Abstract
Background and aim: Alternative medicine is a broad term used to encompass different therapies, including chiropractic. Chiropractic was called “a science of healing without drugs” by its founder, David Daniel Palmer. It is based on the idea that the body has a powerful self-healing ability and that there is a relationship between body structure and function that affects health. In particular, chiropractic assumes that the nervous system controls the human body through nerves branching from the vertebral column and spinal cord. Researchers do not fully understand how chiropractic therapies affect pain, but chiropractic is widely used today to treat chronic pain, such as back pain. Different studies with animal models have demonstrated that chiropractic therapies mediate neuroplasticity, specifically through modulation of neurotrophins. No studies have yet been published on interaction between neurotrophin gene polymorphisms and chiropractic treatment. Methods: We searched PubMed with the following keywords: chiropractic, neuroplasticity, neurotrophin gene polymorphism for a panorama of on the molecular mechanisms of chiropractic therapy. Results: From the material collected, we identified a set of genes and some functional polymorphisms that could be correlated with better response to chiropractic therapy. Conclusions: Further association studies will be necessary to confirm hypotheses of a correlation between single nucleotide polymorphisms in specific genes and better response to chiropractic therapy. (www.actabiomedica.it)
Keywords: chiropractic, chiropractic therapy, neuroplasticity, neurotrophins, polymorphism
Chiropractic – a technique based on manipulation of the spine and peripheral nerve endings
Chiropractic is a form of complementary and alternative medicine that focuses on the relationship between major body structures, such as skeleton, muscles and nerves, and patient health. Basic concepts of chiropractic are: i) the body has powerful self-healing ability and ii) body function and structure, especially the spinal cord, are closely related and this relationship may affect health. These two concepts lead to the idea that chiropractic therapy can normalize the relationship between body structure and function and can help the body to heal. The founder of chiropractic, David Daniel Palmer, called it “a science of healing without drugs” (1). Chiropractic is based on the assumption that the central nervous system controls the human body through peripheral nerves branching from the vertebral column and the spinal cord (2). In the chiropractic view, spinal cord dysfunctions can interfere with the body’s innate capacity to heal. Chiropractic emphasizes spinal manipulation. The branches of chiropractic that seem most interesting from a biological point of view are those concerning spinal manipulation and acupressure/acupuncture of peripheral sensory nerve endings (3).
Recent insights have identified inflammation as a local protective response to microbial invasion or injury. It must be well regulated, because deficiencies or excesses of inflammatory response can cause morbidity and mortality. The discovery that cholinergic neurons can inhibit inflammation has changed the way we understand the link between the nervous system and immune responses. We now know that a basic neural pathway monitors and adjusts the inflammatory response and that an inflammatory input activates a fast subconscious anti-inflammatory response. In his 2002 review, Tracey discussed evidence indicating that stimulation of the “vagus nerve” can prevent inflammation (4). Recent studies of the mechanisms that regulate inflammation have identified a neural mechanism, called the “cholinergic anti-inflammatory pathway”, that inhibits macrophage activation through parasympathetic outflow. The pathway’s name refers to the role of acetylcholine as the principle parasympathetic neurotransmitter, because macrophages exposed to acetylcholine are effectively deactivated (3).
Biological foundations of chiropractic therapy
Massage is a general term for many different techniques involving the application of bodily contact and physical pressure with therapeutic intent. The effects of massage therapy depend on the amount of pressure and the speed of the stroke. For example, slow strokes can evoke systemic relaxation whereas deep strokes increased blood flow to the area. Attempts to define and classify the extensive range of types of massage have sometimes created confusion, but as far as chiropractic is concerned, spinal manipulative therapy (SMT) is the best practice.
One of the effects of spinal manipulation during chiropractic therapy is to stretch spinal muscles. Muscle stretch is a powerful stimulus for up-regulation of a splice product (mechano-growth factor MGF) of the insulin-like growth factor (IGF-1) gene by the stretched muscle. MGF promotes muscle growth and repair (myotrophism) and the growth and repair of neurons (neurotrophism) (5).
Non-noxious mechanical skin stimulation releases nerve growth factor (NGF) in rats. Produced and secreted by brain cortex neurons, NGF is a neurotrophic factor that promotes neuron survival and function (6).
Mechanical vibration massage in rats promotes secretion of NGF by the sub maxillary gland. This secretion accelerates repair of brachial plexus injuries and slows down atrophy of skeletal muscle. Spinal manipulation is a specific hands-on approach commonly used in chiropractic. Thoracic spinal manipulation can lead to different responses involving the sympathetic nervous system, the endocrine system and the hypothalamic-pituitary axis (7).
