Abstract
Secondary forests and human-made forest gaps are conspicuous features of tropical landscapes. Yet, behavioral responses to these aspects of anthropogenically modified forests remain poorly investigated. Here, we analyze the effects of small human-made clearings and secondary forests on tropical bats by examining the guild- and species-level activity patterns of phyllostomids sampled in the Central Amazon, Brazil. Specifically, we contrast the temporal activity patterns and degree of temporal overlap of 6 frugivorous and 4 gleaning animalivorous species in old-growth forest and second-growth forest and of 4 frugivores in old-growth forest and forest clearings. The activity patterns of frugivores and gleaning animalivores did not change between old-growth forest and second-growth, nor did the activity patterns of frugivores between old-growth forest and clearings. However, at the species level, we detected significant differences for Artibeus obscurus (old-growth forest vs. second-growth) and A. concolor (old-growth forest vs. clearings). The degree of temporal overlap was greater than random in all sampled habitats. However, for frugivorous species, the degree of temporal overlap was similar between old-growth forest and second-growth; whereas for gleaning animalivores, it was lower in second-growth than in old-growth forest. On the contrary, forest clearings were characterized by increased temporal overlap between frugivores. Changes in activity patterns and temporal overlap may result from differential foraging opportunities and dissimilar predation risks. Yet, our analyses suggest that activity patterns of bats in second-growth and small forest clearings, 2 of the most prominent habitats in humanized tropical landscapes, varies little from the activity patterns in old-growth forest.
Keywords: Amazon, behavior, forest gaps, niche partitioning, secondary forest, temporal overlap
Across the tropics, human activities are molding landscapes in which second-growth and forest gaps are conspicuous features (Melo et al. 2013; Chazdon 2014). The long-term persistence of tropical biodiversity, although primarily dependent on the conservation of vast tracts of old-growth forest habitat (Barlow et al. 2007; Gibson et al. 2011), will depend on the capacity of these novel ecosystems to accommodate old-growth forest-dwelling species. Secondary forests are now the largest forest cover type across the humid tropics (Chazdon 2014) and although the potential of second-growth to conserve tropical biodiversity remains hotly debated (Gibson et al. 2011; Chazdon 2014), mounting evidence suggests that areas of second-growth provide numerous ecosystem services and natural products to human populations, as well as crucial habitat for numerous taxa (Melo et al. 2013; Chazdon 2014; Rocha et al. 2018; Rozendaal et al. 2019). Alongside second-growth, man-made forest clearings, small to moderate in size, are increasingly widespread in many tropical landscapes (Tyukavina et al. 2018). This is reflected in the proportional decrease in large (>1,000 ha) deforested areas relative to small clearings (6.25–50.00 ha) in the Brazilian Amazon, during the first decade of this millennium (Rosa et al. 2012). Indeed, in 2002–2009, small clearings accounted for over 80% of the annual deforestation in the region (Rosa et al. 2012). Since many old-growth specialist species avoid even narrow clearings (<30 m wide; Laurance et al. 2009), understanding the effects of these small-scale disturbances on primary forest species is paramount to frame effective conservation strategies.
Tropical bats are a species-rich and ecologically diverse group that plays key ecological roles in the maintenance of forest ecosystems (Kunz et al. 2011; Farneda et al. 2018a). Frugivorous bats act as effective seed dispersers, especially of pioneer plant species (Medellin and Gaona 1999; de la Peña-Domene et al. 2014), whereas insectivores play essential roles in the reduction of herbivory levels by controlling arthropod populations (Maas et al. 2016; Kemp et al. 2019). They are also highly responsive to human-induced landscape changes (Meyer et al. 2016), which combined with their ecological diversity, abundance, and relative ease of sampling make bats a potentially useful indicator group to investigate the effects of habitat disturbance on tropical ecosystems (Jones et al. 2009; Ávila-Gómez et al. 2015).
In the neotropics, the responses of phyllostomid bats to second-growth and small-scale disturbances have received increasing attention (e.g., Castro-Luna et al. 2007; Willig et al. 2007; Avila-Cabadilla et al. 2012; Rocha et al. 2017a; Farneda et al. 2018b). The available evidence suggests that in second-growth and moderately disturbed habitats the abundance of frugivorous and nectarivorous phyllostomids tends to increase due to additional food resources (Willig et al. 2007; Farneda et al. 2015). In contrast, the abundance of gleaning animalivores tends to decrease in response to insufficient roosting and prey resources (Rocha et al. 2017b; Willig et al. 2019).
The magnitude of structural contrast between the original habitat and the habitat by which it is replaced has repeatedly been identified as a key factor driving changes in species abundance and richness, 2 commonly used metrics of species sensitivity to habitat disturbance (e.g., Mendenhall et al. 2014; Rocha et al. 2017b). However, analyses seldom address behavioral changes such as alterations in activity patterns and partitioning along the temporal niche axis, which may represent a more sensitive indicator of species’ responses to habitat conversion.
How species distribute their activity over time is an important dimension of animal behavior and provides valuable insights into their ecological niche and into how specific traits augment or constrain the accessibility to resources (Frey et al. 2017). Species’ activity patterns are adapted to local biotic and abiotic conditions and can be adjusted in response to external stimuli such as predation risk, changes in resource availability and agonistic encounters with competitors (Halle 2000). Understanding how human-driven habitat changes impact temporal dynamics is key to uncover the mechanisms affecting niche partitioning and community structure. Furthermore, the discernment of the behavioral decisions that alter a species’ activity can help unveil the degree of plasticity of animal assemblages to anthropogenic stressors. In tropical bats, changes in within-night activity patterns due to changes in habitat structure have been found to be more pronounced in habitats which are structurally very different from old-growth forest, such as open agricultural areas (Presley et al. 2009a) and urban areas (Montaño-Centellas et al. 2015), than in more similar habitats, such as second-growth (Presley et al. 2009a) and forest subjected to reduced-impact logging (Castro-Arellano et al. 2009; Presley et al. 2009b).
Here, we used a periodical reisolation of forest fragments at the Biological Dynamics of Forest Fragment Project in the Brazilian Amazon, to evaluate the effects of second-growth and small human-made forest clearings (i.e., areas created by the clearing of a 100 m wide strip of regrowth around old-growth forest fragments) on the within-night activity patterns of phyllostomid bats. Specifically, we hypothesized that: 1) due to changes in resource availability and predation risk, temporal activity patterns should be habitat-specific; 2) due to different energetic requirements and foraging behaviors, changes in the activity budget should be guild- and species-specific; and, 3) within-guild patterns of temporal overlap should differ between habitat categories. Accordingly, we predicted that 1) decreasing levels of forest cover, from old-growth forest to second-growth and to forest clearings should lead bats to reduce their early evening activity to avoid periods of higher predation risk by visually oriented predators; 2) due to the lower energetic content of fruits compared with animal prey, frugivores should exhibit longer periods of foraging activity than gleaning animalivores; and 3) due to higher structural similarity, the level of temporal overlap should be more similar between old-growth forest and second-growth, than between old-growth forest and forest clearings.
