Abstract
Background and aim of the work: Epidemic influenza is associated with significant morbidity and mortality, particularly in people at risk. The vaccine reduces complications, hospitalization and mortality excess, as well as health care and social costs. Aim of the study was to estimate the influenza vaccine effectiveness (VE) in Emilia-Romagna Region during the 2018/2019 season. Methods: Within the context of virological surveillance conducted at the Regional Reference Laboratory of Parma, nasal/throat swabs were performed by sentinel practitioners and clinicians, on patients with ILI (Influenza-like illness). VE estimates, overall and against subtype A(H1N1)pdm09 and A(H3N2), were evaluated in three periods of the season, using a test-negative case-control design. Results: From November 2018 to April 2019, 2,230 specimens were analyzed: 1,674 (75.1%) performed by clinicians and 556 (24.9%) by sentinel practitioners of the regional network. The season was characterized by the predominant circulation of influenza type A viruses: 57.4% belonged to subtype A(H3N2), 41.2% to subtype A(H1N1)pdm09. 23.5% of patients was vaccinated against influenza with quadrivalent or adjuvate vaccine. The overall VE was -5% (95% CI -33% - 18%) with a decreasing trend during the season. The overall VE against subtype A(H1N1)pdm09 was 39% (95% CI 11% - 58%) and remained stable during the season. The overall VE against subtype A(H3N2) was -43% (95% CI -89% - -9%), and showed an important decreasing trend. Conclusions: The possibility to make accurate and continuous VE estimates during the season will help to better define the composition of the vaccine for the following season. (www.actabiomedica.it)
Keywords: influenza, influenza-like illness, virological surveillance, vaccine effectiveness, test-negative case-control design
Introduction
Epidemic influenza is associated with significant morbidity and mortality, particularly for the elderly and people at risk (1). The European Center for Disease Control (ECDC) estimates that, about 40,000 people, each year, die prematurely due to influenza in the European Union. A large proportion of influenza-related deaths occur in individuals older than 65 years, especially among those with chronic underlying conditions (2, 3). The prevention of influenza represents an important Public Health intervention, involving Health Services every year in the implementation of the vaccination campaign (4). The vaccine significantly reduces complications, hospitalization and mortality excess in those most at risk, as well as health care costs through the reduction of drug consumption, and the social costs associated with the flu epidemic (5-13).
The viral strains in influenza vaccines have to be evaluated and updated regularly because circulating influenza viruses continuously evolve. Annually, an advisory group of experts analyses influenza virus surveillance data generated by the WHO Global Influenza Surveillance and Response System (GISRS), and issues recommendations on the composition of the influenza vaccines for the following influenza season. These recommendations are used by the national vaccine regulatory agencies and the pharmaceutical companies to develop, produce and license influenza vaccines. Approximately 6-8 months are needed to produce vaccines (14). Recommendations for the following influenza season are usually made in February in the Northern Hemisphere and in September in the Southern Hemisphere. According to the virological surveillance activity, in 2019, the formulation of the influenza vaccine for the Northern Hemisphere was postponed by about a month to allow a better definition of the A(H3N2) strain, genetically and antigenically different from the previous vaccine strain (15).
In recent years the need for Public Health to carry out rigorous and repeated studies, to obtain solid estimates of vaccine effectiveness (VE) performed at mid-season “interim” and at the end season, has been highlighted (16-21). Vaccine effectiveness refers to the impact of a vaccine assessed using observational studies (22). Since the 2008/2009 influenza season, in many European countries (8 to 12), including Italy, several studies have been conducted with the Test Negative design (TN) to assess this effectiveness (23-30). Starting from the 2014-2015 season, the Emilia-Romagna Region, with 5 other Italian Regions (Piedmont, Valle D’Aosta, Lombardy, Friuli Venezia Giulia and Puglia), was officially involved in the multicenter case-control observational study “ I-Move ”(Influenza Monitoring Vaccine Effectiveness in Europe) on field effectiveness of influenza vaccines, coordinated by the Istituto Superiore di Sanità (ISS) (31).
During the 2018/2019 season, within the context of integrated virological and epidemiological surveillance coordinated by the ISS and conducted in Emi-lia-Romagna, at the Regional Reference Laboratory of Parma, a test-negative case-control design was established in order to produce seasonal influenza VE estimates, and interim VE estimates.