Spinal manipulative therapy has been demonstrated to be an effective treatment for acute and chronic back pain (8, 9) although the neuronal mechanisms responsible for the pain-reducing effects are not yet understood (10). The idea of a link between SMT and spinal cord neuroplasticity is gaining interest among researchers, and different studies have demonstrated a connection (11, 12). Interestingly, Guzzetta and colleagues demonstrated that increased body massage and multisensory stimulation affects brain development in humans and rat pups. In particular, visual system maturation seems to be increased by “massage therapy” through modulation of levels of endogenous factors such as IGF-1 in infants. In rat pups, massage also proved effective in increasing IGF-1 levels in the cortex. This suggests that IGF-1 could also be a mediator of the effects of massage therapy on visual development in infants (13). The effects of massage in accelerating visual development in rat pups are not due to the simple act of removing pups from the nest, because they are absent in pups separated from the mother for the same amount of time but not massaged. This supports the idea that massage could be a promoter of brain development and the hypothesis that the level of multisensory stimulation provided by licking/grooming is an important regulator of brain development (14-18).
Chiropractic uses acupressure/acupuncture as well as spinal manipulative therapy to decrease pain and for a wide range of other complaints. Acupuncture is a technique of traditional Chinese medicine (19). Acupuncturists insert hair-thin needles in specific points of the body to balance body energy, stimulate healing and promote relaxation. Researchers do not understand how acupuncture can decrease pain, although the technique is widely used in treatment of neurological disorders such as ischemic stroke, cognitive impairment (20), Parkinson’s disease (PD) (21) and prophylaxis of migraine (22).
Interestingly, insertion of a needle in the human body produced a slow increase in the skin pain threshold, peaking in 30 min, followed by exponential decay after removal of the needle (23). This suggests that chemical mediators are involved, a hypothesis validated by observation of transfer of the analgesic effect on cross-infusion of cerebroventricular fluid from a donor rabbit undergoing acupuncture stimulation to a naive recipient rabbit.
There are currently two theories about how massage and acupuncture could work: i) by stimulating release of endorphins, natural pain-relieving chemicals of the body (23) and/or ii) by influencing the nervous system and the release of chemicals that regulate blood flow and pressure, reduce inflammation and calm the brain (24). It was recently shown that peripheral sensory stimulation by electro-acupuncture could improve the availability and utilization of brain nerve growth factor (NGF). The clinical efficacy of acupuncture on pain and inflammation are based on the stimulation of several classes of sensory afferent fibers. The resulting activation of physiological processes seems to be similar to those resulting from physical exercise or deep massage. Acupuncture stimulation may induce variations in neural activity throughout the nervous system, affecting the synthesis and release of different neuromodulators. A relationship between acupuncture and NGF was recently investigated with a view to synergic clinical use. Administration of NGF with acupuncture and/or SMT in neurological, endocrine and immune diseases could be an interesting therapeutic approach (25).
Neuroplasticity and neuro modulator reflex as effector of chiropractic therapy
Various authors have demonstrated that acupuncture and massage mediate neuroplasticity in animal models (26). Neuroplasticity is defined as the ability of the nervous system to reorganize its structure and function in response to intrinsic and/or environmental demands (27). Neuroplasticity may be involved in physiological or pathological conditions: in physiological conditions, it is mainly related to brain development, learning and memory. The most famous form of physiological neuroplasticity is the process known as “adult hippocampal neurogenesis”, whereby the adult hippocampus generates functional neurons throughout life (28). In pathological conditions, neuroplasticity seems to be involved in brain injury healing processes.
Neuroplasticity has been identified in the spinal cord and central nervous system, together with its prolonged forms, known as long-term potentiation (LTP) and long-term depression (LTD). In particular, observations of LTP in neurons of the spinal cord and similarities between LTP cell mechanisms and those associated with central sensitization suggest that LTP may play a significant role in establishing major pain conditions. It has also been identified as an amplifying mechanism within the pain system, suggesting its involvement both in acute and chronic pain states (10). If LTP is an important mechanism in pain system regulation, any mechanism that interferes with LTP is of clinical interest. Long-term depression (LTD), found to depend on glutamate release, activation of its NMDARs, and intracellular Ca2+, is suggested to have a relationship with LTP. LTD was initially discovered in the hippocampus and was later identified in other body regions, such as spinal neurons, cerebellum, cortex, basal ganglia and amygdala. The identification of spinal LTD and the consequent demonstration of LTP reversal suggests that LTD is a cell mechanism able to mediate the effects of certain therapies (10).
So how can massage and acupuncture operate in the treatment of diseases and pain? Different studies attribute the underlying mechanisms of mediation of neuroplasticity by acupuncture and SMT to modulation of neurotrophins (NTs) (26). It is well known that NTs are responsible for the growth of neurons during development and for the maintenance of adult neurons. More recently, however, there has been increasing evidence that NTs are also involved in neuron survival and differentiation and even in axonal regeneration in neurological disorders (29, 30).
Genetic determinants of chiropractic response
The expression of NTs, for example by the brain-derived neurotrophic factor (BDNF) gene, is induced after cerebral ischemia. Expression peaks within minutes or hours of the event, then quickly returning to normal or even below normal levels. BDNF belongs to a family of neurotrophins that include neurotrophin-3 (NT3), neurotrophin-4 (NT4) and nerve growth factor (NGF) (31). Here we list a series of known polymorphisms, most having a demonstrated functional effect, which could be considered in genetic analysis of predisposition to a positive response to chiropractic care (Table 1). The selected polymorphisms belong to genes involved in neurotrophism, myotrophism and pain sensitivity. Polymorphisms with a weak functional effect, as evaluated by in vitro or association studies, were disregarded.
Table 1.
Polymorphisms identified from the literature as potential modifiers of neurotrophin-mediated neuroplastic activity
| Gene | Protein | Class | RefSeq | Nucleotide change | Amino acid change | MAF (GnomAD) | Ref |
| NGF | Beta-nerve growth factor | NTs | rs6330 | c.104C>T | Ala35Val | A=0.37015 (91127/246188) | 39 |
| BDNF | Brain-derived neurotrophic factor | NTs | rs6265 | c.196G>A | Val66Met | T=0.19437 (47821/246030) | 40 |
| NGFR | Beta-nerve growth factor receptor | NTs | rs2072446 | c.614C>T | Ser205Leu | T=0.05165 (12700/245884) | 39 |
| ADRB2 | Beta-2 adrenergic receptor | NTs | rs1042713 | c.46A>G | Gly16Arg | A=0.42081 (103492/245936) | 45 |
| ADRB2 | Beta-2 adrenergic receptor | NTs | rs1042714 | c.79C>G | Gln27Glu | G=0.31729 (78116/246198) | 45 |
| CNTF | Ciliary neurotrophic factor | NTs | rs1800169 | c.115-6G>A | A=0.14950 (35961/240538) | 46 | |
| MSTN | Growth/differentiation factor 8 | recovery | rs1805086 | c.458A>G | Lys153Arg | C=0.02732 (6704/245366) | 53 |
| ACTN3 | Alpha-actinin-3 | recovery | rs1815739 | c.1729C>T | Arg577* | T=0.46060 (113241/245854) | 55 |
| NTRK1 | High affinity nerve growth factor receptor | pain/NTs | rs6334 | c.1656G>A | Gln552His | A=0.22025 (54107/245664) | 57 |
| SCN9A | Sodium channel protein type 9 subunit alpha | pain | rs6746030 | c.3448C>T | Arg1150Trp | A=0.12050 (18374/152480) | 60 |
| COMT | Catechol O-methyltransferase | pain | rs4680 | c.322G>A | Val158Met | A=0.46252 (112239/242666) | 66 |
Legend: NTs, neurotrophins; RefSeq, polymorhism accession number as recorded in the database of single nucleotide polymorphisms; MAF, minor alle frequency; Ref, reference.
NGF was the first target-derived neurotrophic factor identified. It is fundamental for the development and maintenance of peripheral nervous system neurons and for the functional integrity of central nervous system cholinergic neurons (32). Moreover, NGF can affect balanced interplay between the nervous, endocrine and immune systems. It is well known that NGF concentrations increase during stress and play an important role in the hypothalamic-pituitary-adrenal axis (33).
Growing evidence suggests that different polymorphisms in the NGF gene can be considered risk factors for conditions such as vascular hypertension (34) and processes such as atherogenesis (35) and inflammation (36).
A nucleotide change in NGF, c.104C>T, causes an amino acid change in position 35, p.Ala35Val. This amino acid change is referred to as rs6330 and the more common of the two alleles (C) encodes alanine (Ala). Several studies have indicated that this single nucleotide polymorphism (SNP) can lead to dysfunction in intracellular processing and secretion of NGF. The minor T allele seems to be associated with increased susceptibility to anxiety by association with low vagal activity, while the major C allele seems to be associated with attention deficit hyperactivity disorder (ADHD) (37, 38). More interestingly, rs6330 CC homozygotes show significantly higher NGF median plasma levels of NGF than carriers of the minor T allele (39). These findings suggest an association between certain NGF polymorphisms, rs6330 in particular, and response to chiropractic SMT and acupuncture.