Materials and Methods
Study area
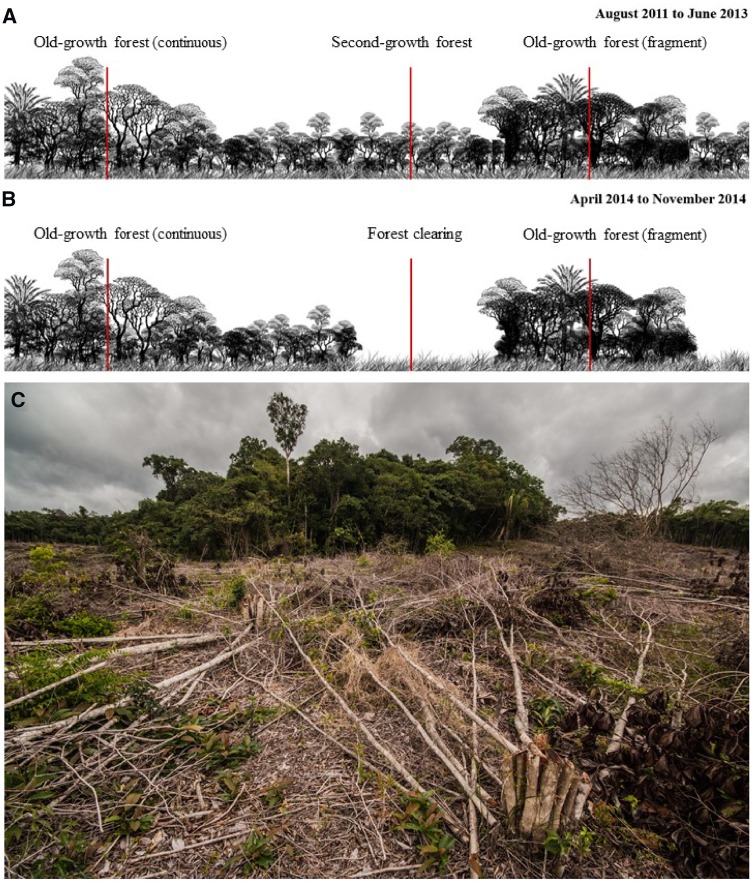
This study was conducted at the Biological Dynamics of Forests Fragments Project (BDFFP), a whole-ecosystem fragmentation experiment located ca. 80 km north of Manaus (2°30′S, 60°W), Amazonas, Brazil (Supplementary Figure S1). The area is characterized by a mosaic of continuous old-growth rainforest (terra firme forest), old-growth forest fragments, and secondary regrowth. Annual rainfall varies from 1,900 to 3,500 mm, with a dry season between July and November (Ferreira et al. 2017). In the early 1980s, 11 old-growth fragments (categorized into size classes of 1, 10 and 100 ha) were isolated from continuous old-growth forest by distances of 80–650 m. The fragments were initially surrounded by cattle pasture, but their rapid abandonment allowed pasture areas to develop into secondary forest. This second-growth matrix is dominated by Vismia spp. in areas that were cleared and burned, and by Cecropia spp. in areas that were cleared without fire (Mesquita et al. 2015). For a more detailed description of the study area and experimental manipulation, see Laurance et al. (2011). Approximately every 10 years, a 100 m-wide strip of second-growth surrounding each of the 11 old-growth forest fragments is felled, forming clearings of areas ranging from 6.4 to 42 ha around each of the experimental forest fragments. Prior to this study, the most recent reisolation of the old-growth forest fragments had occurred between 1999 and 2001 (Laurance et al. 2011). During this study, fragment reisolation took place between November 2013 and March 2014, by clearing but not burning of a 100 m-wide strip of second-growth vegetation around each of the 11 old-growth forest fragments (Figure 1).
Figure 1.
(A) Schematic representation of the study area in 2011–2013, before the creation of the experimental clearings by the felling of 100 m of second-growth vegetation. (B) Schematic representation of the study area in 2014, following the creation of the experimental clearings by the felling of 100 m of second-growth vegetation. (C) Picture of an experimental forest clearing formed by clearing of second-growth vegetation surrounding a 1 ha old-growth forest fragment (picture taken in November 2013 by Adrià Lopéz-Baucells).
Bat sampling
Before the creation of the experimental forest clearings, bats were sampled between August 2011 and June 2013 in 14 old-growth forest sites—6 sites in continuous forest (located in Cabo Frio and 41 km camps) and 8 forest fragments categorized according to their area as “1 ha” (n = 3), “10 ha” (n = 3) and “100 ha” (n = 2) (located in Colosso, Porto Alegre, and Dimona camps; Supplementary Figure S1)—and 8 second-growth sites, located 10–90 m from the forest fragments (in areas to be felled during the creation of the experimental forest clearings). The mean distance between old-growth forest sites was 16,243 ± 11,077 m (standard deviation [SD]) and between second-growth sites was 12,780 ± 9,336 m. We estimated canopy cover (CC) as the average of 4 spherical densiometer readings within 3 100 m2 (5 × 20 m) plots established 5 m from each side of the mist net transects (see Supplementary Table S1 for additional vegetation variables). Since CC was similar across old-growth sites in continuous forest (CC = 83.6 ± 5.3, mean ± 1 SD), 100 ha (CC = 83.6 ± 0.9), 10 ha (CC = 87.4 ± 1.0), and 1 ha fragments (CC = 84.6 ± 2.0), we clustered the 6 sites in continuous forest and 8 forest fragments into the category “old-growth forest sites.”
After the creation of the experimental forest clearings, bats were sampled between April and November 2014 in the same 14 old-growth forest sites previously sampled in 2011–2013 and within the 8 experimental forest clearings formed during the reisolation of the old-growth fragments (these were the exact same sites covered by second-growth in 2011–2013; Figure 1). In total, 22 sites were sampled in both 2011–2013 (before the creation of the experimental clearings) and 2014 (after the creation of the experimental clearings).
Each site was surveyed 8 times before the creation of the experimental clearings and 4 times after the creation of the experimental clearings (respectively, 4 and 2 times per season before and after the creation of the experimental clearings). Each site was surveyed using 14 ground-level mist nets (12 × 2.5 m, 16-mm mesh, ECOTONE, Poland), set next to each other along 2 transects of 7 mist nets each. Transects were ca. 30–80 m apart. Mist nets were opened at dusk and remained open for 6 h, being revised at intervals of 15–20 min. Species identification and taxonomy are in accordance with López-Baucells et al. (2016). Bat capture and handling were conducted following guidelines approved by the American Society of Mammalogists (Sikes and Gannon 2011) and in accordance with Brazilian conservation and animal welfare laws.