Methods
During the 2018/2019 influenza season, 31 General Practitioners (GPs) and 18 Pediatricians (P) from the InfluNet network of Emilia-Romagna Region (Bologna, Ferrara, Forlì-Cesena, Modena, Parma, Piacenza and Reggio Emilia) performed nasal or throat swabs on not hospitalized infants, children and adults with ILI (Influenza-like illness). The Care Units of Piacenza, Parma and Reggio Emilia Hospitals performed nasal or throat swabs on patients admitted with influenza-like symptoms and/or severe acute respiratory diseases. According to operative InfluNet protocol (32), for each sample, information on age, sex, vaccination status, presence/absence of chronic diseases, and Care Unit for hospitalized patients, were collected. For data analysis, the subjects were stratified into 4 age groups: 0-4 years, 5-14 years, 15-64 years and >65 years. Laboratory diagnosis was undertaken by using one-step Real Time retro-transcription PCR assay (rRT-PCR), able to detect circulating influenza A and B viruses and subtypes. For rRT-PCR positive samples, influenza viruses were also isolated in MDCK or MDCK-SIAT1 cells (Madin-Darby Canine Kidney), specific for the growth of influenza viruses. Protocols and materials (kits, primers and probes) indicated by the CDC (Centers for Disease Control and Prevention) and WHO (World Health Organization) were used (33). Further strain characterisation was performed by the Reference Laboratory Network of the Italian National Influenza Center (NIC) on a selected number of influenza virus isolates.
Under the TN design, subjects who seek medical care for ILI and tested positive for influenza virus infection were cases, subjects who seek medical care for ILI and tested negative for influenza virus infection were non case/control.
To estimate the VE, the season in study was divided in three periods, considering the peak epidemic period (peak: 5th - 7th week/2019), the previous weeks (pre-peak: 46th week/2018 - 4th week/2019) and the following weeks (post-peak: 8th - 17th week/2019) at the peak period. VE was estimated as (1- ORadj) x 100 with the relative confidence intervals of 95% (95% CI). In particular, were estimated: the seasonal influenza VE (overall) and the VE against subtype A (H1N1)pdm09 and subtype A(H3N2) (adjusted for epidemic period, age group and sex); the interim VE estimates in the three considered periods of the season (adjusted for age group and sex).
The results were summarized in frequency tables and analyzed with the X2 test with Yates continuity correction when necessary. A logistic regression model was used for the calculation of the adjusted VE for sex, age group and epidemic period. All statistical analyses were performed with SPSS 25.0 (IBM SPSS Inc., Chicago – IL).
Results
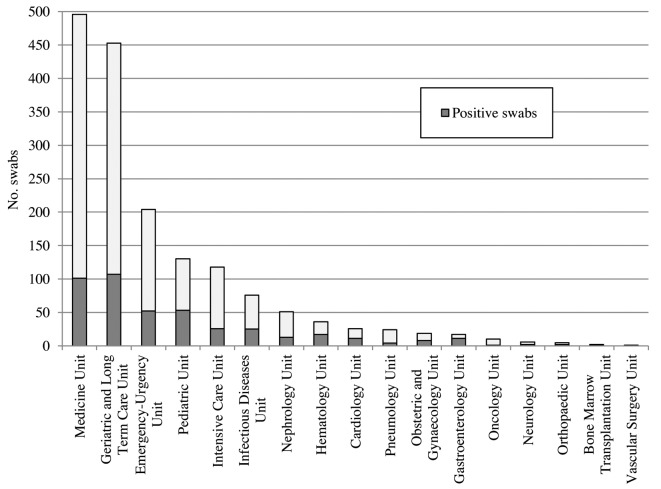
From November 2018 to April 2019, 2,230 specimens were analyzed. 1,674 samples (75.1%) came from hospital Care Units, in particular from the Medicine Unit (29.6%), Geriatrics and Long-term Care Units (27.1%) Emergency-Urgency Unit (12.2%), Pediatrics Unit (7.8%) and Intensive Care Unit (7%) (Figure 1); 556 swabs (24.9%) came from GPs and P of the regional network. Overall, 704 samples were positive (31.6%); 48.6% of swabs performed by sentinel practitioners and 25.9% by hospital Care Units, were positive (Table 1).
Figure 1.
Number of swabs and specimens positive for influenza virus by hospital Care Unit
Table 1.