The BDNF gene encodes a protein found in the brain and spinal cord. This protein promotes neuron survival by playing a role in the growth, differentiation, maturation and maintenance of these cells. BDNF protein is active in synapses. Synapses change and adapt in response to external factors. The BDNF protein helps regulate synaptic plasticity, which is important for learning and memory.
Several polymorphisms have been identified in BDNF. The non-synonymous polymorphism, known as SNP rs6265 or p.Val66Met or c.G>A196, is common and causes a valine (Val) to methionine (Met) substitution at position 66 of the pro-BDNF protein. The replacement of Val by Met impairs neuronal activity-dependent secretion of BDNF (40). Approximately 30-50% of the population is heterozygous or homozygous for this rs6265 polymorphism. The SNP occurs in the 5’ pro-domain of BDNF and does not affect mature BDNF protein function or BDNF constitutive release, but rather activity-dependent BDNF release, thus influencing intracellular trafficking of pro-BDNF (41).
Recent studies suggest a role of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and their receptor, nerve growth factor receptor (NGFR), in neuropsychiatric disorders, especially Alzheimer’s disease (42). The rs2072446 polymorphism in NGFR has been associated with risk of Alzheimer’s disease, but also as a functional SNP that could be involved in gene expression and protein secretion (39, 43).
The ADRB2 gene encodes beta2-adrenergic receptor and maps to the 5q32 chromosomal region. The association between polymorphisms in ADRB2 and risk of other diseases has also been studied (44). Moreover, rs1042713 (p.Arg16Gly) and rs1042714 (p.Gln27Glu), non-synonymous polymorphisms in ADRB2, have been associated with cognitive function and brain white matter integrity (45).
Ciliary neurotrophic factor (CNTF) is a pleiotropic cytokine of the interleukin-6 family whose myotrophic and neurotrophic effects have been extensively studied (46). Rs1800169 is the most widely studied CNTF polymorphism: this SNP leads to a G to A transition close to the boundary between the first intron and second exon. As a result of this change, four nucleotides are inserted in CNTF mRNA, leading to a frameshift mutation and a premature stop codon. By virtue of its functional effect, the rs1800169 polymorphism in CNTF could also be considered in relation to response to chiropractic treatment.
Polymorphisms in muscle-related genes can modify muscle healing after injury, so they could also be related to response to chiropractic treatment. Myostatin, encoded by MSTN, is a muscle inhibitor peptide that regulates myoblast differentiation with direct consequences on muscle mass and strength. MSTN knock-out mice show muscle hypertrophy and greater strength than wild-type mice (47). Null MSTN variants are known to express the same phenotype in humans (48) and other mammals (49, 50). Lack of the protein has also been associated with better muscle regeneration after injury (51, 52).
Among common polymorphisms, rs1805086 is receiving growing attention from researchers, and a number of association studies agree that it has functional significance (53, 54).
The ACTN3 gene, encoding the structural skeletal muscle protein α-actinin-3, locates on chromosome 11 and is one of the most interesting genes associated with athletic performance. Previous studies report that the ACTN3 Arg577* variant (rs1815739) seems to be associated with athletic performance in different races but the function of this SNP is unknown (55, 56). Since it is a null mutation and considering its evocative role in muscle quality, the rs1815739 polymorphism is worth studying in relation to chiropractic response.
The NTRK1 gene, encoding the high-affinity NGF receptor TrkA, seems to be involved in gene expression and protein secretion. Szczepankiewicz and colleagues demonstrated that NGF serum levels may be influenced by the rs6334 polymorphism in NTRK1 and that the interaction between this variant and NGF indicates that this pathway may influence NGF protein levels (57). The polymorphism has also been associated with pain perception during acupunture (58). This involvement led us to consider the rs6334 polymorphism in NTRK1 as a variation that could be involved in chiropractic response.
Pain is a disturbing non-motor symptom in Parkinson disease (PD) and susceptibility to pain varies widely among these patients. SCN9A encodes the NaV1.7 sodium channel and is preferentially expressed in pain-signaling dorsal root ganglion (DRG) neurons (nociceptors) that play a critical role in amplifying small depolarizations, thus increasing the pain signaling gain (59).
Greenbaum and colleagues demonstrated that the non-synonymous rs6746030 polymorphism in SCN9A was associated with PD-related pain susceptibility and with PD central and musculoskeletal pain. Since the non-synonymous rs6746030 polymorphism has also proved experimentally to influence the excitability of nociceptive DRG neurons, thus influencing pain sensitivity and susceptibility to chronic pain (60). We focus on this SNP in relation to chiropractic response (61).
The COMT gene encodes catechol-O-methyltransferase, the enzyme mainly responsible for catecholamine metabolism and known as a major modulator of synaptic dopamine concentrations in the brain. COMT is involved in dopamine degradation at biochemical level and in complex cognitive functions such as cognitive control and working memory (61), and interestingly also pain modulation (62). Indeed, COMT is a key regulator in the pain perception pathway and polymorphisms in the gene have been studied in relation to variability in pain perception between individuals (63, 64).
Among the different polymorphisms identified in COMT, the rs4680 SNP seems to be involved in the increased synaptic dopamine concentrations in Met-allele carriers (65). The experimentally detected functional effect of rs4680 revealed a 40% decrease in COMT activity associated with the Met108 variant as a consequence of reduced COMT protein levels (66). The polymorphism has also been shown to influence the human experience of pain (67). All these findings make it a good candidate for an association study regarding chiropractic response in cases of chronic pain.
Conclusions
As documented by the growing number of scientific papers concerning chiropractic therapy in PubMed over the last 20 years, complementary and alternative medicine and chiropractic in particular are ranking close to traditional medicine, especially but not only in the treatment of chronic pain. Different studies show utilization rates for chiropractic services of 6-12% (68-70). Interestingly, several surveys have found higher use of chiropractic services among middle-aged women (71, 72). As expected, higher chiropractic utilization is reported for patients with chronic pain and back pain (16.1% and 31.0%, respectively) (73, 74). Chiropractors provide a substantial portion of care for patients with various pathologies, including lumbar pain and neck pain (75). Today chiropractic care is available in over 100 countries, most of which have established national chiropractic associations. To the best of our knowledge, no studies regarding interaction between neurotrophin gene polymorphisms and chiropractic have been published; such studies could be interesting for understanding whether certain polymorphisms predispose for response to chiropractic, especially to acupuncture and SMT. Four genes in the family of neurotrophic factors, BDNF, NGF, NTF3 and NTF4, together with other genes, could be considered for a study into the existence of a genetic association between polymorphisms in these genes and the amount of neurotrophic factors released by chiropractic techniques. Concerning the last two genes, we did not find convincing studies on functional polymorphisms in the literature and they were therefore not considered by this review.
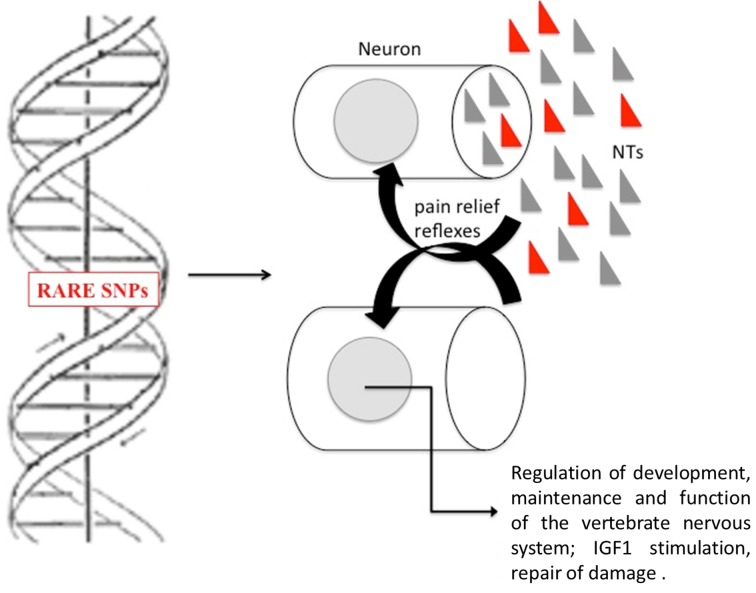
Today it is well known that neuroplasticity plays an important role in neurological rehabilitation. SNPs with a demonstrated effect on neuroplasticity may therefore be relevant for neurological rehabilitation (Figure 1). Different authors suggest that individuals with genetic variants associated with neuroplasticity may respond better to therapies involving neuroplastic processes than individuals without such variants (70-72).
Figure 1.