Statistical analysis
We restricted species-level analyses to the 10 most abundant phyllostomid species (for both 2011–2013 and 2014 only species with >30 captures were evaluated—see “Results” section for species list). A measure of capture rate (bats per mist net hour [mnh]; 1 mnh denoting one 2.5 × 12 m mist net open for 1 h) was obtained by standardizing the capture frequency of each site by the sampling effort (mnh). Captures were pooled into 12 sampling intervals of 30 min—for example, bats captured between 18:00 h and 18:29 h were grouped into the same time interval. Due to unequal sampling sizes within habitat categories, we based the analyses on the per interval relative number of captures such that values for all 12 sampling intervals for each activity pattern amount to 1. Although we use capture frequency as a measure of capture rate, we note that captures might better reflect the distance travelled by bats than their activity per se.
Intraspecific and intraguild temporal activity patterns between habitat types were compared using Kolmogorov–Smirnov 2-sample tests. We used bat capture data from 2011 to 2013 to contrast activity patterns in old-growth forest and second-growth and bat capture data from 2014 to contrast activity patterns in old-growth forest and clearings. Additionally, to investigate any potential temporal changes in the activity patterns in old-growth forest (our baseline habitat), we contrasted the activity patterns in old-growth before the creation of the experimental clearings (2011–2013) and after the creation of the experimental clearings (2014). Furthermore, since the same sites, while covered by secondary forest and after the creation of the experimental clearings contrasted massively in habitat structure, we also compared the activity patterns in second-growth (2011–2013) to the activity patterns in clearings (2014). Kolmogorov–Smirnov 2-sample tests were also used to evaluate within-habitat pairwise differences in activity patterns, both between each pair of species within the same guild and between guilds. The restriction of pairwise comparisons with members of the same trophic guild produces results that are ecologically more meaningful, as activity patterns are likely to reflect interspecific interactions and shared ecological constraints (Halle and Stenseth 2000). For analytical reasons, only pairwise comparisons with an n >30 were evaluated. Kolmogorov–Smirnov 2-sample tests were conducted in R v3.0.2 (R Development Core Team 2013).
For each habitat category, the temporal overlap for frugivorous and gleaning animalivorous species was evaluated with Pianka (Pianka 1973) and Czechanowski (Feinsinger et al. 1981) indices. Assessment of all possible within-guild interspecific pairwise comparisons was conducted using the null model of assemblage-wide temporal overlap algorithm ROSARIO (Castro-Arellano et al. 2010). This algorithm was developed to analyze data of sequential and cyclical nature and works by shifting the continuous distribution of activity patterns over time, whereas retaining the empirical temporal autocorrelation in the activity of each species (Castro-Arellano et al. 2010). ROSARIO was implemented using the software TimeOverlap (Castro-Arellano et al. 2010) by means of 10,000 iterations. Significance (α = 0.05) was determined by relating each empirical index to the proportion of randomizations that obtained an overlap ≥ to that of the empirical overlap value (observed). Significance, therefore, reflects higher temporal overlap than expected by chance.
Results
During 22,070 mnh, we captured 3,971 phyllostomid bats (2,870 before and 1,101 after the creation of the experimental clearings) belonging to 46 species. Before the creation of the experimental clearings, 6 frugivorous (brown fruit-eating bat Artibeus concolor, great fruit-eating bat A. lituratus, dark fruit-eating bat A. obscurus, silky short-tailed bat Carollia brevicauda, Seba’s short-tailed bat C. perspicillata, and dwarf little fruit bat Rhinophylla pumilio) and 4 gleaning animalivorous species (white-throated round-eared bat Lophostoma silvicolum, striped hairy-nosed bat Mimon crenulatum, stripe-headed round-eared bat Tonatia saurophila, and fringe-lipped bat Trachops cirrhosus) met the criterion of >30 captures established for species-level analyses. However, in the period after the creation of the experimental clearings, only 4 frugivorous species had over 30 captures (A. concolor, C. brevicauda, C. perspicillata, and R. pumilio). For most of the aforementioned species and for both periods (before and after the creation of the experimental clearings), there was a peak of activity within the first 2 h after sunset, followed by a clear reduction of activity after that (Supplementary Figures S2 and S3).
Temporal activity responses to habitat type
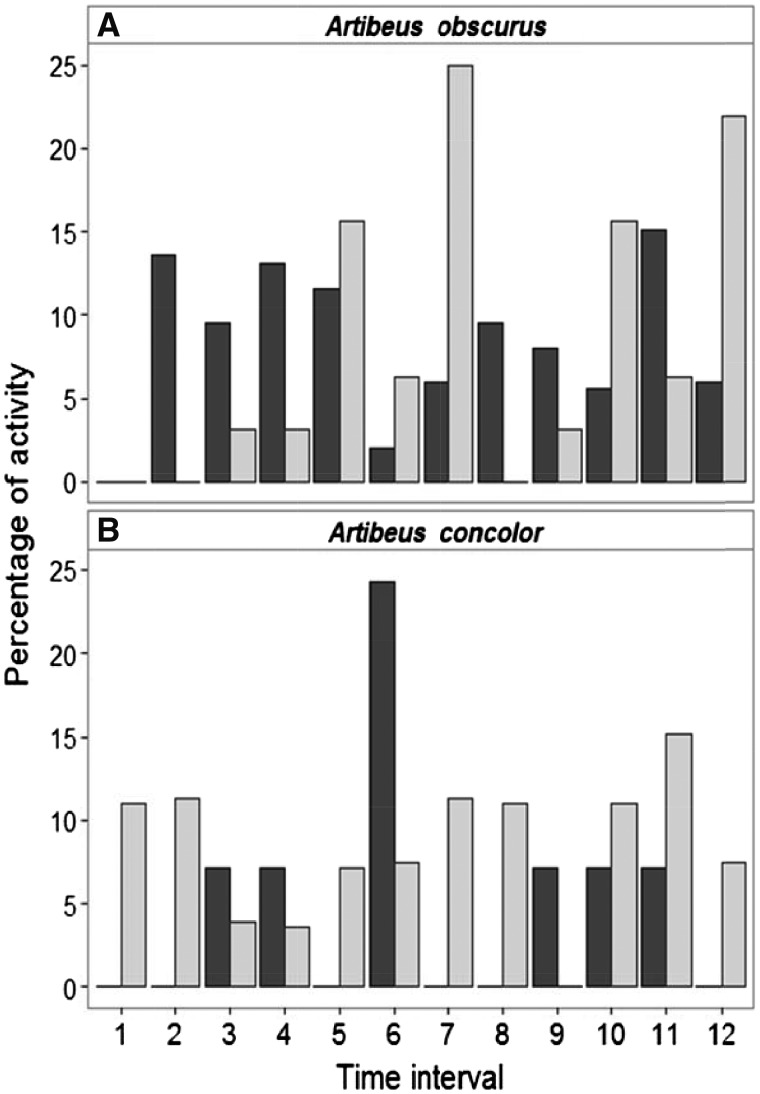
Differences in activity patterns between old-growth forest and second-growth (2011–2013) and between old-growth forest and clearings (2014) were negligible for most species and guilds (Table 1). At the species-level, the only 2 exceptions were the frugivorous A. obscurus (Figure 2A), which exhibited significant differences between old-growth forest and second-growth (with activity being higher in the first 2 h after sunset in old-growth forest compared with secondary forest), and A. concolor (Figure 2B), whose activity patterns differed between old-growth forest and clearings (with activity is higher in the first 2 h after sunset in clearings compared with old-growth). Species-level activity patterns did not change significantly between old-growth forest sites sampled before (2011–2013) and after (2014) the creation of the experimental clearings. Likewise, no differences were detected between sites that were second-growth in 2011–2013 and clearings in 2014 (Supplementary Table S2).
