Abstract
In a number of animal species, individuals differ in their ability to solve cognitive tasks. However, the mechanisms underlying this variability remain unclear. It has been proposed that individual differences in cognition may be related to individual differences in behavior (i.e., personality); a hypothesis that has received mixed support. In this study, we investigated whether personality correlates with the cognitive ability that allows inhibiting behavior in 2 teleost fish species, the zebrafish Danio rerio and the guppy Poecilia reticulata. In both species, individuals that were bolder in a standard personality assay, the open-field test, showed greater inhibitory abilities in the tube task, which required them to inhibit foraging behavior toward live prey sealed into a transparent tube. This finding reveals a relationship between boldness and inhibitory abilities in fish and lends support to the hypothesis of a link between personality and cognition. Moreover, this study suggests that species separated by a relatively large phylogenetic distance may show the same link between personality and cognition, when tested on the same tasks.
Keywords: cognitive abilities, cognitive ecology, fish behavior, individual differences, inhibitory control, personality
Human psychologists have often investigated variation in cognitive abilities (Gustafsson and Undheim 1996), finding that, once they have controlled for age, education level, and other confounding factors, some individuals consistently outperform others on certain cognitive tasks (problem-solving tasks: Simon and Simon 1978; linguistic tasks: Conway 1996; spatial tasks: Hegarty and Waller 2005; mathematical tasks: Halberda et al. 2008). Similarly, researchers have provided evidence that nonhuman mammals, birds, and teleost fish show individual differences in cognition (reviewed in Thornton and Lukas 2012; Lucon-Xiccato and Bisazza 2017). However, cognitive individual differences are less investigated and less understood in these species than in human.
Human individual differences in cognition have often been associated with variability in the capacity to perform basic cognitive tasks (Miyake and Friedman 2012; Diamond 2013), such as inhibitory control (Carlson and Moses 2001; Passolunghi and Siegel 2001; Cain 2007; Gilmore et al. 2013; Mercier et al. 2014). Inhibitory control is the cognitive ability that allows prevailing over internal predispositions and external lures to control attention, behavior, emotions, and thoughts (Diamond 2013). For example, inhibitory control allows to inhibit the temptation to eat sweets while on a diet or stay focused on a single voice at a cocktail party (Diamond 2013). Because inhibitory control is involved in many activities and higher cognitive processes, its variability might cause, along with other factors, individual differences in diverse cognitive tasks (Shamosh et al. 2008). The ability to inhibit behavior has also been observed in nonhuman animals and is thought to derive from cognitive processes akin to human inhibitory control (e.g., MacLean et al. 2014; Beran 2015). For example, ambush predators frequently inhibit their initial prepotent responses toward attacking prey and wait the appropriate moment (MacNulty et al. 2007). Similarly, prey exhibit inhibition of foraging behavior when they detect a potential predator (Ryer and Olla 1991). In some mammalian and avian species, individual differences also seem to be present in the ability to inhibit behavior, similarly to what has been reported on inhibitory control in humans (Boogert et al. 2011; Meier et al. 2017; Beran and Hopkins 2018). For example, Meier et al. (2017) reported individual differences in the ability of pheasants Phasianus colchicus to inhibit their tendency to orient toward a distractor food reward, which could not be reached.
Studies on animals have suggested that individual cognitive abilities are positively correlated with fitness (Cole and Quinn 2012; Cauchard et al. 2013). This is expected to cause directional selection, which would reduce individual differences in cognitive abilities. A direct explanation for the maintenance of individual differences in cognition is that the brain tissues involved in cognitive computation are expensive to maintain (Aiello and Wheeler 1995). Therefore, individuals may develop these tissues at different level according to their condition, which is highly variable among individuals (e.g., Rowe and Houle 1996). Alternatively, cognitive abilities might be associated or in trade-off with other life history traits (Stearns 1989). For example, guppies Poecilia reticulata artificially selected for large brains, which show higher cognitive abilities in several tasks (Kotrschal et al. 2014a, 2014b; Buechel et al. 2018), have been reported to develop small guts and produce few offspring (Kotrschal et al. 2014b).
Recent studies have also suggested the possibility of an indirect explanation: cognitive individual differences may be related to personality traits, that is, consistent individual differences in behavior, such as exploration, boldness, activity, and sociability (Carere and Locurto 2011; Sih and Del Giudice 2012). Personality is maintained by frequency-dependent selection and spatio-temporal fluctuation in the pattern of selection (Dingemanse and Réale 2005). Among great tits Parus major, females with higher exploratory tendencies have higher survival rates in years with scarce resources and the opposite pattern was observed in years with abundant resources (Dingemanse et al. 2004). If individual differences in cognition are related to personality, then the selective pressures that maintain variability in personality may indirectly be responsible of variability in cognitive abilities. Empirical studies furnish partial support for this hypothesis in various taxa (Dougherty and Guillette 2018): in cavies Cavia aperea, bolder and more active individuals exhibit higher learning performance (Guenther et al. 2014); slow-explorer black-capped chickadees Poecile atricapillus exhibit higher accuracy in an instrumental learning task (Guillette et al. 2015); and guppies with higher sociability exhibit a reduced ability to discriminate between the sizes of 2 conspecific shoals and choosing the larger, safer shoal (Lucon-Xiccato and Dadda 2017). Few studies, to date, have addressed whether personality also explains variability in inhibitory abilities. A study on rats found that highly explorative individuals display lower inhibitory abilities (Ferland et al. 2014). On the contrary, personality did not explain inhibitory performance in 5 avian species (Guillette et al. 2015; Stow et al. 2018; van Horik et al. 2018).
