Abstract
Background and aims: Incidence of leprosy in Italy has declined steadily over the last century, but available evidence remains fragmentary. Our review aims to summarize available data on the epidemiology of leprosy cases in Italy. Methods: The following keywords were used to explore PubMed and Embase: leprosy, Hansen’s disease, (Mycobacterium) leprae, Italy, without any chronological restriction. Results: We identified a total of 39 reports, including 7 national reports, 11 international reports, 20 case reports. Notified leprosy cases were: 839 between 1925 and 1948; 434 between 1955 and 1979; 76 cases for the decade 1980-1989; 112 between 1990 and 1999; 62 between 2000 and 2009, and a total of 25 cases since 2009. Since 2003, 53% of all cases occurred in illegal residents. Focusing on individual cases, latency between early signs/symptoms and a proper diagnosis ranged between 2 and 20 years in 52.1% of individual cases. Conclusion: Imported cases of leprosy are responsible for most leprosy incidence in Italy, and social stigma, the unfamiliarity of healthcare professionals with such disorders, and difficulties of some high-risk groups to be appropriately assessed hint to a possible under-diagnosis. Professionals should be made more aware of the potential for leprosy incidence among patients from countries where the disease is endemic. (www.actabiomedica.it)
Keywords: emigration and immigration, incidence, leprosy/epidemiology, retrospective studies, Italy/epidemiology
Introduction
Leprosy is an ancient illness caused by Mycobacterium leprae that mainly affects skin, peripheral nerves and upper respiratory tract (1). Following the introduction of multidrug treatment during the mid-1980s, global prevalence has decreased by over 90%, from 5.3 million cases in 1985 to around 192,713 cases at the end of 2017 (1-3). However, leprosy remains far from being eradicated (4,5). For instance, official statistics show that new cases increased worldwide from 210,758 in 2015 to 214,783 in 2016, and again in 2017 around 210,000 cases were reported from 150 countries (i.e. notification rate of 2.77/100,000 inhabitants) (2,6,7). As the surveillance systems from low-income countries are reportedly affected by significant inaccuracies, even in endemic areas, available figures are supposedly affected by significant underreporting (3,8).
Leprosy began being endemic in Italy during the early Roman Empire (4,9-11), but official epidemiological surveillance data have suggested a steady decrease during the last century, with autochthonous cases progressively disappearing during last decades (9,11,12); current epidemiology is affected by significant uncertainties and conflicting estimates (5,9). In Italy newly detected leprosy cases should be statutorily reported to the Ministry of Health, and a National Leprosy Register has been officially existing since 1923. Even though the last official report was published in 1983 (9), reports following the ongoing migratory crisis have underlined the possibility of reintroduction of leprosy from endemic areas, with a significant share of missed or late diagnoses (5,13).
In order to further understand the actual epidemiology of leprosy in Italy, we conducted this comprehensive literature review addressing all available evidence on Hansen’s disease, specifically focusing on cases occurring after 1983.
Materials and Methods
This systematic review has been conducted following the PRISMA (Prepared Items for Systematic Reviews and Meta-Analysis) guidelines (14). We searched into two different databases (PubMed and Embase) for relevant studies published from their inception to 31/05/2019, without any chronological restriction. The search strategy was a combination of the following keywords (free text and Medical Subject Heading [MeSH] terms): leprosy, Hansen’s disease, (Mycobacterium) leprae, Italy. Records were handled using a references management software (Mendeley Desktop Version 1.19.5, Mendeley Ltd 2019), and duplicates were removed.
Articles eligible for review were original research publications available online or through inter-library loan. Articles had to be written in Italian, English, German, French or Spanish, the languages spoken by the investigators. Studies included were national and international reports, case studies, cohort studies, case-control studies and cross-sectional studies, case reports. Only articles reporting data from Italy, with relevant information on the prevalence of Hansen’s disease were eligible for the full review. Articles were excluded if: (1) full text was not available; (2) articles were written in a language not understood by reviewers; (3) reports lacked significant timeframe (i.e. the year of diagnosis) and demographic data (i.e. sex, age, country of origin of the patient, etc.).