Characteristics of patients in the 2018/19 influenza-season in Emilia Romagna-Region
| Characteristic | Outpatients No. (%) | Inpatients No. (%) |
| Overall | 556 (24.9) | 1674 (75.1) |
| Province | ||
| Bologna | 41 (7.4) | - |
| Ferrara | 72 (13.0) | - |
| Forli-Cesena | 31 (5.6) | - |
| Modena | 52 (9.3) | - |
| Parma | 284 (51.1) | 1370 (81.8) |
| Piacenza | 29 (5.2) | 200 (11.9) |
| Reggio Emilia | 47 (8.4) | 104 (6.3) |
| Sex | ||
| Female | 245 (44.1) | 872 (52.1) |
| Male | 311 (55.9) | 802 (47.9) |
| Age group (years) | ||
| 0-4 | 194 (34.9) | 85 (5.1) |
| 5-14 | 204 (36.7) | 42 (2.5) |
| 15-64 | 144 (25.9) | 484 (28.9) |
| ≥65 | 14 (2.5) | 1063 (63.5) |
| Vaccination Status | ||
| Unvaccinated | 476 (85.7) | 1048 (62.6) |
| Vaccinated | 77 (13.8) | 448 (26.8) |
| Missing information | 3 (0.5) | 178 (10.6) |
| Target group for vaccination | ||
| No | 477 (85.8) | 296 (17.7) |
| Yes | 79 (14.2) | 1316 (78.6) |
| Missing information | 0 (0) | 62 (3.7) |
| Influenza Laboratory Diagnosis | ||
| Positive | 270 (48.6) | 434 (25.9) |
| Negative | 286 (51.4) | 1240 (74.1) |
| Influenza virus type or subtype | ||
| A(H3N2) | 152 (56.3) | 252 (58.1) |
| A(H1N1)pdm09 | 115 (42.6) | 175 (40.3) |
| A unsubtyped | 3 (1.1) | 6 (1.4) |
| Influenza B | 0 (0) | 1 (0.2) |
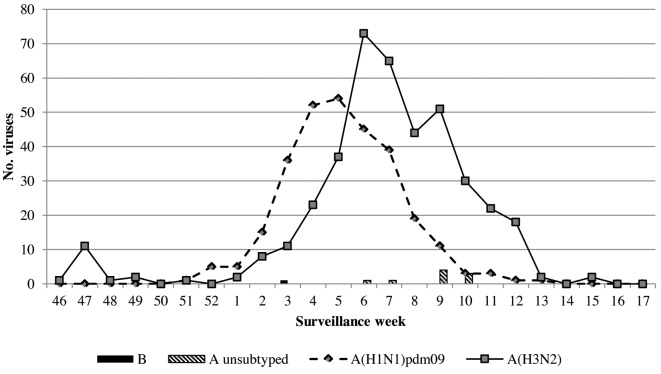
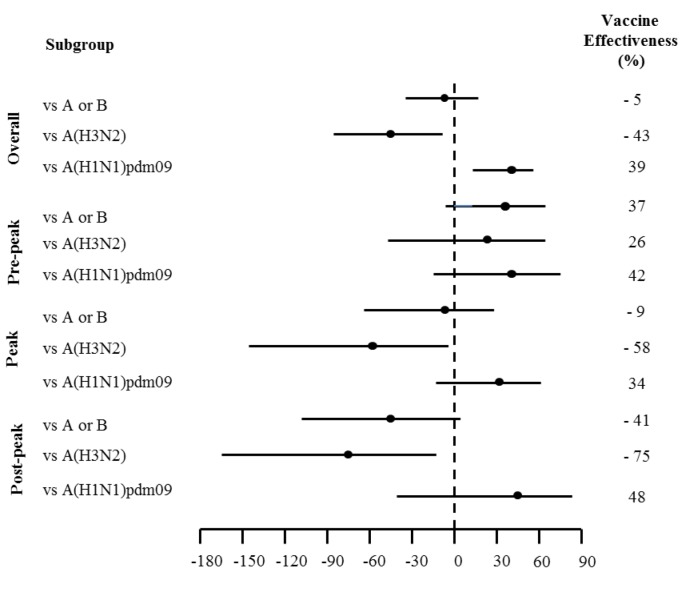
This influenza season was characterized by an initial period of low incidence, until the end of December 2018 and by an intensification of the viral activity at the beginning of the new year, with an incidence rate of 14 cases per 1000 person/years in the 5th week. In 2018/2019 season, the trend of the epidemic showed a peak around the 6th surveillance week. From a virological point of view, the season was characterized by the predominant circulation of influenza type A viruses (99.9%); of these, 57.4% belonged to subtype A(H3N2), 41.2% to subtype A(H1N1)pdm09 and the remaining 1.3% was not subtyped. Within type A, viruses of the two subtypes A(H3N2) and A(H1N1)pdm09 always co-circulated, although the A(H1N1)pdm09 strains were found to be prevalent in the first half of the epidemic season, and the A(H3N2) strains from the second half of February onwards. One virus type B, belonging to the B/Yamagata lineage, was isolated (Figure 2). Subtype A(H3N2) circulated more than subtype A(H1N1)pdm09, both in outpatients (56.3% vs 42.6%) and in inpatients (58.1% vs 40.3%) (Table 1). Although the highest number of swabs was performed on subjects older than 65 years (48.3%), the highest number of positive samples was identified in pediatric ages, 5-14 years (55.3%) and 0-4 years (42.3%). While in the age group 0-4 years, the subtypes A(H3N2) and A(H1N1)pdm09 co-circulated (50% vs 50%), in the classes 5-14 and over 65 years, circulated mainly the A(H3N2) (67.6% vs 30.9% and 69.8% vs 29.4% respectively). Subtype A(H1N1)pdm09, on the other hand, circulated more frequently in the age group 15-64 years (58.5% vs 38.5%). 