In this study, we explored whether personality explains individual differences in the inhibitory abilities of teleost fish, as observed in some mammalian species (Avila and Parcet 2001; Ferland et al. 2014). Moreover, we attempted to understand whether the relationship between personality and inhibitory abilities is constant across species. This relationship has been found in some species, but not in others (Dougherty and Guillette 2018), and may therefore be species specific. However, because the different species have been tested with different paradigms, it is also possible that methodological differences caused the contrasting results. To address our second aim, we performed our study on 2 fish species, the zebrafish Danio rerio and the guppy P. reticulata using the same methodology. We first tested subjects twice in an inhibition task that consisted of presenting a transparent tube containing live prey (Lucon-Xiccato and Bertolucci 2019). Because fish could not reach the prey, they had to inhibit their foraging behavior. In previous studies with this task, we found both study species showing inhibition and rapidly reducing the number of attempts to catch the prey over time (guppies: Lucon-Xiccato and Bertolucci 2019; zebrafish: T.L.-X. and C.B., unpublished data). After the inhibition task, we tested the same individuals twice in an open-field test, commonly used to assess personality in these species (Dadda et al. 2010; Tran and Gerlai 2013). This design allowed us to identify consistent individual differences (i.e., repeatability) in inhibitory ability and in personality, and then, to assess the presence of covariance between individuals’ performance on the 2 tasks (Griffin et al. 2015). Given the scarcity of previous research, we could not draw a priori hypothesis on the direction of the relationship between personality and inhibitory performance.
Materials and Methods
Experimental design
We tested individuals of both species with the same procedure. First, fish performed the inhibition task, which consisted of 3 days of habituation and, on the 4th day, 2 20-min inhibition trials, separated by a 2 h interval. Two hours after the Trial 2 of the inhibition task, we moved the fish into the open field for the Trial 1 of the personality test (duration: 10 min). We performed Trial 2 of the personality test after 48 h, during which we kept the fish in the apparatus of the inhibition task. The long interval between the personality trials was needed, because this test was aimed at measuring subjects’ reaction to a novel, unfamiliar environment. We chose a fixed-order test design, because it is considered helpful to study individual variation without the confounding variance due to randomization of the test order across individuals (Bell 2013).
Subjects
The subjects were 16 adult zebrafish and 16 adult guppies (total: N = 32 fish). The zebrafish belonged to a wild-type strain routinely bred at the zebrafish facility of University of Ferrara. This zebrafish stock consists of 500 individuals and was originated in 2011 (corresponding to ∼20 generations) from 100 zebrafish (sex ratio 50:50) bought from a local shop. We maintained the zebrafish stock by performing periodical reproductions with fish randomly selected from different maintenance tanks. Moreover, twice per year, we added 30–50 new zebrafish (sex ratio 50:50) to the stock. The guppies belonged to a stock of 1,000 domestic fish (“snakeskin cobra green” strain), maintained in the laboratory since 2012, roughly corresponding to 30 generations. We founded our guppy’s stock in 2012 with 200 individuals (sex ratio 50:50) bought from local dealers. Guppies breed spontaneously in their maintenance tanks; to reduce chances of inbreeding, we routinely moved individuals from the different maintained tanks and we occasionally added new guppies bought from the shop. We used these laboratory-reared fish to avoid confounding effects due to different individual experience before the experiments. Moreover, domestic fish seem to habituate faster than wild fish to the training procedure used in cognitive experiments (Lucon-Xiccato and Bisazza 2014).
We took care to ensure that all the conditions experienced by the individuals were identical before the experiment. We kept both species in separated tanks under standardized laboratory conditions: water at 26 ± 1°C, photoperiod of 12 h of light (07.00–19.00 h), and water biological filters to maintain water condition. We provided food flakes and brine shrimps Artemia salina, nauplii to the fish twice per day.
Inhibition task
The inhibition task was developed to study cognition in cuttlefish (Messenger 1973) and we recently adapted it for fish (Lucon-Xiccato and Bertolucci 2019). As stimulus prey, we used brine shrimp A. salina, nauplii. Our fish were routinely fed with brine shrimps during maintenance and therefore recognized them as prey. We have showed that exploratory tendency toward the novel object inserted in the tank (i.e., the tube) did not affect the inhibitory behavior of fish in the tube task. Indeed, fish did not respond to empty tubes: their attack behavior was triggered by the presence of the live prey inside the tube (Lucon-Xiccato and Bertolucci 2019). Moreover, a control experiment showed that fish performed less attacks, and therefore exhibited higher inhibition, toward a tube with a small number of preys (Lucon-Xiccato and Bertolucci 2019). This suggests that habituation did not affect the measure of inhibitory control because habituation is expected to cause the opposite pattern of results, that is, reduced number of attacks toward tubes with a large number of preys. Last, the results of the tube task are likely not affected by extinction because this process arises in case of conditioned responses (Shettleworth 2010). Therefore, inhibition seems to be the cognitive ability that account for performance in the tube task.
We chose the tube task over other tasks developed for land vertebrates for 2 main reasons. First, the test phase was rapid (20 min of testing versus several weeks; Lucon-Xiccato et al. 2017) and did not require to expose the fish to the experimenter during the trials. Long conditioning procedure of other tasks might be stressful for fish and might favor subjects with a personality that adapts faster to the presence of human experimenter. Second, in tasks developed for land vertebrates, the subject can actually reach the food reward (Kabadayi et al. 2018); in the water, the chemical cues of the food likely disperse widely and may help the subject to solve the task using the smell (Santacà et al. 2019b). This factor has been shown to alter the results of comparisons between different fish species, including guppies and zebrafish (Santacà et al. 2019a). In the tube task, the food is sealed inside a tube and thus chemical cues cannot affect subjects’ performance.
Apparatus
We tested the subjects in plastic tanks (33 × 13 cm, 15 cm height) containing 4 L of water. Green plastic panels covered the walls of the tanks, preventing the fish from seeing outside. A panel made of transparent plastic placed above the tanks served as a lid. The lid also allowed to support the tube with the stimuli (see “Stimulus prey” section) thanks to a Ø 1.2 cm circular hole. A warm-white LED strip illuminated the tanks from above (photoperiod 12:12 h). A webcam (Logitech) placed 50 cm above each tank recorded the behavior of the subjects during the experiment and stored the recordings in a computer running custom-made software.