Table 1.
Comparison of bat activity patterns between old-growth forest and second-growth and between old-growth forest and clearings
| Old-growth forest versus second-growth |
Old-growth forest versus clearings |
|||||
|---|---|---|---|---|---|---|
| n | D | P-value | n | D | P-value | |
| Frugivores | ||||||
| Artibeus concolor | 48 | 0.250 | 0.848 | 36 | 0.5833 | 0.034 |
| Artibeus lituratus | 31 | 0.417 | 0.249 | – | – | – |
| Artibeus obscurus | 111 | 0.583 | 0.034 | – | – | – |
| Carollia brevicauda | 84 | 0.333 | 0.518 | 35 | 0.250 | 0.848 |
| Carollia perspicillata | 1714 | 0.333 | 0.536 | 699 | 0.250 | 0.848 |
| Rhinophylla pumilio | 429 | 0.250 | 0.869 | 134 | 0.3333 | 0.518 |
| Gleaning animalivores | ||||||
| Lophostoma silvicolum | 60 | 0.333 | 0.518 | – | – | – |
| Mimon crenulatum | 50 | 0.167 | 0.996 | – | – | – |
| Tonatia saurophila | 50 | 0.417 | 0.249 | – | – | – |
| Trachops cirrhosus | 81 | 0.333 | 0.517 | – | – | – |
| Total | ||||||
| Guild | ||||||
| Frugivores | 0.250 | 0.869 | 0.250 | 0.869 | ||
| Gleaning animalivores | 0.1667 | 0.996 | 0.250 | 0.848 | ||
Significant (P < 0.05) results based on Kolmogorov–Smirnov 2-sample tests are highlighted in bold.
Figure 2.
Temporal activity patterns for the 2 species that exhibited significant differences in activity between old-growth forest (dark grey) and (A) second-growth or (B) clearings (light grey) based on Kolmogorov–Smirnov 2-sample tests.
Differences in temporal activity patterns between species and guilds
Significant within-guild interspecific differences in temporal activity patterns were restricted to the comparison between A. obscurus and R. pumilio in second-growth (R. pumilio’s activity was relatively balanced through the 6 h considered in our analyses, whereas A. obscurus exhibited a distinct peak of activity in the 2nd and 3rd hours after sunset; Figure 3A). At the guild-level, by contrast, differences were only observed for the comparison between frugivores and gleaning animalivores in old-growth and before the creation of the experimental clearings (activity levels of gleaning animalivores were more than double the activity levels of frugivores in the first 30 min after sunset; Figure 3B). Activity patterns for all species and guilds before (2011–2013) and after (2014) the creation of the experimental clearings are presented in Supplementary Figures S2 and S3.
Figure 3.
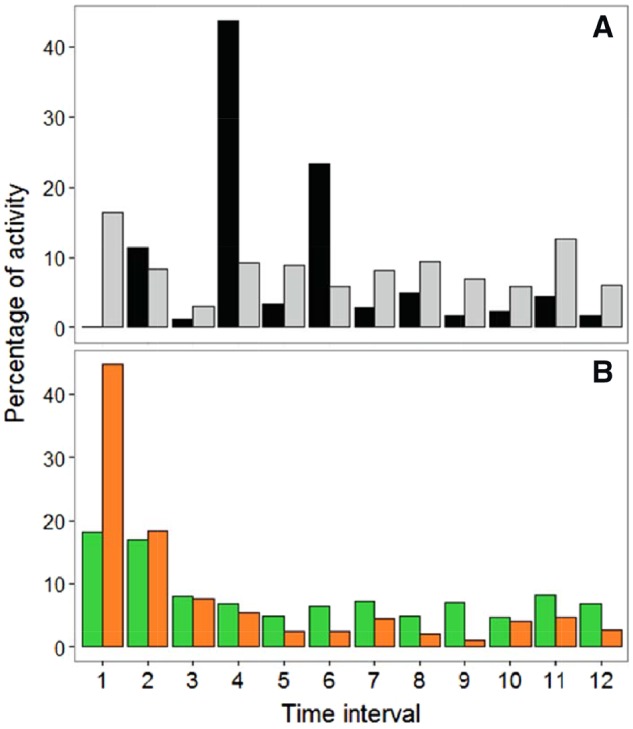
Temporal activity patterns for the pair of species and guilds that exhibited significant differences in activity patterns: (A) A. obscurus (black) and R. pumilio (grey) in second-growth; (B) frugivores (green) and gleaning animalivores (orange) in old-growth forest (before the creation of the experimental clearings).
Temporal overlap
Frugivorous and gleaning animalivorous species presented greater temporal overlap than expected for all habitats both before (2011–2013) and after (2014) the creation of the experimental clearings (Table 2). For frugivores, the degree of temporal overlap (as given by the Pianka and Czechanowski indices) was always lower in old-growth forest than in second-growth and clearings. The contrast was particularly notable for the comparison between old-growth forest and clearings (Table 2). Compared with frugivores, the pattern of temporal overlap for gleaning animalivores between old-growth forest and second-growth was reversed (Table 2).
Table 2.