Two independent researchers reviewed titles, abstracts, and articles. Titles were screened for relevance to the subject of Hansen’s disease. Any articles reporting original studies with information on leprosy in Italy, which did not meet one or more of the exclusion criteria, were retained for full-text review. The investigators independently read full-text versions of eligible articles. Disagreements were resolved by consensus between the two reviewers; where they did not reach consensus, input from a third investigator (MR) was obtained. Further studies were retrieved from reference lists of relevant articles and consultation with experts in the field.
Data abstracted included:
Reports on incidence/prevalence: year of publication; level (i.e. all cases or selected risk groups); timeframe; the number of prevalent cases for index year; the number of incident cases; age at diagnosis; sex ratio; share of foreign-born people; share of lepromatous, tuberculoid or borderline cases.
Case reports: year of publication; year of diagnosis; age at diagnosis; sex; country of origin; clinical characteristics following Ridley and Jopling classification (15); multibacillary vs. paucibacillary status; latency; individual risk factors (i.e. stay in high-risk areas; HIV+ status; refugee status; adopted status from high-risk areas). When Ridley and Jopling’s classification was not openly reported, it was retrospectively defined by analysis of reported data.
Results
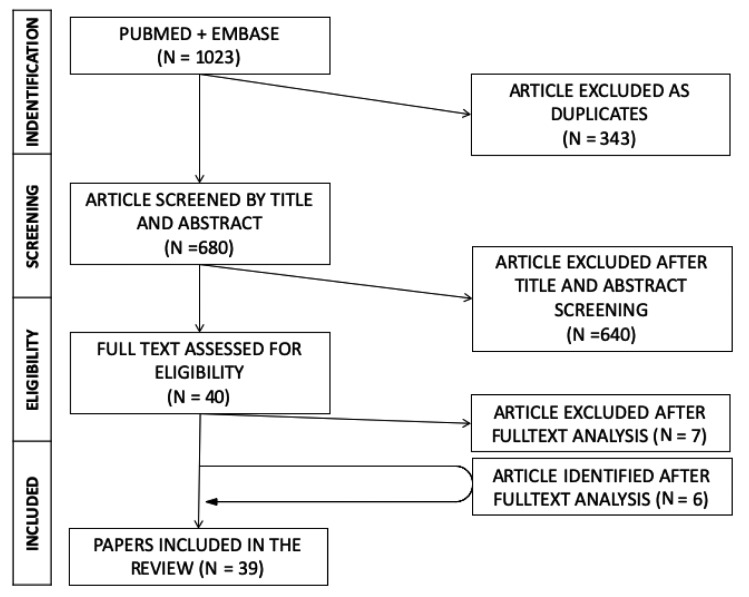
Briefly (Figure 1), a total of 1023 articles were initially identified. After removal of duplicates, titles of 680 remaining articles were screened, identifying a total of 33 publications, and 6 further reports were added following full-text analysis. Eventually, 39 publications were retrieved, encompassing: 8 nationwide reports, 11 international reports (2,5-7,9,11-12,16-27) (Table 1) and 20 case reports (13,28-46) (Table 2).
Figure 1.
Flow of studies through the review
Table 1.