Stimulus prey
We used brine shrimps nauplii as prey, because the fish used in the experiment were accustomed to feed on them (see “Subjects” section). We prepared brine shrimps with a standard protocol: 24 h before the experiment, we mixed 2 g of cysts (Ocean Nutrition, California, HE 240.000 NPG), 2 L of water, and 50 g of salt in a sedimentation cone. We kept the water aerated and at 28°C using a heater and an air pump. With this procedure, the brine shrimps hatched after 24 h. We presented the brine shrimps (4 mL of the solution obtained as before) to the fish by means of a standard glass test tube (length: 10 cm; Ø: 1.2 cm). In a prior study (Lucon-Xiccato and Bertolucci 2019), we counted the number of brine shrimps in the tube, finding that they were 470 ± 48 (mean ± standard deviation [SD]; N = 10). We also measured the activity of brine shrimps in the tube for 20 min in an empty aquarium. Because the brine shrimps showed constant activity after the first minute, in which they settled down (Lucon-Xiccato and Bertolucci 2019), we inserted the stimuli in the tube 2 min before the start of the trial with the fish. This prevented that the first minute of high stimuli activity affected the attack rate of the subject.
Habituation procedure
The inhibition task consisted of 2 phases, habituation and test (Lucon-Xiccato and Bertolucci 2019). During the habituation phase, which lasted 3 days, the subjects familiarized with a feeding habit useful for the test phase. We placed each individual fish into an apparatus and we fed it after few minutes. As food, we used flakes crumbled and mixed to water. We delivered the food using a Pasteur pipette, inserted in the water through the hole in the lid. After 1 h, we fed the fish again. Then, we left the fish undisturbed overnight. We similarly fed the guppies on Day 2 (4 times) and Day 3 of habituation (6 times). We progressively started to deliver the food only when the fish looked at and approached the pipette. Hence, the fish learned to receive food in correspondence of the hole in the lid and usually reached the pipette within 5 s from its immersion.
Test procedure
On the day after the conclusion of the habituation phase, we started the test phase. We presented the tube with the prey for 2 trials separated by 2 h. Each trials lasted 20 min (the test lasted 40 min overall). To maintain similar conditions in the 2 trials, we did not feed the subjects before the trials on the experimental days. We feed them after completion of the inhibitory trials and on the day between the 2 personality trials, according to the maintenance schedule. We presented the tube by inserting it through the hole of the lid, where it stayed in place, suspended in the water column, thanks to a support. Because of the habituation to feed from hole in the lid, subjects usually attempted to reach the prey in the tube with almost no latency (<5 s) and exhibited high frequency of attacks. To ensure accurate scoring, we left the fish undisturbed during the trials and we analyzed their behavior using the video recordings played back at 0.5×. We counted the number of attacks toward the brine shrimps performed by each fish, divided by each minute of each trial. We considered the fish to attack the prey when it touched the glass of the tube with the snout. Because trial duration was fixed, the number of attacks also provided information on the frequency of attacks (i.e., individuals with higher number of attacks also had higher frequency of attacks).
Personality test
The open-field test was originally developed to study anxiety, boldness, and exploration in rodents (Walsh and Cummins 1976), but it is now commonly used in fish (Irving and Brown 2013; Tran and Gerlai 2013; Polverino et al. 2016). The subject is placed in an unfamiliar, empty arena for a brief period and its spontaneous behavior is observed, with the assumption that individuals with different personality will behave differently. Two variables are commonly measured to characterize personality in fish with the open-field test: the activity (measured as distance travelled) and the time spent in the central area of the open field. Regarding activity, some species tend to show reduced activity in the open field (and thus they reduce distance travelled). Activity reduction is a response to risk because the unfamiliar arena is a novel, potentially dangerous environment. As shyer individuals show higher risk copying, they are expected to show lower activity compared with bolder individuals in the open field. In guppies and other fish species, it has been reported an opposite situation: shyer individuals show greater activity because they try to escape from the novel, uncomfortable environment more intensely than bolder individuals (Kotrschal et al. 2014b). Time spent in the central area of the open field is generally higher in bolder and more explorative individuals, because fish tend to avoid the center in which they are more exposed, compared with the edges, with eventual predators (Warren and Callaghan 1975).
Apparatus
The apparatus resembled the one previously used to study open-field behavior in these species (Dadda et al. 2010; Tran and Gerlai 2013). It consisted of a 40 × 40 cm (15 cm height) arena made of white plastic and filled with 10 cm of water. Warm-white LED strips placed 1 m above the water level lighted the arena, which was placed in a dark room. To record fish behavior, we used an automated tracking system designed by Noldus Information Technology. The system consisted of a LED infrared-backlit (λ > 980 nm) table and a camera (Monochrome GigE camera, Basler, D; resolution: 1280 × 1024) placed 1 m above the table and recording at 5 frames per second. A computer connected to the camera run the EthoVision 11.5 tracking software, which collected the data and analyzed the behavior of the subjects.
Procedure
We performed 2 trials in the open field, separated by 48 h. In each trial, we collected an individual subject from the apparatus used for the tube task and we moved it into the open field using an opaque jar filled with 1 L water. We gently released the fish into the middle of the open field and we started the tracking software to record the behavior of the subjects for 10 min. Between the 2 trials, the fish was moved back to the apparatus in which the inhibition task took place. The EthoVision software recorded the distance travelled by the fish and the time spent in the central area of the open field (1 body length far from the edges).
Statistical analysis
We conducted the same analyses on the data of the 2 species. We used R version 3.4.0 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org). Descriptive statistics in the text are mean ± SD.
The analysis was divided in 3 steps. First, we analyzed the data of the inhibition task. The number of attacks followed the event count distribution and was thus analyzed with models adapted to handle these data (Poisson error structure). Data of guppies presented one outlier consisting of a subject that performed 6 times more attacks compared with the average of the other fish. Because diagnostic plots and Cook’s distances showed that this outlier substantially reduced models’ fit, we dropped it from the dataset. We analyzed the raw data (number of attacks per each minute of each trial) using generalized linear mixed-effects models (GLMMs) fitted with trial and minute of the trial as fixed effects, and fish ID as random effect. Then, we estimated repeatability across 2 trials of the inhibition task, which allowed to measure individual level consistency and thus individual differences. We used the function “rptPoisson” of the “rprR” R package (Stoffel et al. 2017). In these models, we fit the sum of the number of attacks in each trial as depended variable, trial as fixed effect and fish ID as random effect, and we estimated 95% confidence interval (CI) with 10,000 bootstraps. Because fish showed to reduce the number of attacks across minutes, especially in Trial 1, we also calculated the regression coefficient of this behavioral pattern for each individual in Trial 1. We used generalized linear models (GLMs) fitted with each individual’s number of attacks as dependent variable and the minute of the test as fixed effect. Then, we used GLMs to test whether individuals’ coefficient covaried with their overall number of attacks.
The second step of our analysis regarded data of the personality test. The variables collected for the personality test, distance travelled, and time spent in the center of the open field, were normally distributed (1-sample Kolmogorov–Smirnov test). We tested repeatability as described above, but we used the “rpt” function, which is suitable for Gaussian data. As the repeatability analysis for 1 variable of zebrafish seemed to be affected by reduced variability between subjects, we rerun the analysis with square-root transformed data, which increased data variance. We also correlated the 2 variables of the personality test (sum of the 2 trials). As this latter analysis suggested that the 2 variables measured different personality traits, we analyzed them separately.
In the last step of our analysis, we looked for a relationship between the number of attacks in the tube task (sum of the 2 trials) and the 2 variables collected in the personality test (sum of the distance travelled and sum of the time spent in the center). Here, we used GLMs with Poisson error distribution fitted with the personality trait of interest as fixed effect.
Ethical note
Experiments were conducted in accordance with the law of the country in which they were performed (Italy, D.L. 4 Marzo 2014, n. 26). The Ethical Committee of University of Ferrara approved the experimental procedures (Pr. TLX n. 2/2018).
Results
Zebrafish
Inhibition task
In the inhibition task, zebrafish attempted to attack the prey inside the tube 19.18 ± 8.54 times overall. The number of attacks significantly decreased from Trial 1 (14.75 ± 7.00) to Trial 2 (4.44 ± 2.78; GLMM: χ21 = 71.716, P < 0.001). The number of attacks performed by zebrafish in the inhibition task was consistent at individual level across the 2 trials (R = 0.509, CI = [0, 0.748], P = 0.037; Figure 1A). The number of attacks also decreased across minutes within trial (GLMM: χ21 = 230.137, P < 0.001), with this decreasing being more marked for Trial 1 compared with Trial 2 (GLMM interaction: χ21 = 12.285, P < 0.001). The slope of the decrease in the number of attacks calculated for each individual was positively correlated with its overall number of attacks (GLM: χ21 = 34.870, P = 0.027): individuals that decreased faster the number of attacks showed an overall smaller number of attacks.
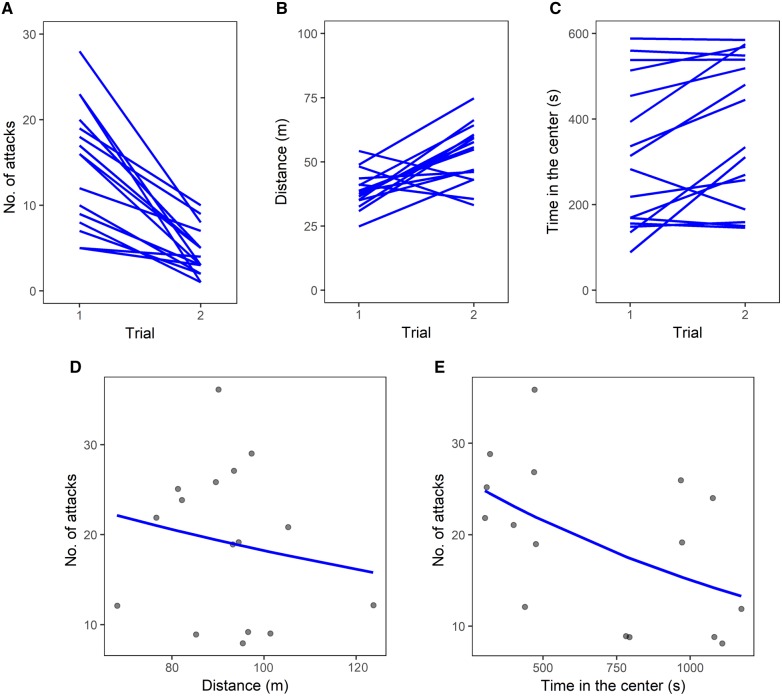
Figure 1.
Experiment on zebrafish. (A) Individual performance in the 2 trials of the inhibition task as number of attacks toward the prey. Individual performance in the 2 trials of the personality test: (B) distance moved and (C) time spent in the center of the open field. (D) Scatterplot of the number of attacks in the inhibition task and the distance moved in the personality test and (E) scatterplot of the number of attacks in the inhibition task and the time spent in the center of the open field in the personality test; lines represent covariance predicted from general linear models.
Personality test
In the personality test, zebrafish swam for 92.16 ± 12.27 m overall, 38.96 ± 7.05 m in Trial 1 and 53.20 ± 11.16 m in Trial 2. The distance moved by zebrafish in the personality test was not consistent at individual level across the 2 trials (R < 0.001, CI = [<0.001, <0.001], P = 1). This latter analysis could be affected by the low variability observed between individuals (Figure 1B). When we repeated the analysis using square-root transformed data, we found a larger CI (R < 0.001, CI = [<0.001, 0.518], P = 0.5), suggesting that we could not estimate repeatability due to low variability in subjects’ behavior. Zebrafish spent 718.18 ± 329.84 s overall (60% testing time) in the center of the open field, 329.52 ± 174.12 s in Trial 1 and 388.67 ± 167.89 s in Trial 2 (55 and 65% trial time, respectively). The time spent in the center was consistent at individual level across the 2 trials (R = 0.854, CI = [0.660, 0.949], P < 0.0001; Figure 1C). There was no significant correlation between the overall distance moved and the time spent in the center of the open field (r14 = −0.268, CI = [−0.674, 0.263]; P = 0.316), suggesting that the variables measured different personality traits.
Covariation between inhibition task and personality test
The distance moved in the personality test did not covary with the number of attacks performed in the inhibition task (GLM: χ21 = 1.668, P = 0.197; Figure 1D). Conversely, the time spent in the center of the open field in the personality test was negatively related with the number of attacks performed in the inhibition task (GLM: χ21 = 15.180, P < 0.001; Figure 1E).
Guppies
Inhibition task
In the inhibition task, guppies attempted to attack the prey inside the tube 66.33 ± 54.00 times overall. The number of attacks significantly decreased from Trial 1 (39.80 ± 43.95) to Trial 2 (26.53 ± 40.08; GLMM: χ21 = 34.668, P < 0.001). The number of attacks performed by guppies in the inhibition task was not consistent at individual level across the 2 trials (R = 0.097, CI = [0, 0.607], P = 0.359; Figure 2A). The number of attacks also decreased across minutes within trial (GLMM: χ21 = 212.130, P < 0.001), with this decreasing being more marked for Trial 1 compared with Trial 2 (GLMM interaction: χ21 = 61.811, P < 0.001). The slope of the decrease in the number of attacks calculated for each individual was positively correlated with its overall number of attacks (GLM: χ21 = 387.81, P < 0.001): individuals that decreased faster the number of attacks showed an overall smaller number of attacks.
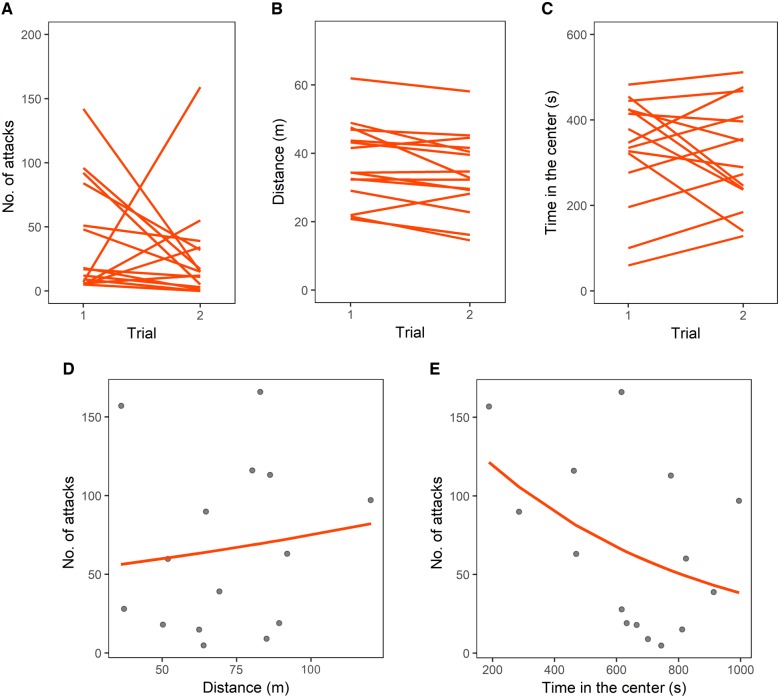
Figure 2.
Experiment on guppies. (A) Individual performance in the 2 trials of the inhibition task as number of attacks toward the prey. Individual performance in the 2 trials of the personality test: (B) distance moved and (C) time spent in the center of the open field. (D) Scatterplot of the number of attacks in the inhibition task and the distance moved in the personality test and (E) scatterplot of the number of attacks in the inhibition task and the time spent in the center of the open field in the personality test; lines represent covariance predicted from general linear models.
Personality test
In the personality test, guppies swam for 71.40 ± 22.68 m overall, 37.40 ± 11.72 m in Trial 1 and 33.99 ± 11.50 m in Trial 2. Guppies spent 646.53 ± 221.71 s overall (54% testing time) in the center of the open field, 332.68 ± 127.29 s in Trial 1 and 313.85 ± 121.99 s in Trial 2 (55% and 52% trial time, respectively). Both the distance moved and the time spent in the center of the open field were consistent at individual level across the 2 trials (distance moved: R = 0.909, CI = [0.761, 0.970], P < 0.001; time in the center of the open field: R = 0.581, CI = [0.162, 0.848], P = 0.006; Figure 2B, C). There was no significant correlation between the overall distance moved and the time spent in the center of the open field (r13 = 0.377, CI = [−0.168, 0.745], P = 0.166), suggesting that the variables measured different personality traits.
Covariation between inhibition task and personality test
The distance moved in the personality test positively covaried with the number of attacks performed in the inhibition task (GLM: χ21 = 9.766, P = 0.002; Figure 2D). As observed in zebrafish, the time spent in the center of the open field in the personality test was negatively related with the number of attacks performed in the inhibition task (GLM: χ21 = 102.120, P < 0.001; Figure 2E).
Discussion
Across several animal taxa, evidence suggests that individuals show different cognitive performance. In some cases, this variability regards tasks in which individuals have to inhibit behavior and in 2 mammalian species, is related to individuals’ personality (Avila and Parcet 2001; Ferland et al. 2014). This study reveals that individuals’ ability to inhibit foraging behavior in 2 teleost fish species is related to their personality.
In zebrafish, we found that 1 of the 2 traits measured in the personality test covaried with the performance in the inhibition task. Zebrafish that spent more time in the center of the open field in Trial 1 also did so in Trial 2, as expected if this variable measured a personality trait. Moreover, zebrafish that spent more time in the center of the open field less frequently attacked the brine shrimps sealed in the tube in the inhibition task. Hence, our data suggest that, in zebrafish, bolder and more explorative individuals have higher inhibitory abilities. The activity in the open field, measured as distanced moved, did not significantly covary with the inhibitory ability of the zebrafish. We are unsure as to whether this variable was unrelated to inhibition in zebrafish. It is also possible that our personality test failed to capture substantial individual differences in activity, as suggested by the lack of significant repeatability.
In guppies, both traits measured in the personality test (activity, i.e., distance moved and time in the center of the open field) were related to the performance on the inhibition task. The 2 personality variables significantly differed among individuals, and they were not related between each other. Therefore, these variables measured individual differences in 2 personality traits of guppies. Similarly to what observed in zebrafish, guppies that spent more time in the center of the open field showed a lower number of attacks in the inhibition task, suggesting that bolder and more explorative guppies display greater inhibitory abilities. Regarding the activity in the open field, more active individuals performed a greater number of attacks in the inhibition task. In guppies, it has been reported that shyer individuals are more active in the open field, perhaps, because they are swimming panicky in the attempt to leave the unfamiliar environment (Kotrschal et al. 2014b). Therefore, this result aligns with that on time spent in the center of the open field and suggests that bolder guppies display higher inhibitory abilities.
Results in zebrafish and guppies concordantly suggest that more active and bolder individuals are more efficient in inhibiting behavior, at least in the context of foraging. Personality may be affected by individuals’ developmental experience (Brown et al. 2007; Bell and Sih 2007) and similar plasticity has been often reported for cognitive abilities (Relyea 2003; Kotrschal et al. 2012; Chivers et al. 2016), although it has not been investigated for inhibition in animals. Because we used subjects reared in the laboratory under controlled conditions, we can exclude the possibility that developmental plasticity due to different individual experiences caused the variability observed in our study. It is also arguable that laboratory rearing and domestication can affect fish personality and, in turn, the results of this study (Sundström et al. 2004; Christensen et al. 2014). However, these factors have been reported to affect the average boldness of a population. Consistent evidence across different species suggests that variability in personality is also present in domestic and laboratory reared strains (D. rerio: Ariyomo et al. 2013; P. reticulata: O’Neill et al. 2018; Salmonids: Huntingford and Adams 2005; Salmo trutta: Sundström et al. 2004; Solea solea: Mas-Muñoz et al. 2011). In agreement with these records, we found substantial repeatability for most of the personality traits. Accordingly, even if laboratory breeding and domestication may have affected average personality in our fish populations, this is unlikely to have affected the measure of individual variability and its relationship with inhibitory abilities.
This study aligns with the growing literature that suggests a relationship between personality and cognition in teleost fish (e.g., Dugatkin and Alfieri 2003; Brown et al. 2013; Trompf and Brown 2014; White et al. 2017). Yet, our study is one of the few evidencing covariance between personality and the cognitive abilities involved in inhibiting behavior outside humans. One of the main values of the relationship between personality and cognitive abilities is that it supports an indirect hypothesis for the evolution and maintenance of individual differences in cognition. Many studies found that personality is maintained by several selective pressures (Dingemanse et al. 2004; Dingemanse and Réale 2005), and this might in turn, indirectly maintain individual differences in inhibitory ability. It is worth noting that this study does not exclude the reversed causal relationship. Selection on individual differences in cognition may maintain personality. However, the evolutionary causes of variability in cognition are still puzzling, supporting the idea that personality causes variability in cognition rather than the opposite.
The reasons for the direction of the relationship that we identified remain to be understood. Theory predicts that individuals with more active and bolder personalities encounter new resources at a faster rate than individuals with shyer and less active personalities (Sih and Del Giudice 2012). In the context of foraging, which is related to our inhibition task, a convenient strategy for bolder and more active individuals may be rapidly switching away from a food source when it is not available or accessible, because these individuals have high chances to encounter new food sources. Conversely, shyer and less active individuals may be more persistent in trying to obtain the food, because they are less likely to encounter other resources soon. This may explain the pattern of results that we observed and can be tested by developing and exploiting tests that measure inhibition outside of the foraging context.
A novelty of our study is that we tested 2 species with the same tasks, whereas previous empirical studies on the link between personality and cognition usually investigated a single species. Therefore, it is possible that the result of prior studies were due to the specific combination of the ecological characteristics of the species and the tasks adopted. With this in mind, it is interesting that the relationship between cognition and personality is present and has the same direction in zebrafish and guppies. These 2 teleost species have some ecological similarities, such as inhabiting warm-water streams (Magurran 2005; Engeszer et al. 2007), that may explain the similarity in the covariation between personality and inhibition. However, the 2 species also exhibit substantial ecological differences. For instance, guppies have a reproductive system characterized by internal fertilization and ovoviviparity, and the offspring are fully independent from the first day of life; conversely, zebrafish produce eggs via spawning, and the offspring hatch after few days, as embryos that do not feed and almost do not move. Moreover, guppies and zebrafish belong to 2 different orders, Cypriniformes and Cyprinodontiformes, respectively, and are separated by ∼200 million years of evolution (Steinke et al. 2006). Therefore, the most reasonable interpretation of our results is that the link between personality and cognition may be similar within teleost fish, in spite of ecological diversity. Future studies should try to confirm our data, by considering different fish species and different populations within species, and it will be important to understand whether the link between cognition and personality is also similar across species in other vertebrate groups.
Acknowledgments
The authors thank Andrea Margutti for technical assistance. They have no competing interests.
Funding
Funding was provided by FAR2018 and FIR2018 grant from University of Ferrara to T.L.-X.