Pianka and Czechanowski indices of temporal overlap for frugivores and gleaning animalivores in old-growth forest, second growth and forest clearings
| Pianka index |
Czechanowski index |
|||
|---|---|---|---|---|
| Mean overlap | P-value | Mean overlap | P-value | |
| Frugivores | ||||
| 2011–2013 | ||||
| Old-growth forest | 0.778 | 0.01 | 0.675 | 0.008 |
| Second-growth | 0.799 | 0.01 | 0.713 | 0.004 |
| 2014 | ||||
| Old-growth forest | 0.572 | 0.022 | 0.513 | 0.013 |
| Forest clearings | 0.824 | 0.033 | 0.715 | 0.048 |
| Gleaning animalivores | ||||
| 2011–2013 | ||||
| Old-growth forest | 0.701 | 0.002 | 0.544 | 0.001 |
| Second-growth | 0.551 | 0.041 | 0.453 | 0.022 |
Significant (P < 0.05) results indicating greater temporal overlap than expected by chance are highlighted in bold.
Discussion
Numerous studies have reported detrimental effects of habitat modification on tropical forest bats (reviewed in Meyer et al. 2016). At the BDFFP, and despite the “soft” matrix composed of advanced secondary vegetation, bat species richness, abundance, evenness, and assemblage composition varies across old-growth forest and the second-growth matrix (Farneda et al. 2015; Ferreira et al. 2017; Rocha et al. 2017b; Nuñez et al., Forthcoming). However, despite population-, guild-, and assemblage-level responses in occupancy and abundance, here we show that evidence for behavioral changes in the activity pattern of phyllostomid bats in response to variations in habitat structure between old-growth forest and both second-growth and forest clearings is limited.
Temporal activity responses to habitat type
Intraspecific differences in temporal activity patterns between old-growth forest and modified habitats were restricted to A. obscurus in second-growth and A. concolor in forest clearings. In lowland Amazonian rainforest, Presley et al. (2009a) found the activity patterns of 5 out of 8 frugivorous bat species differed between open agricultural areas and forested habitats in Iquitos (Peru), whereas Castro-Arellano et al. (2009) found that the temporal behavior of 3 out of 7 analyzed bat species differed between reduced-impact logging sites and unlogged forest controls in Pará (Brazil). In both studies, the greatest differences were observed soon after sunset and the authors proposed the alterations to reflect twilight avoidance of habitats with open CC due to increased illumination and consequently higher susceptibility to bird predation. Reduced bat activity has been repeatedly linked with increased predation risk in habitats with reduced vegetation cover (Weinbeer et al. 2006), during twilight (Lima and O'keefe 2013) and periods of brighter moonlight (Meyer et al. 2004; Ribeiro Mello 2009, but see Musila et al. 2019). Indeed, during the study period, only 3 events of bird predation upon entangled bats took place in the 1st minutes after sunset (Rocha and López-Baucells 2014; Serra-Gonçalves et al. 2017). However, despite a noticeable reduction in activity of A. obscurus in the first 2 h after sunset in second-growth relative to old-growth forest, we did not detect significant differences between the activity patterns in old-growth forest versus clearings for any species other than A. concolor, for which the activity during the first 2 h after sunset was higher in clearings than in old-growth forest. Our results thus do not lend strong support to the hypothesis of twilight avoidance in more open habitats due to increased predation risk.
Several, non-exclusive explanations can explain the apparent non-alteration of the activity patterns between old-growth forest and second-growth and particularly between old-growth forest and forest clearings. First, despite ample evidence of bat predation by nocturnal and diurnal birds and other visually oriented predators (de Moraes Costa et al. 2016; Mikula 2015; Mikula et al. 2016), levels of predation pressure might vary little between old-growth forest and second-growth, since the secondary forest matrix in our study system is <30 years old (Carreiras et al. 2014). Indeed, the maturation of the second-growth at the BDFFP is eroding the sharp differences in species composition previously found between old-growth and early second-growth habitats (Powell 2013; Quintero and Roslin 2005; Stouffer et al. 2011; Farneda et al. 2018b; Rocha et al. 2018) and, for at least 2 of the most common owl species (Lophostrix cristata and Glaucidium hardyi), occupancy levels are now indistinguishable between old-growth and second-growth forest (Sberze et al. 2010). Secondly, bats might adjust their flight patterns to avoid predation and fly closer to the understory vegetation, particularly in areas of reduced CC. This “vegetation hugging” behavior has been reported for several European vespertilionids (e.g., Schaub and Schnitzler 2007) and, in our system, could explain the increased capture rate of canopy species in the experimental clearings (Rocha et al. 2017c). This change in flight behavior can be one of the possible explanations for the perceived higher activity of A. concolor in clearings than in old-growth forest during the 1st 2 h after sunset. Lastly, given that surveys in 2014 began nearly immediately after the creation of the experimental clearings, local avian predators of bats might not have had enough time to adjust their predatory behavior to the new landscape features.
Differences in temporal activity patterns between species and guilds
Similar to Presley et al. (2009a), no differences in activity patterns were detected between species pairwise comparisons in old-growth forest. However, at the guild-level, frugivores and gleaning animalivores were found to exhibit different activity dynamics, with the latter presenting a more marked activity peak during the first half-hour following sunset. In fact, >60% of the captured gleaning animalivores in old-growth forest were mist-netted within the 1st hour after dark whereas <40% of the sampled frugivores were captured over the same period. The poor nutritional and caloric content of fruits requires frugivores to consume the equivalent of twice their body weight in fruit each night (Charles-Dominique 1986) and, as a result, much of their activity budget is devoted to foraging (Henry and Kalko 2007). On the contrary, gleaning animalivores feed on more energy-rich insects and small vertebrates (e.g., Bernard 2002; Munin et al. 2012; Rocha et al. 2016) through a mix of hunting in continuous flight and sallying from perches (“hang-and-wait” strategy; Kalko et al. 1999). Their activity peak seems to be related to the time of departure from the roost, which telemetry studies in Panama have revealed to be ∼30 and ∼60 min after sunset for, respectively, T. cirrhosus and L. silvicolum, 2 of our focal species (Kalko et al. 1999).
At the species level, the sole significant pairwise comparison was found for A. obscurus and R. pumilio in second-growth. The activity pattern of R. pumilio had already been recorded to differ from the temporal activity of other frugivorous bats in both open agricultural areas and second-growth in Peru (Presley et al. 2009a) and in closed canopy reduced-impact logging sites in the Brazilian Amazon (Presley et al. 2009b). Large frugivorous bats of the genus Artibeus are specialized in mass-producing fruiting trees that are often far apart, demanding long (>2–10 km) commuting distances. However, once a fruiting tree has been located and the bat initiates feeding, it seldom moves during the rest of the night (De Foresta et al. 1984; Henry and Kalko 2007). In contrast, the foraging strategy of the small R. pumilio (∼9 g) relies on short (40–120 m) search flights for scattered epiphytic infructescences within a small foraging area (3.5–14.1 ha; Henry and Kalko 2007). This, alongside smaller flight energetic efficiency in small-sized bats compared with larger ones (Speakman and Thomas 2003), explains the little variation in activity observed for R. pumilio during the 6 h of nightly sampling.
Temporal overlap
Temporal niche overlap was greater than expected by chance in all sampled habitats for both frugivorous and animalivorous bats. However, we found that the similarity between the temporal overlap in frugivores in old-growth forest and second-growth was greater than the similarity between old-growth forest and clearings. Additionally, there was a reduction in temporal overlap of gleaning animalivores in second-growth, comparatively to old-growth forest.
Temporal partitioning is 1 of the 3 primary mechanisms allowing the coexistence of closely related species (the other 2 being partitioning of space and food resources; Schoener 1974) and, it is likely molded by ecological determinants linked with species’ survival and reproduction (Presley et al. 2009a). The observed similar values for both Pianka and Czechanowski indexes in old-growth forest and second-growth suggests that for frugivorous bats, the second-growth matrix at the BDFFP may currently offer similar food availability to old-growth habitats. Consequently, frugivores may not need to adjust their activity budgets to compensate for scarcer or more scattered food resources. Indeed, the second-growth matrix at the BDFFP has a ∼15 m tall canopy that although forming a dense layer that partly inhibits sunlight to reach the understory, stills allows the understory to receive more sunlight than that of the old-growth (Bentos et al. 2013), therefore possibly allowing for higher fruit productivity. As such, potential higher predatory risks associated with reduced CC might be counterbalanced by higher food availability, leveling the values for temporal niche overlap between old-growth forest and second-growth. The same justification can be given for the observed higher temporal overlap of this guild at clearings relative to old-growth forest. Forest gaps increase the abundance of fruits of early-successional plants targeted by frugivorous bats (Presley et al. 2009b). The experimental clearings targeted by this study were surveyed too soon after tree felling to support a high abundance of fruits. However, increased light availability can augment growth and reproduction in tropical trees (Graham et al. 2003) and thus standing fruiting trees at the edge of the clearings might have increased fruit productivity due to additional exposition to sunlight. This food surplus might have lessened competition and consequently reduced temporal displacements. These results mimic the findings from bat assemblages inhabiting a disturbance gradient in the tropical Bolivian Andes, in which temporal niche overlap among generalist frugivores was found to increase with disturbance (Montaño-Centellas et al. 2015).
For gleaning animalivores, the values of temporal overlap between old-growth forest and second-growth were considerably less similar than for the frugivore guild. This aligns with the findings of lower abundance of gleaning animalivorous bats in the BDFFP second-growth habitats (Rocha et al. 2017b) and indicates that while the Vismia- and Cecropia-dominated secondary vegetation allows for additional food resources for frugivorous bats, the regrowth habitats are still less suitable for gleaning animalivores than old-growth forest.
Conservation implications
The analysis of behavioral responses in terms of changes in activity patterns in relation to forest disturbance complements studies focusing on the effects of habitat modification on species occupancy and abundance (Meyer et al. 2016). Consequently, this study contributes to a more comprehensive understanding of the impacts of the ongoing wave of forest loss and deterioration plaguing the humid tropics. Here, we show that although small differences can be detected for the disturbance-sensitive gleaning animalivorous bats, the activity patterns of most species in second-growth and small forest clearings, 2 of the most prominent habitat types in humanized tropical landscapes, vary little from the activity patterns in old-growth forest. This suggests that, at least for the most common species in our study landscape, regenerating second-growth seems to offer enough resources, allowing species to retain similar activity budgets between old-growth and regrowth habitats. Our results thus reinforce the conservation potential of secondary rainforests by showing that old (>30 years) second-growth has little effect on the activity budgets of 10 of the most abundant Amazonian phyllostomids.
Supplementary Material
Acknowledgments
We would like to thank the many students, volunteers, and field assistants who helped us during fieldwork; Paulo E.D. Bobrowiec, José L.C. Camargo, Rosely Hipólito, and Ary Jorge Ferreira for logistic support and 2 anonymous reviewers for helpful comments. This research was conducted under ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) ermit (26877-2) and constitutes the publication number 766 of the BDFFP technical series.
Funding
Funding was provided by the Portuguese Foundation for Science and Technology to C.F.J.M. (PTDC/BIA-BIC/111184/2009), R.R. (SFRH/BD/80488/2011), and A.L.-B (FCT PD/BD/52597/2014). F.Z.F. was supported by a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Additional funding was provided by Bat Conservation International student research fellowships to A.L.-B and R.R.
References
- Avila-Cabadilla LD, Sanchez-Azofeifa GA, Stoner KE, Alvarez-Anorve MY, Quesada M. et al. , 2012. Local and landscape factors determining occurrence of phyllostomid bats in tropical secondary forests. PLoS ONE 7:e35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila-Gómez ES, Moreno CE, García-Morales R, Zuria I, Sánchez-Rojas G. et al. 2015. Deforestation thresholds for phyllostomid bat populations in tropical landscapes in the Huasteca region, Mexico. Trop Conserv Sci 8:646–661. [Google Scholar]
- Barlow J, Gardner TA, Araujo IS, Ávila-Pires TC, Bonaldo AB. et al. , 2007. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci USA 104:18555–18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentos TV, Nascimento HEM, Williamson GB, 2013. Tree seedling recruitment in Amazon secondary forest: importance of topography and gap micro–site conditions. For Ecol Manage 287:140–146. [Google Scholar]
- Bernard E, 2002. Diet, activity and reproduction of bat species (Mammalia, Chiroptera) in Central Amazonia, Brazil. Rev Bras Zool 19:173–188. [Google Scholar]
- Carreiras JM, Jones J, Lucas RM, Gabriel C, 2014. Land use and land cover change dynamics across the Brazilian Amazon: insights from extensive time-series analysis of remote sensing data. PLoS ONE 9:e104144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Arellano I, Lacher TE, Willig MR, Rangel TF, 2010. Assessment of assemblage-wide temporal niche segregation using null models. Methods Ecol Evol 1:311–318. [Google Scholar]
- Castro-Arellano I, Presley SJ, Willig MR, Wunderle JM, Saldanha LN, 2009. Reduced-impact logging and temporal activity of understorey bats in lowland Amazonia. Biol Conserv 142:2131–2139. [Google Scholar]
- Castro-Luna AA, Sosa IJ, Castillo-Campos G, 2007. Quantifying phyllostomid bats at different taxonomic levels as ecological indicators in a disturbed tropical forest. Acta Chiropt 9:219–228. [Google Scholar]
- Charles-Dominique P, 1986. Inter-relations between frugivorous vertebrates and pioneer plants: Cecropia, birds and bats in French Guyana In: Estrada A, Fleming TH, editors. Frugivores and Seed Dispersal. Dordrecht: Springer Netherlands; 119–135. [Google Scholar]
- Chazdon RL, 2014. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation. Chicago: University of Chicago Press. [Google Scholar]
- De Foresta H, Charles-Dominique P, Erard C, Prevost MF, 1984. Zoochorie et premiers stades de la régénération naturelle après coupe en forêt guyanaise. Revue D'Écologie 39:369–400. [Google Scholar]
- de la Peña-Domene M, Martinez-Garza C, Palmas-Perez S, Rivas-Alonso E, Howe HF, 2014. Roles of birds and bats in early tropical-forest restoration. PLoS ONE 9:e104656.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes Costa L, de Oliveira Tabosa L, Luz JL, de Carvalho WD, 2016. Predadores naturais de morcegos no Brasil. Bol Soc Bras Mastozool 77:131–142. [Google Scholar]
- Farneda FZ, Rocha R, López-Baucells A, Groenenberg M, Silva I. et al. , 2015. Trait-related responses to habitat fragmentation in Amazonian bats. J Appl Ecol 52:1381–1391. [Google Scholar]
- Farneda FZ, Rocha R, López-Baucells A, Sampaio EM, Palmeirim JM. et al. , 2018a. The road to functional recovery: temporal effects of matrix regeneration on Amazonian bats. Trop Conserv Sci 11:1–4. [Google Scholar]
- Farneda FZ, Rocha R, López-Baucells A, Sampaio EM, Palmeirim JM. et al. , 2018b. Functional recovery of Amazonian bat assemblages following secondary forest succession. Biol Conserv 218:192–199. [Google Scholar]
- Feinsinger P, Spears EE, Poole RW, 1981. A simple measure of niche breadth. Ecology 62:27–32. [Google Scholar]
- Ferreira DF, Rocha R, López-Baucells A, Farneda FZ, Carreiras JM. et al. 2017. Season-modulated responses of Neotropical bats to forest fragmentation. Ecol Evol 7:4059–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Fisher JT, Burton AC, Volpe JP, 2017. Investigating animal activity patterns and temporal niche partitioning using camera-trap data: challenges and opportunities. Remote Sens Ecol Conserv 3:123–132. [Google Scholar]
- Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA. et al. , 2011. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378.. [DOI] [PubMed] [Google Scholar]
- Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ, 2003. Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc Natl Acad Sci USA 100:572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S, 2000. Ecological relevance of daily activity patterns In: Halle S, Stenseth NC, editors. Activity Patterns in Small Mammals: An Ecological Approach. Berlin: Springer; 67–90. [Google Scholar]
- Halle S, Stenseth NC, 2000. Introduction In: Halle S, Stenseth NC, editors. Activity Patterns in Small Mammals: An Ecological Approach. Berlin: Springer; 3–17. [Google Scholar]
- Henry M, Kalko EKV, 2007. Foraging strategy and breeding constraints of Rhinophylla pumilio (Phyllostomidae) in the Amazon Lowlands. J Mammal 88:81–93. [Google Scholar]
- Jones G, Jacobs DS, Kunz TH, Willig MR, Racey PA, 2009. Carpe noctem: the importance of bats as bioindicators. Endanger Species Res 8:93–115. [Google Scholar]
- Kalko EKV, Friemel D, Handley CO, Schnitzler H-U, 1999. Roosting and foraging behavior of two neotropical gleaning bats Tonatia silvicola and Trachops cirrhosus (Phyllostomidae). Biotropica 31:344–353. [Google Scholar]
- Kemp J, López-Baucells A, Rocha R, Wangensteen OS, Andriatafika Z. et al. , 2019. Bats as potential suppressors of multiple agricultural pests: a case study from Madagascar. Agric Ecosyst Environ 269:88–96. [Google Scholar]
- Kunz TH, Braun de Torrez E, Bauer D, Lobova T, Fleming TH, 2011. Ecosystem services provided by bats. Ann N Y Acad Sci 1223:1–38. [DOI] [PubMed] [Google Scholar]
- Laurance WF, Camargo JL, Luizão RC, Laurance SG, Pimm SL. et al. , 2011. The fate of Amazonian forest fragments: a 32-year investigation. Biol Conserv 144:56–67. [Google Scholar]
- Laurance WF, Goosem M, Laurance SGW, 2009. Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 24:659–669. [DOI] [PubMed] [Google Scholar]
- Lima SL, O'keefe JM, 2013. Do predators influence the behaviour of bats? Biol Rev 88:626–644. [DOI] [PubMed] [Google Scholar]
- López-Baucells A, Rocha R, Bobrowiec PED, Bernard E, Palmeirim JM. et al. , 2016. Field Guide to Amazonian Bats. Manaus: National Institute of Amazonian Research (INPA; ). [Google Scholar]
- Maas B, Karp DS, Bumrungsri S, Darras K, Gonthier D. et al. , 2016. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol Rev 91:1081–1101. [DOI] [PubMed] [Google Scholar]
- Medellin RA, Gaona O, 1999. Seed dispersal by bats and birds in forest and disturbed habitats of Chiapas, Mexico. Biotropica 31:478–485. [Google Scholar]
- Melo FPL, Arroyo-Rodríguez V, Fahrig L, Martínez-Ramos M, Tabarelli M, 2013. On the hope for biodiversity-friendly tropical landscapes. Trends Ecol Evol 28:462–468. [DOI] [PubMed] [Google Scholar]
- Mendenhall CD, Karp DS, Meyer CF, Hadly EA, Daily GC, 2014. Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 509:213.. [DOI] [PubMed] [Google Scholar]
- Mesquita R, Massoca P, Jakovac CC, Bentos TV, Williamson GB, 2015. Amazon rain forest succession: stochasticity or land-use legacy? BioScience 65:849–861. [Google Scholar]
- Meyer CF, Schwarz CJ, Fahr J, 2004. Activity patterns and habitat preferences of insectivorous bats in a West African forest-savanna mosaic. J Trop Ecol 20:397–407. [Google Scholar]
- Meyer CFJ, Struebig MJ, Willig MR, 2016. Responses of tropical bats to habitat fragmentation, logging, and deforestation In: Voigt CC, Kingston T, editors. Bats in the Anthropocene: Conservation of Bats in a Changing World. Cham: Springer International Publishing; 63–103. [Google Scholar]
- Mikula P, 2015. Fish and amphibians as bat predators. Eur J Ecol 1:71–80. [Google Scholar]
- Mikula P, Morelli F, Lučan RK, Jones DN, Tryjanowski P, 2016. Bats as prey of diurnal birds: a global perspective. Mamm Rev 46:160–174. [Google Scholar]
- Montaño-Centellas F, Moya MI, Aguirre LF, Galeón R, Palabral O. et al. , 2015. Community and species-level responses of phyllostomid bats to a disturbance gradient in the tropical Andes. Acta Oecol 62:10–17. [Google Scholar]
- Munin RL, Fischer E, Gonçalves F, 2012. Food habits and dietary overlap in a phyllostomid bat assemblage in the Pantanal of Brazil. Acta Chiropt 14:195–204. [Google Scholar]
- Musila S, Bogdanowicz W, Syingi R, Zuhura A, Chylarecki P. et al. , 2019. No lunar phobia in insectivorous bats in Kenya. Mamm Biol 95:77–84. [Google Scholar]
- Nuñez SF, López-Baucells A, Rocha R, Bobrowiec PED, Palmeirim JM. et al. , Forthcoming. Echolocation and wing morphology: key trait correlates of vulnerability of insectivorous bats to tropical forest fragmentation. Front Ecol Evol. [Google Scholar]
- Pianka ER, 1973. The structure of lizard communities. Annu Rev Ecol Evol Syst 4:53–74. [Google Scholar]
- Powell L, 2013. Recovery of Understory Bird Movement in Post-Pasture Amazonia [PhD thesis]. University of Maine.
- Presley SJ, Willig MR, Castro-Arellano I, Weaver SC, 2009a. Effects of habitat conversion on temporal activity patterns of phyllostomid bats in lowland Amazonian rain forest. J Mammal 90:210–221. [Google Scholar]
- Presley SJ, Willig MR, Saldanha LN, Wunderle JM Jr. et al. , 2009b. Reduced-impact logging has little effect on temporal activity of frugivorous bats (Chiroptera) in lowland Amazonia. Biotropica 41:369–378. [Google Scholar]
- Quintero I, Roslin T, 2005. Rapid recovery of dung beetle communities following habitat fragmentation in Central Amazonia. Ecology 86:3303–3311. [Google Scholar]
- R Development Core Team, 2013. R: A Language and Environment for Statistical Computing Available from: http://www.r-project.org (assessed on 30 June 2013).
- Ribeiro Mello MA, 2009. Temporal variation in the organization of a Neotropical assemblage of leaf-nosed bats (Chiroptera: Phyllostomidae). Acta Oecol 35:280–286. [Google Scholar]
- Rocha R, Ferreira DF, López-Baucells A, Farneda FZ, Carreiras JM. et al. , 2017a. Does sex matter? Gender-specific responses to forest fragmentation in Neotropical bats. Biotropica 49:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha R, Gordo M, Lopez-Baucells A, 2016. Completing the menu: addition of Scinax cruentommus and Scinax cf. garbei (Anura: Hylidae) to the diet of Trachops cirrhosus (Chiroptera: Phyllostomidae) in Central Amazon. North-West J Zool 12:199–204. [Google Scholar]
- Rocha R, López-Baucells A, 2014. Opportunistic predation by crested owl Lophostrix cristata upon Seba’s short-tailed bat Carollia perspicillata. Rev Bras Ornitol 22:58–60. [Google Scholar]
- Rocha R, López-Baucells A, Farneda FZ, Groenenberg M, Bobrowiec PE. et al. , 2017b. Consequences of a large-scale fragmentation experiment for Neotropical bats: disentangling the relative importance of local and landscape-scale effects. Landsc Ecol 32:31–45. [Google Scholar]
- Rocha R, Ovaskainen O, López-Baucells A, Farneda FZ, Ferreira DF. et al. , 2017c. Design matters: an evaluation of the impact of small man-made forest clearings on tropical bats using a before-after-control-impact design. For Ecol Manage 401:8–16. [Google Scholar]
- Rocha R, Ovaskainen O, López-Baucells A, Farneda FZ, Sampaio EM. et al. , 2018. Secondary forest regeneration benefits old-growth specialist bats in a fragmented tropical landscape. Sci Rep 8:3819.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa I, Souza C, Ewers RM, 2012. Changes in size of deforested patches in the Brazilian Amazon. Conserv Biol 26:932–937. [DOI] [PubMed] [Google Scholar]
- Rozendaal DM, Bongers F, Aide TM, Alvarez-Dávila E, Ascarrunz N. et al. , 2019. Biodiversity recovery of neotropical secondary forests. Sci Adv 5:3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sberze M, Cohn-Haft M, Ferraz G, 2010. Old growth and secondary forest site occupancy by nocturnal birds in a neotropical landscape. Anim Conserv 13:3–11. [Google Scholar]
- Schaub A, Schnitzler H-U, 2007. Flight and echolocation behaviour of three vespertilionid bat species while commuting on flyways. J Comp Physiol A 193:1185–1194. [DOI] [PubMed] [Google Scholar]
- Schoener TW, 1974. Resource partitioning in ecological communities. Science 185:27–39. [DOI] [PubMed] [Google Scholar]
- Serra-Gonçalves C, López-Baucells A, Rocha R, 2017. Opportunistic predation of a silky short-tailed bat Carollia brevicauda by a tawny-bellied screech-owl Megascops watsonii, with a compilation of predation events upon bats entangled in mist-nets. J Bat Res and Conserv 10:41–46. [Google Scholar]
- Sikes RS, Gannon WL, 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Thomas DW, 2003. Physiological ecology and energetics of batss In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago (IL: ): University of Chicago Press; 430–490. [Google Scholar]
- Stouffer PC, Johnson EI, Bierregaard RO Jr, Lovejoy TE, 2011. Understory bird communities in Amazonian rainforest fragments: species turnover through 25 years post-isolation in recovering landscapes. PLoS ONE 6:e20543.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyukavina A, Hansen MC, Potapov P, Parker D, Okpa C. et al. , 2018. Congo Basin forest loss dominated by increasing smallholder clearing. Sci Adv 4:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbeer M, Meyer CFJ, Kalko EKV, 2006. Activity pattern of the trawling phyllostomid bat Macrophyllum macrophyllum in Panamá. Biotropica 38:69–76. [Google Scholar]
- Willig MR, Presley SJ, Bloch CP, Hice CL, Yanoviak SP. et al. , 2007. Phyllostomid bats of lowland Amazonia: effects of habitat alteration on abundance. Biotropica 39:737–746. [Google Scholar]
- Willig MR, Presley SJ, Plante JL, Bloch CP, Solari S. et al. , 2019. Guild-level responses of bats to habitat conversion in a lowland Amazonian rainforest: species composition and biodiversity. J Mammal 100:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.