Summary of the available reports on prevalence and incidence data for Leprosy in Italy (1925 - 2019). Notes: * = year of data analysis; FBP = foreign-born people; MB = multibacillary
| Incidence Data | Prevalence Data | |||||||||||||
| Reference | Level | Timeframe | All notified cases | (No.) cases by year | Year* | Prevalent cases (No.) | Age at diagnosis (mean) | Males (%) | FBP (%) | Lepromatous (%) | Tuberculoid (%) | Borderline (%) | Indefinite (%) | MB (%) |
| Greco D, Galanti MR (9) | Nationwide, all cases | 1920-1980 | 734 | 12.3 | 1979 | 547 | 37.8 | 60.0% | 17.2% | 62.2% | 15.4% | 9.5% | 2.9% | - |
| Greco D, Galanti MR, Moro ML (12) | Nationwide, all cases | 1920-1980 | 678 | 11.1 | 1980 | 547 | 37.7 | 59.9% | 19.3% | 69.3% | 10.4% | 10.4% | 3.2% | - |
| National Health Institute (24) | Nationwide, all cases | 1955-1979 | 424 | 17.4 | 1981 | 543 | - | 61.1% | 18.8% | 73.6% | 20.2% | 6.4% | - | - |
| Terni M, Signorini FL (11) | Nationwide, all cases | 1925-1948 | 839 | 35.0 | 1947 | 364 | - | 59.0% | 23.1% | - | - | - | - | - |
| Massone C et al. (5) | Nationwide, Only FBP | 2003-2009 | 59 | 9.8 | 2011 | 59 | 30.2 | 69.5% | 100% | 27.1% | 3.4% | 69.5% | - | 51% |
| AIFO 2007 (25) | Nationwide, all cases | 1970-2006 | 351 | 9.5 | 2007 | 351 | - | - | 47.0% | - | - | - | - | - |
| AIFO 2006 (26) | Nationwide, all cases | 1970-2004 | 337 | 9.6 | 2006 | 337 | 36.5 | 64.0% | 44.8% | - | - | - | - | - |
| ISTAT (27) | Nationwide, all cases | 1992-2001 | 23 | 2.3 | - | - | - | - | - | - | - | - | - | - |
| WHO (2,6,23,7,16-22) | Nationawide, all cases | 2005-2017 | 27 | 2.1 | - | - | - | - | - | - | - | - | - | - |
Table 2.
Summary of single cases reported from Italy (1925 - 2019). Notes. * = year of actual diagnosis; BB = borderline borderline; TT = tuberculoid leprosy; TB = tuberculoid borderline; LL = lepromatous leprosy; LB = lepromatous borderline; N/A not specified; MB = multibacillary; IBP = Italian Born People; FBP = Foreign Born People
| Reference | Year* | Age (years) | Gender | Country of origin | Familiarity | Diagnosis | MB | Latency (years) | Risk group |
| Fiallo P et al. (33) | 1992 | 38 | M | Italy | N | TB | - | 2 | Long stay, high-risk area |
| Passarini B et al (34) | 2001 | 52 | M | Italy | N | LL | - | 2 | - |
| Visco-Comandini U et al. (35) | 2004 | 32 | M | Brazil | N/A | TT | - | 2 | HIV positive |
| Mozzillo R et al. (36) | 2006 | 68 | M | Italy | N/A | LL | - | 15 | Long stay, high-risk area |
| Zammarchi L et al. (13) | 2006- | 29 | M | Philippines | Y | TT | - | 14 | - |
| 2010 | 49 | M | Sudan | N/A | LL | - | N/A | Refugee | |
| Bongiorno MR et al. (32) | 2008 | 43 | M | Italy | N | LL | X | 3 | Long stay, high-risk area |
| Fiallo P et al. (31) | 2008 | 51 | W | Philippines | N/A | TT | - | 3 | Long stay, high-risk area |
| Filippetti R et al. (30) | 2008 | 30 | W | Italy | N | BB | - | < 1 | Long stay, high-risk area |
| Aridon P et al. (29) | 2009 | 15 | M | Senegal | N/A | TT | - | < 1 | Refugee |
| Rongioletti F et al. (46) | 2009 | 43 | W | Brazil | Y | BB | X | 1.5 | - |
| Giacomet V et al. (45) | 2010 | 14 | M | Brazil | N/A | LL | - | < 1 | Adopted |