23.5% of the subjects was vaccinated; considering subjects belonging to the age group greater than 65 years and/or with chronic diseases, for whom vaccination is strongly recommended, 36.6% of these, was vaccinated. According to the indications of Italian Ministry of Health (34), all vaccinated subjects were immunized with a quadrivalent or adjuvated (trivalent) vaccine; 29.1% of these, contracted influenza, and in particular 74.5% were positive for subtype A(H3N2) and 24.8% for subtype A(H1N1)pdm09. The overall VE was -5% (95% CI -33% - 18%) with a decreasing trend during the season: 37% (95% CI -3% - 62%) in the weeks preceding the epidemic peak, -9% (95% CI -63% - 27%) during the peak weeks and -41% (95% CI -109% - 5%) in the post-peak weeks. The overall VE against subtype A(H1N1)pdm09 was 39% (95% CI 11% - 58%) and remained stable during the season. The overall VE against subtype A(H3N2) was -43% (95% CI -89% - -9%), and showed a decreasing trend from values of 26% (95% CI -45% - 62%) at the beginning of the season (pre-peak), to -75% (95% CI - 168 - -15%) in the weeks following the peak period (Figure 3).
Figure 2.
Number of specimens positive for influenza virus, by type or subtype and week of specimen collection
Figure 3.
Adjusted estimates of Influenza Vaccine Effectiveness (VE) against virus type or subtype, overall and stratified according to epidemic period
Molecular and phylogenetic analyses carried out on the HA (Haemagglutinin) gene of A(H3N2) strains (35) circulating in Emilia-Romagna, identified at the beginning of the season, have shown that A(H3N2) viruses were mainly grouped in subclade 3C.2a1b (vaccine reference strain: A/Singapore/INFIMH-16-0019/2016) and, in a small proportion, in subclade 3C.2a2; however, in the following weeks, A(H3N2) viruses belonging to clade 3C.3a started to circulate more widely. Viruses belonging to subclade 3C.3a are defined by the aminoacid substitutions S91N, N144K, F193S and K326R in HA1. Molecular and phylogenetic analyses carried out on the HA gene of A(H1N1)pdm09 strains (35), from January onwards, have shown that they belong to subclade 6B.1A, defined, in HA1, by three additional aminoacid substitutions, S74R, S164T and I295V, compared to the vaccine strain A/Michigan/45/2015. Most of the A(H1N1)pdm09 strains analyzed, present further substitution, S183P, as the new vaccine strain selected for the 2019/2020 season, A/Brisbane/02/2019.