Presence of specific SNPs in genes related to brain function could increase production of neurotrophins (NTs) by neurons and consequently their neuroplasticity, leading to a better response to chiropractic therapy through regulation of development, maintenance and function of the vertebrate nervous system. These specific SNPs could also increase IGF-1 blood levels after stimulation by massage
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Ernst E. Chiropractic: a critical evaluation. J Pain Symptom Manage. 2008;35:544–62. doi: 10.1016/j.jpainsymman.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Haavik H, Murphy B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J Electromyogr Kinesiol. 2012;22:768–76. doi: 10.1016/j.jelekin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 4.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 5.Johnson IP. Hypothesis: upregulation of a muscle-specific isoform of insulin-like growth factor-1 (IGF-1) by spinal manipulation. Med Hypothese. 2008;71:715–21. doi: 10.1016/j.mehy.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Hotta H, Watanabe N, Piché M, Hara S, Yokawa T, Uchida S. Non-noxious skin stimulation activates the nucleus basalis of Meynert and promotes NGF secretion in the parietal cortex via nicotinic ACh receptors. J Physiol Sci. 2014;64:253–60. doi: 10.1007/s12576-014-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampath KK, Botnmark E, Mani R, et al. Neuroendocrine response following a thoracic spinal manipulation in healthy men. J Orthop Sports Phys Ther. 2017;47:617–27. doi: 10.2519/jospt.2017.7348. [DOI] [PubMed] [Google Scholar]
- 8.Shekelle P, Adams A, Chassin M, Hurwitz E, Brook R. Spinal manipulation for low-back pain. Ann Intern Med. 1992;117:590–8. doi: 10.7326/0003-4819-117-7-590. [DOI] [PubMed] [Google Scholar]
- 9.Koes BW, Assendelft WJJ, van der Heijden G, Bouter LM. Spinal manipulation for low back pain. An updated systematic review of randomized clinical trials. Spine. 1996;21:2860–73. doi: 10.1097/00007632-199612150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004;27:314–26. doi: 10.1016/j.jmpt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Guzzetta A, D’Acunto MG, Carotenuto M, et al. The effects of preterm infant massage on brain electrical activity. Dev Med Child Neurol. 2011;53:46–51. doi: 10.1111/j.1469-8749.2011.04065.x. [DOI] [PubMed] [Google Scholar]
- 12.Waters-Banker C, Dupont-Versteegden EE, Kitzman PH, Butterfield TA. Investigating the mechanisms of massage efficacy: the role of mechanical immunomodulation. J Athl Train. 2014;49:266–73. doi: 10.4085/1062-6050-49.2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzzetta A, Baldini S, Bancale A, et al. Massage accelerates brain development and the maturation of visual function. J Neurosci. 2009;29:6042–51. doi: 10.1523/JNEUROSCI.5548-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 15.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 16.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver IC, D’Alessio AC, Brown SE, et al. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374–83. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 20.Jiang C, Yang S, Tao J, et al. Clinical efficacy of acupuncture treatment in combination with RehaCom cognitive training for improving cognitive function in stroke: a 2 x 2 factorial design randomized controlled trial. J Am Med Dir Assoc. 2016;17:1114–22. doi: 10.1016/j.jamda.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Kluger BM, Rakowski D, Christian M, et al. Randomized, controlled trial of acupuncture for fatigue in Parkinson’s disease. Mov Disord. 2016;31:1027–32. doi: 10.1002/mds.26597. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Chen J, Li Y, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. 2017;177:508–15. doi: 10.1001/jamainternmed.2016.9378. [DOI] [PubMed] [Google Scholar]
- 23.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258–61. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Sui J, Shi H. Control modeling and Chinese acupuncture treatment on cerebral circulation. Technol Health Care. 2015;23:77–82. doi: 10.3233/thc-150934. [DOI] [PubMed] [Google Scholar]
- 25.Manni L, Rocco ML, Barbaro Paparo S, Guaragna M. Electroacupucture and nerve growth factor: potential clinical applications. Arch Ital Biol. 2011;149:247–55. doi: 10.4449/aib.v149i2.1365. [DOI] [PubMed] [Google Scholar]
- 26.Xiao LY, Wang XR, Yang Y, et al. Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation. 2018;21:762–76. doi: 10.1111/ner.12724. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche MA, Muller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 2012;590:4641–62. doi: 10.1113/jphysiol.2012.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolb B, Mychasiuk R, Muhammad A, Gibb R. Brain plasticity in the developing brain. Prog Brain Res. 2013;207:35–64. doi: 10.1016/B978-0-444-63327-9.00005-9. [DOI] [PubMed] [Google Scholar]
- 29.Altar CA, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 30.Larpthaveesarp A, Ferriero DM, Gonzalez FF. Growth factors for the treatment of ischemic brain injury (growth factor treatment) Brain Sci. 2015;5:165–77. doi: 10.3390/brainsci5020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–8. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 32.Aloe L, Iannitelli A, Bersani G, et al. Haloperidol administration in humans lowers plasma nerve growth factor level: evidence that sedation induces opposite effects to arousal. Neuropsychobiology. 1997;36:65–8. doi: 10.1159/000119364. [DOI] [PubMed] [Google Scholar]
- 33.Aloe L, Alleva E, Fiore M. Stress and nerve growth factor: findings in animal models and humans. Pharmacol Biochem Behav. 2002;73:159–66. doi: 10.1016/s0091-3057(02)00757-8. [DOI] [PubMed] [Google Scholar]
- 34.Sherer TB, Neff PS, Hankins GR, Tuttle JB. Mechanisms of increased NGF production in vascular smooth muscle of the spontaneously hypertensive rat. Exp Cell Res. 1998;241:186–93. doi: 10.1006/excr.1998.4043. [DOI] [PubMed] [Google Scholar]
- 35.Chaldakov GN, Stankulov IS, Fiore M, Ghenev PI, Aloe L. Nerve growth factor levels and mast cell distribution in human coronary atherosclerosis. Atherosclerosis. 2001;159:57–66. doi: 10.1016/s0021-9150(01)00488-9. [DOI] [PubMed] [Google Scholar]
- 36.Villoslada P, Hauser SL, Bartke I, et al. Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of T helper cell type 1 and 2 cytokines within the central nervous system. J Exp Med. 2000;191:1799–806. doi: 10.1084/jem.191.10.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syed Z, Dudbridge F, Kent L. An investigation of the neurotrophic factor genes GDNF, NGF and NT3 in susceptibility to ADHD. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:375–8. doi: 10.1002/ajmg.b.30459. [DOI] [PubMed] [Google Scholar]
- 38.Lang UE, Hellweg R, Bajbouj M, Gaus V, Sander T, Gallinat J. Gender-dependent association of a functional NGF polymorphism with anxiety-related personality traits. Pharmacopsychiatry. 2008;41:196–9. doi: 10.1055/s-0028-1082070. [DOI] [PubMed] [Google Scholar]
- 39.Zakharyan R, Atshemyan S, Gevorgyan A, Boyajyan A. Nerve growth factor and its receptor in schizophrenia. BBA Clin. 2014;1:24–9. doi: 10.1016/j.bbacli.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 41.Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil. 2009;16:282–99. doi: 10.1310/tsr1604-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cozza A, Melissari E, Iacopetti P, et al. SNPs in neurotrophin system genes and Alzheimer’s disease in an Italian population. J Alzheimers Dis. 2008;15:61–70. doi: 10.3233/jad-2008-15105. [DOI] [PubMed] [Google Scholar]
- 43.Stepanyan A, Zakharyan R, Simonyan A, Tsakanova G, Arakelyan A. Involvement of polymorphisms of the nerve growth factor and its receptor encoding genes in the etiopathogenesis of ischemic stroke. BMC Med Genet. 2018;19:33. doi: 10.1186/s12881-018-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Y, Yan T, Zhou L, Yin W, Lu J. A single-nucleotide polymorphism of the beta 2-adrenergic receptor gene can predict pathological complete response to taxane- and platinum-based neoadjuvant chemotherapy in breast cancer. Breast Cancer. 2018;10:201–6. doi: 10.2147/BCTT.S189197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyall DM, Lopez LM, Bastin ME, et al. ADRB2, brain white matter integrity and cognitive ageing in the Lothian Birth Cohort 1936. Behav Genet. 2013;43:13–23. doi: 10.1007/s10519-012-9570-x. [DOI] [PubMed] [Google Scholar]
- 46.Vergara C, Ramirez B. CNTF, a pleiotropic cytokine: emphasis on its myotrophic role. Brain Res Brain Res Rev. 2004;47:161–73. doi: 10.1016/j.brainresrev.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 47.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 48.Schuelke M, Wagner KR, Stolz LE, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–8. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 49.Grobet L, Martin LJ, Poncelet D, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–4. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 50.Mosher DS, Quignon P, Bustamante CD, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102:2519–24. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nozaki M, Li Y, Zhu J, et al. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am J Sports Med. 2008;36:2354–62. doi: 10.1177/0363546508322886. [DOI] [PubMed] [Google Scholar]
- 53.Garatachea N, Pinós T, Cámara Y, et al. Association of the K153R polymorphism in the myostatin gene and extreme longevity. Age. 2013;35:2445–54. doi: 10.1007/s11357-013-9513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Wang SJ, Tan SC, et al. The A55T and K153R polymorphisms of MSTN gene are associated with the strength training-induced muscle hypertrophy among Han Chinese men. J Sports Sci. 2014;32:883–91. doi: 10.1080/02640414.2013.865252. [DOI] [PubMed] [Google Scholar]