| Massone C et al. (44) | 2010 | 28 | M | Nigeria | N/A | LL | - | N/A | Refugee |
| 2010 | 22 | M | Columbia | N/A | BB | - | N/A | - | |
| 2010 | 14 | M | Brazil | N/A | BB | - | 1 | Adopted | |
| Simeoni S et al. (43) | 2011 | 20 | M | India | Y | LL | X | 4 | - |
| Piras AM et al. (42) | 2011 | 26 | M | Nigeria | N/A | BB | - | N/A | Refugee |
| Massone C et al. (41) | 2012 | 77 | M | Italy | N | LB | - | 20 | - |
| Liguori R et al. (40) | 2015 | 59 | M | Italy | N | TT | - | 2 | - |
| Maritati M, Contini C (39) | 2015 | 22 | M | Ghana | N/A | BB | - | N/A | Refugee |
| Marotta M et al. (28) | 2017 | 29 | M | Nigeria | N | LL | X | 3 | Refugee |
| Cusini M et al. (37) | 2017 | 75 | M | Italy | N | LB | - | 2 | - |
| Beltrame A at al. (38) | 2017 | 78 | M | Italy | N/A | TT | - | 4 | Missionary, high risk area |
| Summary | Tot = 23 | 39.7 y ± 20.6 | M: 20, 87.0% F: 3, 13.0% | IBP: 8, 34.8% FBP: 15, 65.2% | Y: 3, 13.0% N: 8, 34.8% N/A: 12, 52.2% |
TT: 6, 26.1% TB: 1, 4.3% BB: 6, 26.1% LB: 2, 8.6% LL: 8, 34.8 % |
4, 17.4% | N/A: 5, 21.7% < 1y: 3, 13.0% 2y: 4, 17.4% 3y: 3, 13.0% 4y: 2, 8.7% ≥5y: 3, 13.0% | None: 8, 34.8% Refugee: 6, 26.1% Adopted: 2, 8.7% Long stay, high-risk area: 5, 21.7% Missionary, high-risk area: 1, 4.3% HIV positive: 1, 4.3% |
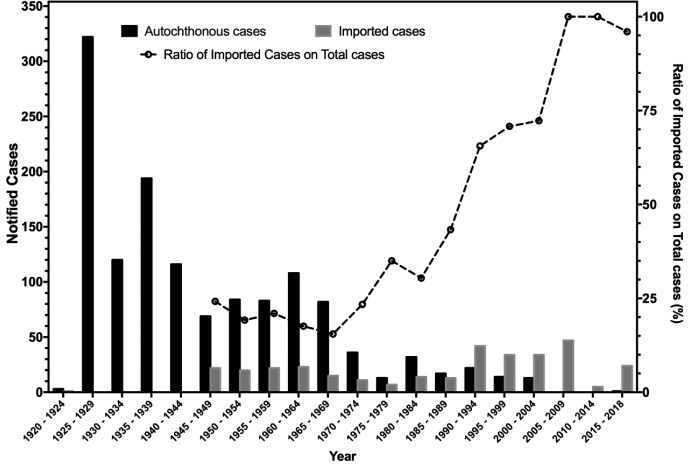
Despite some inconsistencies for the years from 1920 to 1948, available data suggest that a total of 1658 leprosy cases have been notified between 1920 and 2018 (i.e. 16.7 cases/year). The number of reported cases decreased from 847 for the time period 1920 - 1949 (i.e. 28.2 cases/year), to 437 between 1950 and 1979 (14.6 cases/year), and eventually 307 between 1980 and 2018 (i.e. 6.3 cases/year) (9,11,12,24), with corresponding incidence rates ranging from 0.005 in 1979 to 0.25 in 1961 (9,11,24) (Figure 2).
Figure 2.
Epidemic curve of notification of leprosy cases in Italy by year and source of infection, 1920 - 2018 (2,5,19-27,6,7,9,11,12,16-18)
Interestingly enough, after 1980 no official reports from National Health authorities are available. While a report of the National Institute for Statistics hints to a total of 23 new cases (mean: 2.3, range 0 to 9) between 1992 and 2001 (27), analysis of data published by the Italian Society for Hansenology (SIHAN) suggests a mean of 9.5-9.8 cases/year between 1970 and 2006, and more precisely 76 cases for the decade 1980-1989 (mean: 7.6, range 2 - 10), 112 new diagnoses for 1990-1999 (mean: 11.2, range 6 - 17), and 62 new cases between 2000 and 2009 (mean: 4.9, range 0 - 14) (5,25,26). Since 2010, a total of 25 cases have been officially reported to the World Health Organization (mean: 2.1, range 0 - 12) (2,6,23,7,16-22), including 5 cases notified during 2018 (personal communication of the National Health Ministry). However, available data are heavily fragmentary. In fact, while no cases were officially reported between 2005 and 2006, 2008 and 2009, and then between 2011 and 2015, analysis of case reports that occurred during the index years suggests that such figure may be affected by significant underreporting (29,31,39-41,43,45).