Conclusions
The 2018-2019 influenza season was particularly intense, with a high number of ILI cases and specimens collected, lower only than the 2009-2010 pandemic season. After 2 seasons in which the epidemic peak was anticipated by about 4 weeks, the trend of the epidemic returned to the usual timing, with a peak around the 6th week of surveillance; during this week there was the highest number of swabs performed and viral isolations. In Emilia-Romagna Region A(H1N1)pdm09 and A(H3N2) have co-circulated, with a greater prevalence of the A(H3N2) (57.4% vs 41.2%); the highest number of throat swabs was performed in people over 65 years, most of whom were hospitalized patients with influenza-like symptoms; however, the highest percentage of viral isolation concerned pediatric age groups. The first influenza viruses were identified in hospitalized patients and, only several weeks later, they also appeared in outpatients.
This study has some limitations: although the TN design controls for health care seeking behaviour bias, the VE estimates may not be generalizable to entire population (22). We adjusted the VE estimates for age, sex and epidemic season period. However, for a more correct estimate of the VE, it will be necessary to consider, in the future, also a severity score, based on the clinical symptomatology of the disease for each patient.
Our results, although referring to only one Region, suggest that the 2018/19 seasonal vaccine conferred a moderate protection against influenza viruses. The overall seasonal influenza VE was very moderate and showed a rapid decrease from the start of the season, throughout the peak period, until the end of the season.
A good VE against A(H1N1)pdm09 with stable trend in the 3 different periods of the season and a lack of protection against A(H3N2), due to antigenic and genetic mismatch between circulating A(H3N2) and the respective 2018/19 vaccine strain, were observed.
These results reflect what has been observed at national level and in most European Countries, and confirm a wide circulation of A(H3N2) variants antigenically distinct from the vaccine virus A/Singapore/INFIMH-16-0019/2016 (36). Phylogenetic analyses carried out in our Laboratory and at the NIC of the ISS, relating to the HA gene of a selection of viruses of subtype A(H3N2) isolated in Parma, have shown how, while in the first part of the season have circulated strains similar to the vaccine, with a moderate value of VE (26%), in the middle weeks of the season began to circulate in Parma, as well as Italy and in other parts of the world (35, 36), strains belonging to different genetic subgroups, and in particular to the subclade 3C.3a (reference strain: A/Kansas/14/2017) recently indicated by the WHO as an A(H3N2) component for the 2019/2020 influenza vaccine in the Northern Hemisphere.
In Italy, since the start of this influenza season, 8,104.000 cases of influenza syndrome have been reported; 809 severe cases of confirmed influenza have been reported in subjects with SARI (Severe Acute Respiratory Infection) and/or ARDS (Acute Respiratory Distress Syndrome) admitted to Intensive Care Units; among these, 198 died. In Emilia-Romagna Region, 72 severe cases of confirmed influenza and 53 deaths were reported (37).
The contribution given by Virological and Epidemiological Surveillance Programmes allows the correct identification of any variations (minor and major) in the circulating strains and, therefore, the preparation of more targeted vaccines, the effectiveness of which derives from the correct alignment between circulating viruses and antigens contained in the vaccine. The viruses characterization complemented with other available epidemiological and disease information, form the evidence base for Public Health decisions on epidemic response and pandemic preparedness, including seasonal vaccine virus selection and zoonotic influenza candidate vaccine virus development (38). Moreover, the timely identification of sick people and their contacts could contain the epidemic, at local level, and direct GPs and P towards more targeted therapies, reducing the risk of evolution in complicated cases, hospitalizations and deaths; in closed communities, and in inpatients it could reduce the risk of infections related to care (Healthcare Associated Infection), especially in subjects at risk for age or chronic disease.
The possibility to make accurate and continuous effectiveness estimates during the season, thanks to the availability of an acquired methodology based on the integration of virological and epidemiological data, combined with sensitive and standardized molecular biology methods, will help to better define the composition of the vaccine for the following season.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.World Health Organization (WHO) Global Influenza Programme. A Manual for Estimating Disease Burden Associated With Seasonal Influenza. Sept 2015 [Google Scholar]
- 2.European Center for Disease Control (ECDC) Influenza remains a threat. Available from: https://ecdc.europa.eu/en/seasonal-influenza/facts/key-messages) (last accessed on 12 June 2019) [Google Scholar]
- 3.Smetana J, Chlibek R, Shaw J, Splino M, Prymula R. Influenza vaccination in the elderly. Hum Vaccin Immunother. 2018 Mar 4;14(3):540–549. doi: 10.1080/21645515.2017.1343226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Circolare n.9/2018. Prevenzione e controllo dell’influenza. Raccomandazioni per la stagione 2018/2019. Available from: http://salute.regione.emilia-romagna.it/documentazione/materiale-informativo/schede-informative/vaccinazione-influenza . [Google Scholar]
- 5.CDC. Estimated influenza illnesses, medical visits, and hospitalizations averted by vaccination. Available from: https://www.cdc.gov/flu/about/disease/burden-averted-vaccination.htm. (last accessed on 12 June 2019) [Google Scholar]
- 6.Rolfes MA, Flannery B, Chung JR, et al. Effects of Influenza Vaccination in the United States During the 2017–2018 Influenza Season. Clin Infect Dis. doi: 10.1093/cid/ciz075. Published online: 02 February 2019. https://doi.org/10.1093/cid/ciz075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito S, Principi N. European Society of Clinical Microbiology Infectious Diseases (ESCMID) Vaccine Study Group (EVASG). Influenza vaccination and prevention of antimicrobial resistance. Expert Rev Vaccines. 2018 Oct;17(10):881–888. doi: 10.1080/14760584.2018.1525298. [DOI] [PubMed] [Google Scholar]
- 8.Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018 Jun 22;36(27):3960–3966. doi: 10.1016/j.vaccine.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Lai PL, Panatto D, Ansaldi F, et al. Burden of the 1999-2008 seasonal influenza epidemics in Italy: comparison with the H1N1v (A/California/07/09) pandemic. Hum Vaccin. 2011 Jan-Feb;(7 Suppl):217–2514. doi: 10.4161/hv.7.0.14607. [DOI] [PubMed] [Google Scholar]
- 10.Plans-Rubió P. Prevention and control of influenza in persons with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(1):41–53. doi: 10.2147/copd.2007.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichol KL. Influenza vaccination in the elderly: impact on hospitalisation and mortality. Drugs Aging. 2005;22(6):495–515. doi: 10.2165/00002512-200522060-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini R, Pozzi T, Bonanni P, Fragapane E, Montomoli E, Lucioni C. Valutazione dei costi di un’epidemia influenzale nella popolazione lavorativa di Siena. Giornale di Farmacoeconomia. 2000;4(13):3–9. [Google Scholar]
- 13.Nichol KL, D’Heilly SJ, Greenberg ME, Ehlinger E. Burden of influenza-like illness and effectiveness of influenza vaccination among working adults aged 50-64 years. Clin Infect Dis. 2009 Feb 1;48(3):292–817. doi: 10.1086/595842. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Available from: https://www.who.int/influenza/vaccines/virus/recommendations/201902_qanda_recommendation_ah3n2.pdf?ua=1. (last accessed on 12 June 2019) [Google Scholar]
- 15.World Health Organization (WHO) Available from: https://www.who.int/influenza/vaccines/virus/recommendations/201902_recommendation_addendum.pdf?ua=1. (last accessed on 12 June 2019) [Google Scholar]
- 16.Kissling E, Rose A, Emborg HD, et al. European IVE Group. Interim 2018/19 influenza vaccine effectiveness: six European studies, October 2018 to January 2019. Euro Surveill. 2019 Feb;24(8) doi: 10.2807/1560-7917.ES.2019.24.1900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flannery B, Chung JR, Belongia E, et al. Interim Estimates of 2017-18 Seasonal Influenza Vaccine Effectiveness - United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018 Feb 16;67(6):180–185. doi: 10.15585/mmwr.mm6706a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skowronski DM, Chambers C, De Serres G, et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill. 2018 Feb;23(5) doi: 10.2807/1560-7917.ES.2018.23.5.18-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan SG, Chilver MB, Carville KS, et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro Surveill. 2017 Oct;22(43) doi: 10.2807/1560-7917.ES.2017.22.43.17-00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiménez-Jorge S, Pozo F, Larrauri A. cycEVA Study Team. Interim influenza vaccine effectiveness: A good proxy for final estimates in Spain in the seasons 2010-2014. Vaccine. 2015 Jun 26;33(29):3276–80. doi: 10.1016/j.vaccine.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Skowronski DM, Janjua NZ, De Serres G, et al. Interim estimates of influenza vaccine effectiveness in 2012/13 from Canada’s sentinel surveillance network, January 2013. Euro Surveill. 2013 Jan 31;18(5) doi: 10.2807/ese.18.05.20394-en. [DOI] [PubMed] [Google Scholar]