References
- Aiello LC, Wheeler P, 1995. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 36:199–221. [Google Scholar]
- Ariyomo TO, Carter M, Watt PJ, 2013. Heritability of boldness and aggressiveness in the zebrafish. Behav Gen 43:161–167. [DOI] [PubMed] [Google Scholar]
- Avila C, Parcet MA, 2001. Personality and inhibitory deficits in the stop-signal task: the mediating role of Gray’s anxiety and impulsivity. Pers Indiv Differ 31:975–986. [Google Scholar]
- Bell A, 2013. Randomized or fixed order for studies of behavioral syndromes? Behav Ecol 24:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM, Sih A, 2007. Exposure to predation generates personality in threespined sticklebacks Gasterosteus aculeatus. Ecol Lett 10:828–834. [DOI] [PubMed] [Google Scholar]
- Beran MJ, 2015. The comparative science of “self–control”: what are we talking about? Front Psychol 6:51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Hopkins WD, 2018. Self-control in chimpanzees relates to general intelligence. Curr Biol 28:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S, 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim Behav 81:1209–1216. [Google Scholar]
- Brown C, Burgess F, Braithwaite VA, 2007. Heritable and experiential effects on boldness in a tropical poeciliid. Behav Ecol Sociobiol 62:237–243. [Google Scholar]
- Brown GE, Ferrari MCO, Malka PH, Fregeau L, Kayello L. et al. 2013. Retention of acquired predator recognition among shy versus bold juvenile rainbow trout. Behav Ecol Sociobiol 67:43–51. [Google Scholar]
- Buechel SD, Boussard A, Kotrschal A, van der Bijl W, Kolm N, 2018. Brain size affects performance in a reversal-learning test. Proc Biol Sci 285:20172031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K, 2007. Individual differences in children’s memory and reading comprehension: an investigation of semantic and inhibitory deficits. Memory 14:553–569. [DOI] [PubMed] [Google Scholar]
- Carere C, Locurto C, 2011. Interaction between animal personality and animal cognition. Curr Zool 57:491–498. [Google Scholar]
- Carlson SM, Moses LJ, 2001. Individual differences in inhibitory control and children’s theory of mind. Child Dev 72:1032–1053. [DOI] [PubMed] [Google Scholar]
- Cauchard L, Boogert NJ, Lefebvre L, Dubois F, Doligez B, 2013. Problem-solving performance is correlated with reproductive success in a wild bird population. Anim Behav 85:19–26. [Google Scholar]
- Chivers DP, Mitchell MD, Lucon-Xiccato T, Brown GE, Ferrari MC, 2016. Background risk influences learning but not generalization of predators. Anim Behav 121:185–189. [Google Scholar]
- Christensen KA, Brunelli JP, Wheeler PA, Thorgaard GH, 2014. Antipredator behavior QTL: differences in rainbow trout clonal lines derived from wild and hatchery populations. Behav Gen 44:535–546. [DOI] [PubMed] [Google Scholar]
- Cole EF, Quinn JL, 2012. Personality and problem-solving performance explain competitive ability in the wild. Proc Biol Sci 279:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway AR, 1996. Individual differences in working memory capacity: more evidence for a general capacity theory. Memory 4:577–590. [DOI] [PubMed] [Google Scholar]
- Dadda M, Domenichini A, Piffer L, Argenton F, Bisazza A, 2010. Early differences in epithalamic left-right asymmetry influence lateralization and personality of adult zebrafish. Behav Brain Res 206:208–215. [DOI] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive functions. Ann Rev Psychol 64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Tinbergen JM, 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc Biol Sci 271:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Réale D, 2005. Natural selection and animal personality. Behaviour 142:1159–1184. [Google Scholar]
- Dougherty LR, Guillette LM, 2018. Linking personality and cognition: a meta-analysis. Philos Biol Sci 373:20170282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin LA, Alfieri MS, 2003. Boldness, behavioral inhibition and learning. Ethol Ecol Evol 15:43–49. [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM, 2007. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4:21–40. [DOI] [PubMed] [Google Scholar]
- Ferland JMN, Zeeb FD, Yu K, Kaur S, Taves MD. et al. , 2014. Greater sensitivity to novelty in rats is associated with increased motor impulsivity following repeated exposure to a stimulating environment: implications for the etiology of impulse control deficits. Eur J Neurosci 40:3746–3756. [DOI] [PubMed] [Google Scholar]
- Gilmore C, Attridge N, Clayton S, Cragg L, Johnson S. et al. , 2013. Individual differences in inhibitory control, not non-verbal number acuity, correlate with mathematics achievement. PLoS ONE 8:e67374.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AS, Guillette LM, Healy SD, 2015. Cognition and personality: an analysis of an emerging field. Trend Ecol Evol 30:207–214. [DOI] [PubMed] [Google Scholar]
- Guenther A, Brust V, Dersen M, Trillmich F, 2014. Learning and personality types are related in cavies Cavia aperea. J Comp Psychol 128:74–81. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Hahn AH, Hoeschele M, Przyslupski AM, Sturdy CB, 2015. Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim Cogn 18:165–178. [DOI] [PubMed] [Google Scholar]
- Gustafsson JE, Undheim JO, 1996. Individual differences in cognitive functions In: Berliner DC, Calfee RC, editors. Handbook of Educational Psychology. New York: Macmillan; 186–242. [Google Scholar]
- Halberda J, Mazzocco MM, Feigenson L, 2008. Individual differences in non-verbal number acuity correlate with maths achievement. Nature 455:665–668. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Waller D, 2005. Individual differences in spatial abilities. In: Shah P, Miyake A, editors. The Cambridge Handbook of Visuospatial Thinking. Cambridge: Cambridge University Press. 121–169.
- Huntingford F, Adams C, 2005. Behavioural syndromes in farmed fish: implications for production and welfare. Behaviour 142:1213–1228. [Google Scholar]
- Irving E, Brown C, 2013. Examining the link between personality and laterality in a feral guppy Poecilia reticulata population. J Fish Biol 83:311–325. [DOI] [PubMed] [Google Scholar]
- Kabadayi C, Bobrowicz K, Osvath M, 2018. The detour paradigm in animal cognition. Anim Cogn 21:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrschal A, Corral-Lopez A, Amcoff M, Kolm N, 2014a. A larger brain confers a benefit in a spatial mate search learning task in male guppies. Behav Ecol 26:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrschal A, Lievens EJ, Dahlbom J, Bundsen A, Semenova S. et al. , 2014b. Artificial selection on relative brain size reveals a positive genetic correlation between brain size and proactive personality in the guppy. Evolution 68:1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrschal A, Rogell B, Maklakov AA, Kolm N, 2012. Sex-specific plasticity in brain morphology depends on social environment of the guppy Poecilia reticulata. Behav Ecol Sociobiol 66:1485–1492. [Google Scholar]
- Lucon-Xiccato T, Bertolucci C, 2019. Guppies show rapid and lasting inhibition of foraging behaviour. Behav Process in Press doi: 10.1016/j.beproc.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2014. Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biol Lett 10:20140206. [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2017. Individual differences in cognition among teleost fishes. Behav Process 141:184–195. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Dadda M, 2017. Personality and cognition: sociability negatively predicts shoal size discrimination performance in guppies. Front Psychol 8:1118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Gatto E, Bisazza A, 2017. Fish perform like mammals and birds in inhibitory motor control tasks. Sci Rep 7:13144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Hare B, Nunn CL, Addessi E, Amici F. et al. , 2014. The evolution of self-control. Proc Natl Acad Sci USA 111: E2140–E2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNulty DR, Mech LD, Smith DW, 2007. A proposed ethogram of large–carnivore predatory behavior, exemplified by the wolf. J Mamm 88:595–605. [Google Scholar]
- Magurran AE, 2005. Evolutionary Ecology: The Trinidadian Guppy. Oxford: Oxford University Press. [Google Scholar]
- Mas-Muñoz J, Komen H, Schneider O, Visch SW, Schrama JW, 2011. Feeding behaviour, swimming activity and boldness explain variation in feed intake and growth of sole Solea solea reared in captivity. PLoS ONE 6:e21393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C, Pant SR, van Horik JO, Laker PR, Langley EJ. et al. , 2017. A novel continuous inhibitory-control task: variation in individual performance by young pheasants Phasianus colchicus. Anim Cogn 20:1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier J, Pivneva I, Titone D, 2014. Individual differences in inhibitory control relate to bilingual spoken word processing. Biling Lang Cogn 17:89–117. [Google Scholar]
- Messenger JB, 1973. Learning in the cuttlefish Sepia. Anim Behav 21:801–826. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, 2012. The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci 21:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SJ, Williamson JE, Tosetto L, Brown C, 2018. Effects of acclimatisation on behavioural repeatability in two behaviour assays of the guppy Poecilia reticulata. Behav Ecol Sociobiol 72:166. [Google Scholar]
- Passolunghi MC, Siegel LS, 2001. Short-term memory, working memory, and inhibitory control in children with difficulties in arithmetic problem solving. J Exp Child Psychol 80:44–57. [DOI] [PubMed] [Google Scholar]
- Polverino G, Ruberto T, Staaks G, Mehner T, 2016. Tank size alters mean behaviours and individual rank orders in personality traits of fish depending on their life stage. Anim Behav 115:127–135. [Google Scholar]
- Relyea RA, 2003. Predators come and predators go: the reversibility of predator-induced traits. Ecology 84:1840–1848. [Google Scholar]
- Rowe L, Houle D, 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc Biol Sci 263:1415–1421. [Google Scholar]
- Ryer CH, Olla BL, 1991. Information transfer and the facilitation and inhibition of feeding in a schooling fish. Environ Biol Fish 30:317–323. [Google Scholar]
- Santacà M, Busatta M, Lucon-Xiccato T, Bisazza A, 2019a. Sensory differences mediate species variation in detour task performance. Anim Behav 155:153–162. [Google Scholar]
- Santacà M, Busatta M, Savaşçı BB, Lucon-Xiccato T, Bisazza A, 2019b. The effect of experience and olfactory cue in an inhibitory control task in guppies. Poecilia reticulata. Anim Behav 151:1–7. [Google Scholar]
- Sih A, Del Giudice M, 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc Lond B Biol Sci 367:2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DP, Simon HA, 1978. Individual differences in solving physics problems In Siegler RS, editor. Children’s Thinking: What Develops? Hillsdale (NJ: ): Lawrence Erlbaum Associates, Inc; 325–348. [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR. et al. , 2008. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci 19:904–911. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ, 2010. Cognition, Evolution, and Behavior. Oxford: Oxford University Press. [Google Scholar]
- Stearns SC, 1989. Trade-offs in life-history evolution. Funct Ecol 3:259–268. [Google Scholar]
- Steinke D, Salzburger W, Meyer A, 2006. Novel relationships among ten fish model species revealed based on a phylogenomic analysis using ESTs. J Mol Evol 62:772–784. [DOI] [PubMed] [Google Scholar]
- Stoffel MA, Nakagawa S, Schielzeth H, 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Method Ecol Evol 8:1639–1644. [Google Scholar]
- Stow MK, Vernouillet A, Kelly DM, 2018. Neophobia does not account for motoric self-regulation performance as measured during the detour-reaching cylinder task. Anim Cogn 21:565–574. [DOI] [PubMed] [Google Scholar]
- Sundström LF, Petersson E, Höjesjö J, Johnsson JI, Järvi T, 2004. Hatchery selection promotes boldness in newly hatched brown trout Salmo trutta: implications for dominance. Behav Ecol 15:192–198. [Google Scholar]
- Thornton A, Lukas D, 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Philos Trans Royal Soc Lond B Biol Sci 367:2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Gerlai R, 2013. Individual differences in activity levels in zebrafish Danio rerio. Behav Brain Res 257:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompf L, Brown C, 2014. Personality affects learning and trade-offs between private and social information in guppies Poecilia reticulata. Anim Behav 88:99–106. [Google Scholar]
- van Horik JO, Langley EJ, Whiteside MA, Laker PR, Beardsworth CE. et al. , 2018. Do detour tasks provide accurate assays of inhibitory control? Proc Royal Soc Lond B Biol Sci 285:20180150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA, 1976. The open-field test: a critical review. Psychol Bull 83:482.. [PubMed] [Google Scholar]
- Warren EW, Callaghan S, 1975. Individual differences in response to an open field test by the guppy Poecilia reticulata (Peters). J Fish Biol 7:105–113. [Google Scholar]
- White SL, Wagner T, Gowan C, Braithwaite VA, 2017. Can personality predict individual differences in brook trout spatial learning ability? Behav Process 141:220–228. [DOI] [PubMed] [Google Scholar]