Demographics of patients significantly changed over the years. While the majority of cases were consistently reported in males, ranging from 59.0% for 1925-1948 (11) and 69.5% in the report of Massone et al. (5), their mean age decreased from 37.7-37.8 years (1920 to 1980 timeframe) (9,12) and 36.5 years (1970-2004) (26) to 30.2 years (5). Such a trend possibly mirrored the increased prevalence of foreign-born patients, whose share increased from 17.8% of earlier reports to the 47.8% of the nationwide estimates 1970-2007 (5,25,26). After 2003, not only 67.0% to nearly all reported cases were identified among people having a migration background (91.8% of all case reports for the same timeframe), but a report from Massone et al. suggests that the majority of cases (i.e. 53%) occurred in illegal residents, with 28.6% of individual cases in refugees (26.1% in total) (5). Not coincidentally, analysis of individual cases reported between 1992 and 2017 identified an older mean age (39.7 years ± 20.6), and such figures included a significant share of Italian-born cases (34.8%), whose mean age was 65.0 years (range 30 - 78) (30,32-34,36-38,40,41) (Table 2).
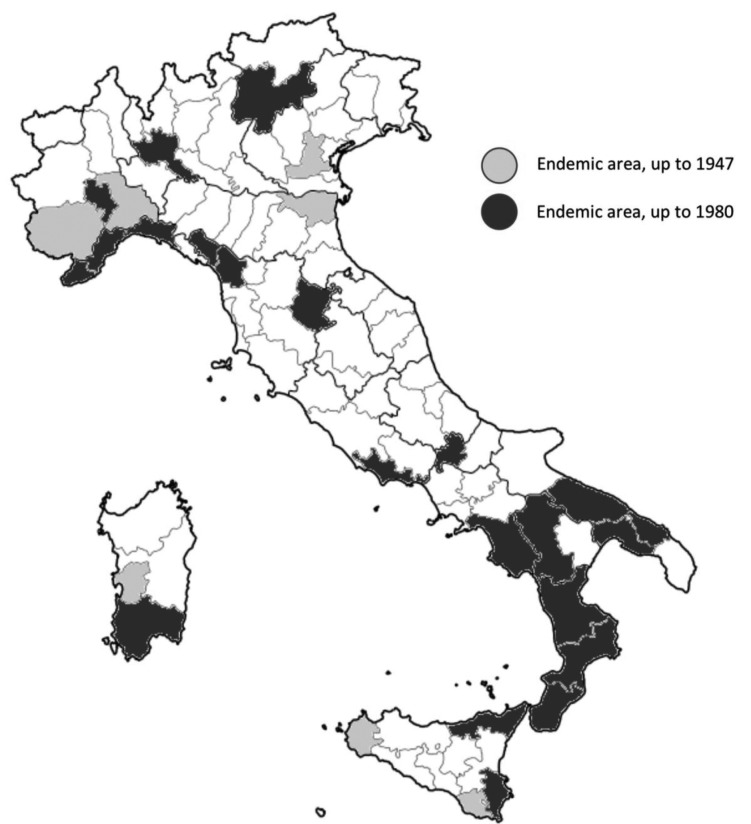
In other words, after 2003 autochthonous cases occurred only in subjects who had spent abroad significant time in high-risk areas, either as adopted children, expatriate or missionaries, or who had been presumptively infected several decades ago, when leprosy was still endemic (32-34,36-38,40,41). As a consequence, while endemic areas were initially scattered across the Italian peninsula, having their roots in the medieval outbreaks of the Hansen’s disease, in 1980 circulation remained significant only in Northern Tuscany, