Abstract
Background and aim of the work: Recent seroprevalence studies in different population groups have shown low antibody titers against poliomyelitis, especially in young adults. This, together with the reduction of vaccination rates, could favor the reintroduction of poliovirus in long-time polio-free countries. Within the Surveillance system of acute flaccid paralysis, a prevalence study was conducted to estimate the immunological status associated with poliomyelitis in young migrants. Methods: Local Health Authority collected serum samples in young migrants, without vaccination documentation. Antibodies levels were assessed with a long incubation neutralization assay. Subjects were stratified by age and by WHO region. Seroprotection was defined by a titer equal or above 1:8 and titers > 1:2 were log-transformed and evaluated as geometric mean titers (GMTs). Results: From January 2004 to August 2017, 1138 blood samples were collected. Mean age was 13.3 years with no differences between WHO regions. The percentage of antibody titers below 1:8 was 6.0% versus poliovirus 1 (PV1), 7.7% versus poliovirus 2 (PV2) and 15% versus poliovirus 3 (PV3). The GMTs were 45.5, 29.5 and 20 towards PV1, PV2 and PV3 respectively. In each WHO region, the GMTs towards PV3 were consistently the lowest, and the Europeans showed the lowest GMTs both towards PV2 and PV3 (27.5 and 15.3 respectively). GMTs decreased with age. Conclusion: The low GMTs and the clear tendency to decrease with increasing age of the subjects, especially against to PV1, confirm the framework of attention that polio is receiving at national and international level. (www.actabiomedica.it)
Keywords: serological survey, seroprevalence, immunity, migrants, poliomyelitis, WHO region
Introduction
Poliomyelitis epidemiology has radically changed since the introduction of intensive vaccination programs against the three polioviruses (PVs) (1,2). The last native case of polio due to wild-type poliovirus (WPV) infection detected in Italy occurred in 1982. At the time, the mandatory vaccination was performed entirely with trivalent oral poliovirus vaccine with Sabin strains (tOPV). In 1999, tOPV was substituted with a sequential schedule: two doses of enhanced inactivated polio vaccine (eIPV) followed by two doses of tOPV. When, in 2002, the European Region was declared “polio-free country” (the last case of indigenous wild poliomyelitis had occurred in Eastern Turkey in 1988) (3), Italy finally decided to adopt the four doses eIPV schedule as well as other high income Countries (4). Several seroprevalence studies, in which the level of neutralizing antibodies against poliovirus 1 (PV1), poliovirus 2 (PV2) and poliovirus 3 (PV3) are considered correlates of protection, conducted in Italy since the Eighties, both in general population and in selected subgroups, showed decreased protective values in terms of geometric mean titers (GMT) and titers considered protective by WHO (equal or higher than 1:8). These studies have also shown, despite good levels of seroprotection in the general population, a reduction in protection among adolescents and subsequently among young adults, probably due to the lack of natural boosters 10-15 years after the primary vaccination cycle (5-16). In addition, over the last years, the Italian Ministry of Health observed a lower vaccination coverage nationwide, explained by a loss of trust of the Italian population in these preventive measures. Due to vaccination hesitancy (17,18), anti-polio vaccination coverage dropped from 96.1% in 2013 to 93.4% in 2015, therefore below 95%, which is the requested threshold for polio elimination and to ensure herd immunity (19). For these reason, the 2017-19 National Immunization Prevention Plan confirmed the mandatory vaccination for children, alongside with a fifth booster dose of eIPV for adolescence (20).
Lower immunization rates, in fact, expose the Italian population, at least hypothetically, to a reintroduction of WPV or vaccine-derived polioviruses (cVDPV). Since 2005, when Environmental surveillance (ES, testing sewage for polioviruses) was introduced in Italy, becoming an important tool for early detection of silent reintroduction and circulation of polioviruses, no WPVs were spotted, although there have been several detections of Sabin-like PVs (21-26).
Migration flows towards Europe and Italy have constantly increased since the early Nineties. In many of the cases, migrants come from countries were OPV schedule is still recommended. Unfortunately in some of these areas there is a strong decline of vaccine coverage due to social disruption caused by civil war, Health Services collapse due to major epidemics, or even religious opposition by fundamentalists culminating with acts of violence against polio vaccination workers.
European countries registered an outbreak of 71 cases (59 paralytic and 2 death) in an unvaccinated religious community in the Netherlands in 1992 (27), whereas other 3 cases were identified among Roma children in Bulgaria in 2001 (28). A large outbreak caused by WPV1 imported from India in late 2009, with 463 laboratory-confirmed and 47 polio-compatible cases, took place in 2010 in Tajikistan and spread to neighbouring countries, Kazakhstan, Russia, Turkmenistan and Uzbekistan (29). Episodes like these ought to remind us that reintroduction of polioviruses cannot be completely ruled out (19).
Migrants who arrive in Italy legally, for work or study reasons, for international adoption or for family reunification and who decide to live permanently in the Italian territory, represent an important population group. Although immunization policies for migrants and refugees vary widely within the WHO European Region (30,31), the Italian Ministry of Health recommends to vaccinate, according to age, all refugee children who have never been vaccinated or who have insufficient documentation regarding prior vaccinations. Additionally, adults with the same characteristics should receive polio vaccination.
The aim of the present study was to estimate the prevalence of antibodies against the three poliomyelitis viruses in subjects of recent immigration who approached the vaccination services for the regularization of their vaccination calendars, to make them coherent with the polio eradication goal.
Methods
Study population
From January 2004 to August 2017, as part of the active surveillance of acute flaccid paralysis (AFP) and of the polio eradication process, all foreign migrants recently arrived in Italy, without or with insufficient vaccination documentation, who have turned to vaccination services of the Local Health Authority of Parma (a city with 190,000 inhabitants, in northern Italy) for the regularization of the vaccination schedule, were subjected to the determination of the antibody titers towards poliomyelitis. The survey was conducted according to the Good Clinical Practice Guidelines: the data collected - age, sex, period elapsed from arrival in Italy and country of origin - were treated anonymously for research purposes. This convenience sample was grouped into the six WHO regions: African Region (AFR), Region of the Americas (AMR), South-East Asia Region (SEAR), European Region (EUR), Eastern Mediterranean Region (EMR), and Western Pacific Region (WPR); by age groups (less than 2 years, 2 to 6 years, 7 to 18 years and equal or more than 19 years). To express graphically the trend of the GMTs in relation to age, instead, the distribution in quintiles of the age, treated as continuous variable, was used.
Serological analysis
Sterile serum samples were collected and kept at -20°C until they were examined. The determination of the three polioviruses antibodies levels was carried out with a long-incubation neutralization assay using 100 TCID50, respectively, of poliovirus type 1 (Mahoney), poliovirus type 2 (Mef-1) and poliovirus type 3 (Saukett).
The search for neutralising antibodies (a) and the titration of the viruses (b) were carried out using a laryngeal carcinoma continuous cell line (HEP-2).
(a) The sera, heated to 56°C for 30 minutes, were tested simultaneously in triplicate at dilutions from 1:2 to 1:1024 with polioviruses type 1, type 2 and type 3, respectively. The serum/virus mixtures (0.025 mL each) were then incubated at 37°C for 6 hours in an appropriately humidified CO2 incubator and then at 4°C for 18 hours.
(b) Aliquots of 0.050 mL of a cellular suspension (5–6 x 104 HEP-2) were added to each well. While being incubated at 37 °C, the microplates were microscopically observed for cytopathic effects (CPEs) on the third and fourth days. The titers of the sera were calculated as the highest dilution capable of neutralising the CPEs. Each reaction included controls of the viral titer, the cells and the sera (32).
Statistical analysis
Seroprotection was defined as a titer equal to or above 1:8. Subjects with antibody titers <1:8 for all the three serotypes were classified as “triple negatives”. Titers > 1:2 were log-transformed and evaluated as GMTs. Continuous variables were summarised as the mean, standard deviation (SD) and minimum–maximum values. The Analysis of Variance (Two-Way ANOVA) and Student’s t-test were performed when appropriate; to verify the association between GMT and quintile distribution of age, a linear regression test was carried out. A p-value of 0.05 was considered significant. All statistical analyses were performed with SPSS 24.0 (IBM SPSS Inc., Chicago, IL).
Results
From January 2004 to August 2017, 2,138 samples were analyzed to determine immunization levels in migrants recently moved to Italy. Such group was mostly composed of male subjects (59.07%), average age was 13.3 years old (sd 6.1), range, 1– 55 yrs, median 13.6 yrs, with no statistically significant differences regarding the WHO region of origin. The most represented age group was the one in school age (Table 1). Median time interval between arrival in Italy and sampling date was 3 months (range = 15 days-5 yrs), resulting higher in population arriving from the European Region (median, 7 months).
Table 1.
Characteristics of the study sample
| WHO Region* | Age | Age group (%) | |||||||
| Subjects (No.) | Mean (sd) | Median | Min | Max | < 2 years | 2 - 6 years | 7 - 18 years | =>19 years | |
| AMR | 195 | 13.00 (5.38) | 13.00 | 1 | 51 | 0.5% | 9.9% | 83.9% | 5.7% |
| AFR | 1,038 | 13.52 (6.01) | 14.00 | 1 | 55 | 1.3% | 10.8% | 80.7% | 7.2% |
| SEAR | 223 | 12.61 (5.63) | 12.2 | 2 | 44 | 0.0% | 12.7% | 80.1% | 7.2% |
| EUR | 240 | 13.56 (7.70) | 14.00 | 1 | 49 | 1.3% | 16.4% | 72.3% | 10.1% |
| EMR | 271 | 13.18 (6.48) | 13.40 | 1 | 40 | 0.7% | 17.2% | 73.9% | 8.2% |
| WPR | 171 | 12.38 (4.76) | 13.00 | 0 | 31 | 2.4% | 11.2% | 82.2% | 4.1% |
| Overall | 2,138 | 13.25 (6.11) | 13.63 | 0 | 55 | 1.1% | 12.4% | 79.2% | 7.3% |
* See abbreviations in the text
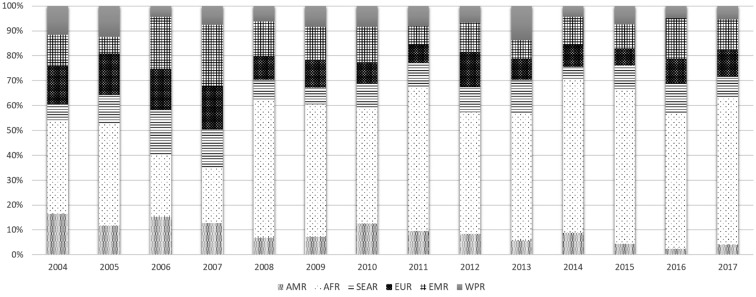
The African Region was the most represented with an elevated number of subjects coming from Senegal, Ivory Coast, Ghana and Nigeria (which is still an endemic country), followed by the EMR which includes two still endemic countries (Pakistan and Afghanistan). SEAR was extensively represented by the Indian sub-continent. Over time, the relative percentage of subjects from the African continent has increased, while the number of subjects coming from AMR has decreased (Figure 1).
Figure 1.
WHO regions of origin: distribution of subjects per year of study
The percentage of antibody titers below 1:8 was 6.0% versus poliovirus 1, 7.7% versus poliovirus 2 and 15% versus poliovirus 3. Twenty-seven subjects resulted triple negatives (antibody titers <1:8 for all the three serotypes).
Stratifying population by WHO region of origin, the WPR had the highest percent of non-seroprotected subjects against poliovirus 1 (8.8%), while the European Region had the highest percent of non-seroprotected against polio 2 and 3 (respectively 11.7% and 24.6%). Overall, the European subjects showed the highest percentages of seronegativity towards one or more serotypes, in fact only 70% of them, at the same time, showed protective antibodies to the three polio viruses. (Table 2).
Table 2.
Numbers and percentages of subjects with protective (≥ 1: 8) and non-protective (<1: 8) antibodies and numbers and percentages of subjects without antibodies to one or more of the polioviruses, by WHO regions
| WHO regions | Poliovirus 1 | Poliovirus 2 | Poliovirus 3 | Triple positives | 1/3 negatives | 2/3 negatives | Triple negatives | |||||
| ≥ 1:8 | < 1:8 | ≥ 1:8 | < 1:8 | ≥ 1:8 | < 1:8 | |||||||
| AMR | No. % | 195 | 185 | 10 | 180 | 15 | 159 | 36 | 147 | 37 | 9 | 2 |
| 94.9% | 5.1% | 92.3% | 7.7% | 81.5% | 18.5% | 75.4% | 19.0% | 4.6% | 1.0% | |||
| AFR | No. % | 1038 | 974 | 64 | 953 | 85 | 896 | 142 | 826 | 141 | 63 | 8 |
| 93.8% | 6.2% | 91.8% | 8.2% | 86.3% | 13.7% | 79.6% | 13.6% | 6.1% | 0.8% | |||
| SEAR | No. % | 223 | 212 | 11 | 213 | 10 | 206 | 17 | 195 | 19 | 8 | 1 |
| 95.1% | 4.9% | 95.5% | 4.5% | 92.4% | 7.6% | 87.4% | 8.5% | 3.6% | 0.4% | |||
| EUR | No. % | 240 | 226 | 14 | 212 | 28 | 181 | 59 | 168 | 48 | 19 | 5 |
| 94.2% | 5.8% | 88.3% | 11.7% | 75.4% | 24.6% | 70.0% | 20.0% | 7.9% | 2.1% | |||
| EMR | No. % | 271 | 257 | 14 | 254 | 17 | 230 | 41 | 221 | 33 | 12 | 5 |
| 94.8% | 5.2% | 93.7% | 6.3% | 84.9% | 15.1% | 81.5% | 12.2% | 4.4% | 1.8% | |||
| WPR | No. % | 171 | 156 | 15 | 161 | 10 | 145 | 26 | 137 | 23 | 5 | 6 |
| 91.2% | 8.8% | 94.2% | 5.8% | 84.8% | 15.2% | 80.1% | 13.5% | 2.9% | 3.5% | |||
| Overall | No. % | 2138 | 2010 | 128 | 1973 | 165 | 1817 | 321 | 1694 | 301 | 116 | 27 |
| 94.0% | 6.0% | 92.3% | 7.7% | 85.0% | 15.0% | 79.2% | 14.1% | 5.4% | 1.3% | |||
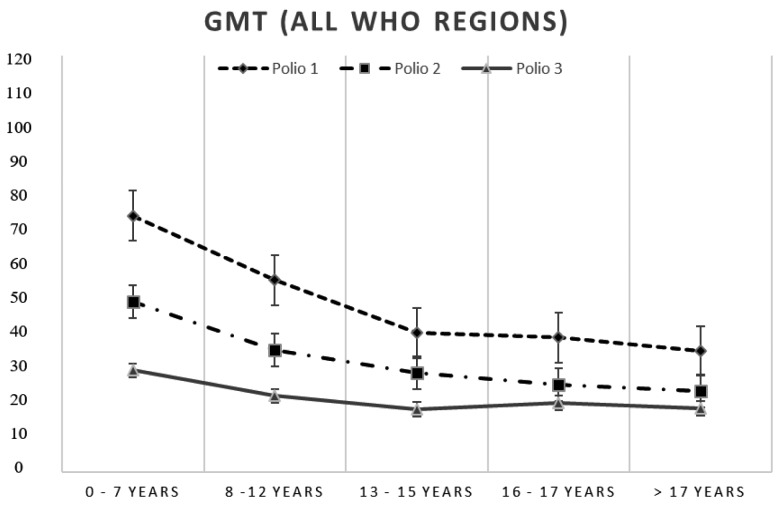
The GMTs towards the 3 polioviruses were 45.5 for PV1, 29.5 for PV2 and 20 for PV3 respectively (Table 3). In each WHO region, the GMTs for PV3 were consistently the lowest, and even in this case the EUR prevailing subjects showed the lowest GMTs for both PV2 and PV3 (respectively 27.5 and 15.3). The GMTs referring to each of the 14 years of study have experienced strong fluctuations (from 21.3 to 89.3 for the PV1, from 16.8 to 55.6 for the PV2, from 12.4 to 36.6 for the PV3). The analysis conducted on the distribution in quintiles of the ages, confirmed the reduction of GMTs that show a decrease in relation to age classes especially those towards polio 1 and polio 2. (Figure 2). The age group below 2 years of age showed the greatest prevalence of non-seroprotected subjects towards the 3 polioviruses; 34.8% of subjects had no protection against at least one of the 3 serotypes. Even the very large group of children and adolescents showed a high percentage of subjects lacking protective antibodies, in particular towards poliovirus 3 (15.4%) (Table 4).
Table 3.
GMTs toward PV1, PV2, PV3, by WHO region
| WHO Region | Subjects (No.) | GMT (Poliovirus 1) | GMT (Poliovirus 2) | GMT (Poliovirus 3) |
| AMR | 195 | 44.8 | 31.1 | 17.2 |
| AFR | 1038 | 43.0 | 28.6 | 21.2 |
| SEAR | 223 | 50.9 | 35.0 | 22.8 |
| EUR | 240 | 56.0 | 27.5 | 15.3 |
| EMR | 271 | 50.7 | 29.3 | 20.6 |
| WPR | 171 | 35.1 | 30.2 | 19.2 |
| Overall | 2138 | 45.5 | 29.5 | 20.0 |
Figure 2.
GMTs calculated by quintile of age group
Table 4.
Numbers and percentages of subjects with protective (> 1: 8) and non-protective (<1: 8) antibodies, percentages of subjects without antibodies to one or more of the polioviruses and GMTs by age class
| Age | Subjects (No.) | Poliovirus 1 | Poliovirus 2 | Poliovirus 3 | All strains | |||||||
| titres ≥1:8 | titres <1:8 | GMT | titres ≥1:8 | titres <1:8 | GMT | titres ≥1:8 | titres <1:8 | GMT | triple positives | triple negatives | ||
| < 2 years | 23 | 82.6% | 17.4% | 70.1 | 87.0% | 13.0% | 40.7 | 65.2% | 34.8% | 14.6 | 65.2% | 13.0% |
| 2 - 6 years | 263 | 96.6% | 3.4% | 79.6 | 95.1% | 4.9% | 47.8 | 87.8% | 12.2% | 28.0 | 86.3% | 1.9% |
| 7 - 18 years | 1,679 | 94.0% | 6.0% | 42.5 | 92.3% | 7.7% | 27.8 | 84.6% | 15.4% | 18.9 | 78.5% | 0.9% |
| =>19 years | 155 | 90.3% | 9.7% | 34.5 | 73.1% | 6.1% | 25.1 | 87.1% | 12.9% | 22.2 | 76.1% | 2.6% |
| Overall | 2,120 | 94.0% | 6.0% | 45.5 | 88.4% | 11.6% | 29.6 | 85.0% | 15.0% | 20.0 | 79.2% | 1.3% |
Conclusions
Sub-optimal vaccination coverage, often the result of the disintegration of social and health systems due to ongoing conflicts, may be responsible for the circulation or reintroduction of wild polioviruses in polio-free populations as evidenced by recent episodes in Tajikistan (2010) or in the Arab Republic of Syria (2013-2014) (29,33).
In this survey, 79.2% of subjects showed protective antibodies to the three polioviruses. As in investigations of the past and in recent seroprevalence studies on the Italian population, PV1 antigen was the most immunogenic with GMTs constantly higher than PV2 and PV3 during the 14 years of the survey and considering the WHO regions of origin. Fifteen percent of the subjects, on the other hand, were found not to have protective antibodies against PV3. In particular, subjects from the European region showed high percentages of low protection both towards PV2 (11.7%) and PV3 (24.6%). Children under the age of 2 were poorly represented (23 overall): they showed elevated GMTs, but a high percentage of unprotected subjects towards at least one of the 3 poliovirus.
GMTs tend to decrease significantly with age, especially PV1 and PV2 and, as in the case of the Italian population, low titers could depend on the absence of natural boosters.
The sample considered, coming from the 6 WHO regions and from 78 different countries, showed a low prevalence of subjects without antibodies; in 14 years of investigation only 27 subjects (1.3%) were triple-negatives.
However, a substantial percentage of sample showed not optimal antibodies levels as considered by the WHO, in a scenario of possible circulation of wild polioviruses. The low GMTs and the clear tendency to decrease with the increasing age of the subjects, especially against PV1, confirm the framework of attention that polio is receiving at national and international level.
The main limitation of this study is the convenience sample represented by the most stable foreign population that, for study and work reasons, turns to the Local Health Services to regularize its vaccination situation. Furthermore, due to the absence of vaccination documentation it was not possible to trace the type of vaccine used, however most of the subjects (> 95%) came from Countries where OPV Sabin is still used and, in this case, all subjects were hypothetically vaccinated with the trivalent vaccine (tOPV) before the switch to bivalent OPV (bOPV), which occurred between April and May 2016, due to the disappearance of PV 2 worldwide and the consequent removal of the type 2 component (OPV2) from immunization programmes. (34).
The population residing in Italy, vaccinated with eIPV, no longer exposed to vaccine polioviruses since 2002, if not those eventually imported by subjects with recent vaccination, could have GMTs and seroprotection levels lower than those found in our study (15,16).
The addition of a 5th dose of eIPV to the adolescent vaccination calendar, could be evaluated on serum epidemiological data collected in controlled investigations on representative population samples identified on the basis of age, origin and vaccination status.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Giovanardi A. Effect of Sabin poliovirus vaccine on incidence of poliomyelitis in Italy. JAMA. 1969;209(4):525–8. [PubMed] [Google Scholar]
- 2.Nathanson N, M. Kew O. From Emergence to Eradication: The Epidemiology of Poliomyelitis Deconstructed. Am J Epidemiol. 2010;172:1213–29. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. (2002). Certification of Poliomyelitis Eradication: European Region declared “polio-free”. Presented at the Fifteenth meeting of European regional Certification Commission. Copenhagen. Last accessed on 12 June 2019 http://www.euro.who.int/__data/assets/pdf_file/0003/79374/E88105.pdf . [Google Scholar]
- 4.Italian Ministry of Health Permanent Conference for relations between the State, Regions and Autonomous Provinces of Trento and Bolzano. Agreement between the Ministry of Health, the Regions and Autonomous Provinces of Trento and Bolzano on amendments to the anti-polio vaccination schedule. Decree 18 June 2002. Official Bulletin of Italian Republic (Gazzetta Ufficiale della Repubblica Italiana, G.U.R.I.) General Series, n. 163, 13 July 2002 [Italian] [Google Scholar]
- 5.Reali D, Carducci A, Ruschi MA. Serum antibodies to polioviruses in a Tuscan population. Italy. Eur J of Epidemiol. 1990;6(3):309–12. doi: 10.1007/BF00150438. [DOI] [PubMed] [Google Scholar]
- 6.Triassi M, Ribera G, Barruffo L, Barbone S, Medda E, Grandolfo M E. Persistence of immunity to poliomyelitis among a southern population that received four doses of OPV 5 to over 15 years before. Eur J of Epidemiol. 1996;12(1):5–8. doi: 10.1007/BF00144420. [DOI] [PubMed] [Google Scholar]
- 7.Mastroeni I, Patti AM, Fabrizi A, et al. Immunity status against poliomyelitis in persons 13-14 years old living in Rome. Vaccine. 1997;15:747–50. doi: 10.1016/s0264-410x(96)00208-3. [DOI] [PubMed] [Google Scholar]
- 8.Patti AM, Santi AL, Bellucci C, et al. Serological survey on immunity status against polioviruses in Italian young adults and in immigrants. Ann Ig. 1999;11(5):353–9. [PubMed] [Google Scholar]
- 9.Affanni P, Veronesi L, Rizziero S, Bizzoco S, Bracchi MT, Tanzi ML. Status of immunity against poliomyelitis: a study among european and extra-european young immigrants living in Parma. Acta Biomed. 2005;76:157–63. [PubMed] [Google Scholar]
- 10.Pires de Miranda M, Carmo Gomes M, Rebelo de Andrade H. 2007. Seroprevalence of antibodies to poliovirus in individuals living in Portugal, 2002. Euro Surveill. 12;6:E7–E8. doi: 10.2807/esm.12.06.00717-en. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi L, Virdis R, Bizzoco S, et al. Vaccination status and prevalence of enteric viruses in internationally adopted children. The case of Parma, Italy. Acta Biomed. 2011;82(3):208–13. [PubMed] [Google Scholar]
- 12.Baldo V, Baldovin T, Cocchio S, et al. Seroepidemiology of Polioviruses among University Students in Northern Italy. Clin Vaccine Immunol. 2012;19(8):1292–5. doi: 10.1128/CVI.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinheimer C, Friedrichs I, Rabenau HF, Doerr HW. Deficiency of immunity to poliovirus type 3: a lurking danger. BMC Infect. Dis. 2012;12:24–9. doi: 10.1186/1471-2334-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veronesi L, Affanni P, Verrotti di Pianella C, Colucci ME, Tanzi ML. Immunity status against poliomyelitis in childbearing women in a province of northern Italy. A cross-sectional analysis. Ann Ig. 2013;25(5):427–33. doi: 10.7416/ai.2013.1944. [DOI] [PubMed] [Google Scholar]
- 15.Giammanco GM, Bechini A, Urone N, et al. Is Italian population protected from Poliovirus? Results of a seroprevalence survey in Florence, Italy. Hum Vaccin Immunother. 2018;14(9):2248–53. doi: 10.1080/21645515.2018.1475812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupi S, Stefanati A, Baldovin T, Roman A, Baldo V, Gabutti G. Assessment of seroprevalence against poliovirus among Italian adolescents and adults. Hum Vaccin Immunother. 2019;15(3):677–82. doi: 10.1080/21645515.2018.1547608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Signorelli C, Odone A, Cella P, Iannazzo S, D’Ancona F, Guerra R. Infant immunization coverage in Italy (2000-2016) Ann Ist Super Sanita. 2017;53(3):231–7. doi: 10.4415/ANN_17_03_09. [DOI] [PubMed] [Google Scholar]
- 18.Signorelli C, Guerra R, Siliquini R, Ricciardi W. Italy’s response to vaccine hesitancy: An innovative and cost effective National Immunization Plan based on scientific evidence. Vaccine. 2017;35(33):4057–9. doi: 10.1016/j.vaccine.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Stefanelli P, Buttinelli G, Rezza G. Poliomyelitis: residual hurdles to global eradication. Commentary. Ann Ist Super Sanita. 2016;52(4):469–71. doi: 10.4415/ANN_16_04_01. [DOI] [PubMed] [Google Scholar]
- 20.Ministero della salute. Piano Nazionale Prevenzione Vaccinale 2017-2019. Last accessed on 12 June 2019 http://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2571 . [Google Scholar]
- 21.Patti AM, Santi AL, Fiore L, et al. Environmental surveillance of poliovirus in Italy: pilot study. Ann Ig. 2003;15(2):97–105. [PubMed] [Google Scholar]
- 22.Cesari C, Colucci M.E, Veronesi L, et al. Detection of enteroviruses from urban sewage in Parma. Acta Biomed. 2010;81(1):40–6. [PubMed] [Google Scholar]
- 23.Battistone A, Buttinelli G, Fiore S, et al. Sporadic Isolation of Sabin-Like Polioviruses and High-Level Detection of Non-Polio Enteroviruses during Sewage Surveillance in Seven Italian Cities, after Several Years of Inactivated Poliovirus Vaccination. Appl Environ Microbiol. 2014;80(15):4491–4501. doi: 10.1128/AEM.00108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrinelli L, Binda S, Chiaramonte I, et al. Detection and distribution of culturable Human Enteroviruses through environmental surveillance in Milan. Italy. J Appl Microbiol. 2013;115(5):1231–9. doi: 10.1111/jam.12321. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrinelli L, Bubba L, Primache V, et al. Surveillance of poliomyelitis in Northern Italy: Results of acute flaccid paralysis surveillance and environmental surveillance. 2012-2015. Hum Vaccin Immunother. 2017;13(2):332–8. doi: 10.1080/21645515.2017.1264726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delogu R, Battistone A, Buttinelli G, et al. Poliovirus and Other Enteroviruses from Environmental Surveillance in Italy, 2009-2015. Food Environ Virol. 2018 Dec;10(4):333–42. doi: 10.1007/s12560-018-9350-8. [DOI] [PubMed] [Google Scholar]
- 27.Oostvogel PM, van Wijngaarden JK, van der Avoort HG, et al. Poliomyelitis outbreak in an unvaccinated community in the Netherlands 1992-93. Lancet. 1994;344(8923):665–70. doi: 10.1016/s0140-6736(94)92091-5. [DOI] [PubMed] [Google Scholar]
- 28.Korsun N, Kojouharova M, Vladimirova N, et al. Three cases of paralytic poliomyelitis associated with type 3 vaccine poliovirus strains in Bulgaria. J Med Virol. 2009;81(9):1661–7. doi: 10.1002/jmv.21545. [DOI] [PubMed] [Google Scholar]
- 29.Yakovenko ML, Gmyl AP, Ivanova OE, et al. The 2010 outbreak of poliomyelitis in Tajikistan: epidemiology and lessons learnt. Euro Surveill. 2014;19(7):20706. doi: 10.2807/1560-7917.es2014.19.7.20706. [DOI] [PubMed] [Google Scholar]
- 30.Mipatrini D, Stefanelli P, Severoni S, Rezza G. Vaccinations in migrants and refugees: a challenge for European health systems. A systematic review of current scientific evidence. Pathog Glob Health. 2017 Mar;111(2):59–68. doi: 10.1080/20477724.2017.1281374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vito E, Parente P, de Waure C, Poscia A, Ricciardi W. A review of evidence on equitable delivery. access and utilization of immunization services for migrants and refugees in the WHO European Region. Copenhagen: WHO Regional Office for Europe 2017. Last accessed 12 June 2019: http://www.ncbi.nlm.nih.gov/books/NBK475647/ [PubMed] [Google Scholar]
- 32.World Health Organization. Guidelines for WHO/EPI collaborative studies on poliomyelitis. Standard procedure for determining immunity to poliovirus using the microneutralization test. WHO/EPI/GEN/ 93.9. 1993 World Health Organization, Geneva, Switzerland [Google Scholar]
- 33.Aylward RB, Alwan A. Polio in Syria. Lancet. 2014;383(9916):489–91. doi: 10.1016/S0140-6736(14)60132-X. [DOI] [PubMed] [Google Scholar]
- 34.Polio Eradication and Endgame Strategic Plan 2013-2018. Last accessed 12 June 2019. https://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/en/ [Google Scholar]