- 55.Persi A, Maltese PE, Bertelli M, et al. Polymorphisms of alpha-actinin-3 and ciliary neurotrophic factor in national-level Italian athletes. Panminerva Med. 2013;55:217–24. [PubMed] [Google Scholar]
- 56.Zhang Q, Cao Y, Chen J, et al. ACTN3 is associated with children’s physical fitness in Han Chinese. Mol Genet Genomics. 2019;294:47–56. doi: 10.1007/s00438-018-1485-7. [DOI] [PubMed] [Google Scholar]
- 57.Szczepankiewicz A, Rachel M, Sobkowiak P, et al. Neurotrophin serum concentrations and polymorphisms of neurotrophins and their receptors in children with asthma. Respir Med. 2013;107:30–6. doi: 10.1016/j.rmed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 58.Li N, Duan G, Sun J, et al. Associations between single-nucleotide polymorphisms in the NTRK1 gene and basal pain sensitivity in young Han Chinese women. Neurosci Lett. 2018;662:312–7. doi: 10.1016/j.neulet.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Waxman SG. A channel sets the gain on pain. Nature. 2006;444:831–2. doi: 10.1038/444831a. [DOI] [PubMed] [Google Scholar]
- 60.Estacion M, Harty TP, Choi JS, Tyrrell L, Dib-Hajj SD, Waxman SG. A sodium channel gene SCN9A polymorphism that increases nociceptor excitability. Ann Neurol. 2009;66(6):862–6. doi: 10.1002/ana.21895. [DOI] [PubMed] [Google Scholar]
- 61.Greenbaum L, Tegeder I, Barhum Y, Melamed E, Roditi Y, Djaldetti R. Contribution of genetic variants to pain susceptibility in Parkinson disease. Eur J Pain. 2012;16:1243–50. doi: 10.1002/j.1532-2149.2012.00134.x. [DOI] [PubMed] [Google Scholar]
- 62.Kim JY, Tillu DV, Quinn TL, et al. Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism. J Neurosci. 2015;35:6307–17. doi: 10.1523/JNEUROSCI.3481-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadhasivam S, Chidambaran V, Olbrecht VA, et al. Genetics of pain perception, COMT and postoperative pain management in children. Pharmacogenomics. 2014;15:277–84. doi: 10.2217/pgs.13.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belfer I, Segall SK, Lariviere WR, et al. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain. 2013;154:1368–76. doi: 10.1016/j.pain.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schacht JP. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenomics J. 2016;16:430–8. doi: 10.1038/tpj.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shield AJ, Thomae BA, Eckloff BW, Wieben ED, Weinshilboum RM. Human catechol O-methyltransferase genetic variation: gene resequencing and functional characterization of variant allozymes. Mol Psychiatry. 2004;9:151–60. doi: 10.1038/sj.mp.4001386. [DOI] [PubMed] [Google Scholar]
- 67.Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 68.Lawrence DJ, Meeker WC. Chiropractic and CAM utilization: a descriptive review. Chiropr Osteopat. 2007;15:2. doi: 10.1186/1746-1340-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper KL, Harris PE, Relton C, Thomas KJ. Prevalence of visits to five types of complementary and alternative medicine practitioners by the general population: a systematic review. Complement Ther Clin Pract. 2013;19:214–20. doi: 10.1016/j.ctcp.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Beliveau PJH, Wong JJ, Sutton DA, et al. Chiropractic & Manual Therapies. 2017;25:35. doi: 10.1186/s12998-017-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisenberg D, Davis R, Ethner S, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 2008;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 72.Woolton J, Sparber A. Surveys of complementary and alternative medicine: part I. General trends and demographic groups. J Altern Complement Med. 2001;7:195–208. doi: 10.1089/107555301750164307. [DOI] [PubMed] [Google Scholar]
- 73.Elder C, DeBar L, Ritenbaugh C, et al. Acupuncture and chiropractic utilization among chronic musculoskeletal pain patients at a health maintenance organization. BMC Complement Altern Med. 2012;12:P279. [Google Scholar]
- 74.Zheng Z, Xue CCL. Pain research in complementary and alternative medicine in Australia: A Critical Review. J Altern Complement Med. 2013;19:81–91. doi: 10.1089/acm.2011.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Druss BG, Rosenheck RA. Association between use of unconventional therapies and conventional medical services. JAMA. 1999;282:651–6. doi: 10.1001/jama.282.7.651. [DOI] [PubMed] [Google Scholar]
- 76.de Boer RGA, Spielmann K, Heijenbrok-Kal MH, et al. The role of the BDNF Val66Met polymorphism in recovery of aphasia after stroke. Neurorehabil Neural Repair. 2017;31:851–7. doi: 10.1177/1545968317723752. [DOI] [PubMed] [Google Scholar]
- 77.Leech KA, Hornby TG. High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. J Neurotrauma. 2017;34:1240–8. doi: 10.1089/neu.2016.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue JK, Winkler EA, Rick JW, et al. DRD2C957T polymorphism is associated with improved 6-month verbal learning following traumatic brain injury. Neurogenetics. 2017;18:29–38. doi: 10.1007/s10048-016-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]