- 22.Ainslie KEC, Haber M, Orenstein WA. Challenges in estimating influenza vaccine effectiveness. Expert Rev Vaccines. 2019 Jun;18(6):615–628. doi: 10.1080/14760584.2019.1622419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenciano M, Ciancio B, Moren A. First steps in the design of a system to monitor vaccine effectiveness during seasonal and pandemic influenza in EU/EEA Member States. Euro Surveill. 2008 Oct;13(43) doi: 10.2807/ese.13.43.19015-en. [DOI] [PubMed] [Google Scholar]
- 24.ECDC. Stockholm: European Centre for Disease Prevention and Control; 2010. Protocol for case control studies to measure pandemic and seasonal vaccine effectiveness in the European Union and European Economic Area. Availble from: http://www.ecdc.europa.eu/en/publications/Publications/0907TEDInfluenzaAH1N1MeasuringInfluenzaVaccineEffectivenessProtocolCaseControlStudies.pdf. (last accessed on 12 June 2019) [Google Scholar]
- 25.Kissling E, Valenciano M, Falcao J, et al. “I-MOVE” towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multicentric case-control study in Europe, 2008-9. Euro Surveill. 2009;14(44) [PubMed] [Google Scholar]
- 26.Savulescu C, Valenciano M, De Mateo S, Larrauri A. Estimating the influenza vaccine effectiveness in elderly on a yearly basis using the Spanish influenza surveillance network-Pilot case-control studies using different control groups 2008–2009 season, Spain. Vaccine. 2010 Feb doi: 10.1016/j.vaccine.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 27.Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine. 2010;28(46):7381–7388. doi: 10.1016/j.vaccine.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Valenciano M, Kissling E, Team I. Early estimates of seasonal influenza vac-cine effectiveness in Europe: results from the I-MOVE multicentre case–control study, 2012/13. Euro Surveill. 2013;18(7):3. [PubMed] [Google Scholar]
- 29.Kissling E, Valenciano M, Larrauri A, Oroszi B, Cohen JM, Nunes B, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case–control study. Euro Surveill. 2013;18(5) doi: 10.2807/ese.18.05.20390-en. [DOI] [PubMed] [Google Scholar]
- 30.Van Doorn E, Darvishian M, Dijkstra F, et al. Influenza vaccine effectiveness estimates in the Dutch population from 2003 to 2014: The test-negative design case-control study with different control groups. Vaccine. 2017 May 15;35(21):2831–2839. doi: 10.1016/j.vaccine.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzo C, Bella A, Alfonsi V, et al. Influenza vaccine effectiveness in Italy: Age, subtype-specific and vaccine type estimates 2014/15 season. Vaccine. 2016 Jun 8;34(27):3102–8. doi: 10.1016/j.vaccine.2016.04.072. [DOI] [PubMed] [Google Scholar]
- 32.Sorveglianza Epidemiologica e Virologica dell’Influenza. Protocollo Operativo influNet - Stagione 2018/2019. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2786_allegato.pdf . [Google Scholar]
- 33.World Health Organization (WHO) Manual for the laboratory diagnosis and virological surveillance of Influenza. 2011 [Google Scholar]
- 34.Italian Ministry of Health. Flu season 2018/2019. Available from: http://www.salute.gov.it/portale/influenza/homeInfluenza.jsp. (last accessed on 12 June 2019) [Google Scholar]
- 35.InfluNet: Sorveglianza Virologica. Report n. 24 - 02/05/2019. Available from: http://old.iss.it/binary/fluv/cont/Agg.Vir_02_05_19.pdf . [Google Scholar]
- 36.European Center for Disease Control (ECDC) Influenza virus characterisation. Summary Europe, April 2019. Available from: https://www.ecdc.europa.eu/sites/portal/files/documents/influenza-virus-characterisation-april-2019.pdf . [Google Scholar]
- 37.EpiCentro - ISS. FluNews Italia. Influenza Integrated Surveillance Report. Available from: https://www.epicentro.iss.it/influenza/FluNews#casi. (last accessed on 12 June 2019) [Google Scholar]
- 38.Chiapponi C, Ebranati E, Pariani E, et al. Genetic analysis of human and swine influenza. A viruses isolated in Northern Italy during 2010-2015. Zoonoses Public Health. 2018 Feb;65(1):114–123. doi: 10.1111/zph.12378. [DOI] [PubMed] [Google Scholar]