286:20182821.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen KK, Madsen J, 2016. Philopatry in a changing world: response of pink-footed geese Anser brachyrhynchus to the loss of a key autumn staging area due to restoration of Filsø Lake, Denmark. J Ornithol 157:229–237. [Google Scholar]
- Conklin JR, Battley PF, Potter MA, Fox JW, 2010. Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat Commun 1:67.. [DOI] [PubMed] [Google Scholar]
- Cramp S, 1998. The Complete Birds of the Western Palearctic on CD-ROM. Oxford: Oxford University Press. [Google Scholar]
- Crespo A, Rodrigues M, Telletxea I, Ibáñez R, Díez F. et al. 2016. No habitat selection during spring migration at a meso-scale range across mosaic landscapes: a case study with the woodcock Scolopax rusticola. PLoS ONE 11:e0149790.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Wolf M, 2013. Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim Behav 85:1031–1039. [Google Scholar]
- Duriez O, Eraud C, Barbraud C, Ferrand Y, 2005. Factors affecting population dynamics of Eurasian woodcocks wintering in France: assessing the efficiency of a hunting-free reserve. Biol Conserv 122:89–97. [Google Scholar]
- Ferrand Y, Gossmann F, 2009. Ageing and sexing the Eurasian woodcock Scolopax rusticola. Wader Study Group Bull 116:75–79. [Google Scholar]
- Giunchi D, Baldaccini NE, Lenzoni A, Luschi P, Sorrenti M. et al. 2019. Spring migratory routes and stopover duration of satellite‐tracked Eurasian Teals Anas crecca wintering in Italy. Ibis 161:117–130. [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJ, 1998. A DNA test to sex most birds. Molec Ecol 7:1071–1075. [DOI] [PubMed] [Google Scholar]
- Hake M, Kjellén N, Alerstam T, 2003. Age‐dependent migration strategy in honey buzzards Pernis apivorus tracked by satellite. Oikos 103:385–396. [Google Scholar]
- Hasselquist D, Montràs‐Janer T, Tarka M, Hansson B, 2017. Individual consistency of long‐distance migration in a songbird: significant repeatability of autumn route, stopovers and wintering sites but not in timing of migration. J Avian Biol 48:91–102. [Google Scholar]
- Hoodless AN, Coulson JC, 1994. Survival rates and movements of British and continental woodcock Scolopax rusticola in the British Isles. Bird Study 41:48–60. [Google Scholar]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A, 2008. Implementing a class of permutation tests: the coin package. J Stat Softw 28:1–23.27774042 [Google Scholar]
- Klaassen RH, Ens BJ, Shamoun-Baranes J, Exo KM, Bairlein F, 2012. Migration strategy of a flight generalist, the Lesser Black-backed Gull Larus fuscus. Behav Ecol 23:58–68. [Google Scholar]
- Lank DB, Butler RW, Ireland J, Ydenberg RC, 2003. Effects of predation danger on migration strategies of sandpipers. Oikos 103:303–319. [Google Scholar]
- Le Rest K, Hoodless A, Heward C, Cazenave JL, Ferrand Y, 2019. Effect of weather conditions on the spring migration of Eurasian woodcock and consequences for breeding. Ibis doi: 10.1111/ibi.12657. [Google Scholar]
- Lin B, Su J, 2008. One-way distance: for shape based similarity search of moving object trajectories. GeoInformatica 12:117–142. [Google Scholar]
- Lockwood R, Swaddle JP, Rayner JM, 1998. Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. J Avian Biol 29:273–292. [Google Scholar]
- Lok T, Overdijk O, Tinbergen JM, Piersma T, 2011. The paradox of spoonbill migration: most birds travel to where survival rates are lowest. Anim Behav 82:837–844. [Google Scholar]
- López-López P, García-Ripollés C, Urios V, 2014. Individual repeatability in timing and spatial flexibility of migration routes of trans-Saharan migratory raptors. Curr Zool 60:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço PM, Mandema FS, Hooijmeijer JC, Granadeiro JP, Piersma T, 2010. Site selection and resource depletion in black‐tailed godwits Limosa l. limosa eating rice during northward migration. J Anim Ecol 79:522–528. [DOI] [PubMed] [Google Scholar]
- Marques PA, Sowter D, Jorge PE, 2010. Gulls can change their migratory behavior during lifetime. Oikos 119:946–951. [Google Scholar]
- Meade J, Biro D, Guilford T, 2005. Homing pigeons develop local route stereotypy. Proc Biol Sci 272:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Klaassen RH, Alerstam T, 2013. Differences in speed and duration of bird migration between spring and autumn. Am Nat 181:837–845. [DOI] [PubMed] [Google Scholar]
- Perez-Tris J, Bensch S, Carbonell R, Helbig A, Tellería JL, 2004. Historical diversification of migration patterns in a passerine bird. Evolution 58:1819–1832. [DOI] [PubMed] [Google Scholar]
- Piper WH, 2011. Making habitat selection more “familiar”: a review. Behav Ecol Sociobiol 65:1329–1351. [Google Scholar]
- Pulido F, Widmer M, 2005. Are long-distance migrants constrained in their evolutionary response to environmental change? Causes of variation in the timing of autumn migration in a blackcap S. atricapilla and two garden warbler Sylvia borin populations. Ann N Y Acad Sci 1046:228–241. [DOI] [PubMed] [Google Scholar]
- Ranacher P, Tzavella K, 2014. How to compare movement? A review of physical movement similarity measures in geographic information science and beyond. Cart Geogr Inform Sci 41:286–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubolini D, Spina F, Saino N, 2004. Protandry and sexual dimorphism in trans-Saharan migratory birds. Behav Ecol 15:592–601. [Google Scholar]
- Senner NR, Verhoeven MA, Abad‐Gómez JM, Gutiérrez JS, Hooijmeijer JC. et al. 2015. When Siberia came to the Netherlands: the response of continental black‐tailed godwits to a rare spring weather event. J Anim Ecol 84:1164–1176. [DOI] [PubMed] [Google Scholar]
- Senner NR, Verhoeven MA, Abad-Gómez JM, Alves JA, Hooijmeijer JC. et al. 2019. High migratory survival and highly variable migratory behaviour in black-tailed godwits. Front Ecol Evol 7:96. [Google Scholar]
- Spina F, Volponi S, 2008. Atlante Della Migrazione Degli Uccelli in Italia. 1. non-Passeriformi. Rome: MATTM - ISPRA. [Google Scholar]
- Stanley CQ, MacPherson M, Fraser KC, McKinnon EA, Stutchbury BJ, 2012. Repeat tracking of individual songbirds reveals consistent migration timing but flexibility in route. PLoS ONE 7:e40688.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel MA, Nakagawa S, Schielzeth H, 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed‐effects models. Methods Ecol Evol 8:1639–1644. [Google Scholar]
- Strasser H, Weber C, 1999. On the asymptotic theory of permutation statistics. Math Methods Stat 8:220–250. [Google Scholar]
- van Wijk RE, Bauer S, Schaub M, 2016. Repeatability of individual migration routes, wintering sites, and timing in a long‐distance migrant bird. Ecol Evol 6:8679–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardanis Y, Nilsson J-Å, Klaassen RH, Strandberg R, Alerstam T, 2016. Consistency in long-distance bird migration: contrasting patterns in time and space for two raptors. Anim Behav 113:177–187. [Google Scholar]
- Vidal-Mateo J, Mellone U, López-López P, de la Puente J, Garcia-Ripolles C. et al. 2016. Wind effects on the migration routes of trans-Saharan soaring raptors: geographical, seasonal and interspecific variation. Curr Zool 62:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincze O, Vágási CI, Pap PL, Palmer C, Møller AP, 2019. Wing morphology, flight type and migration distance predict accumulated fuel load in birds. J Exp Biol 222:jeb183517.. [DOI] [PubMed] [Google Scholar]
- Wilson J, 1983. Wintering site fidelity of woodcock in Ireland In: Kalchreuter H, editor. 2nd European Woodcock and Snipe Workshop. Fordingbridge: International Waterfowl Research Bureau; 18–27. [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS, 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.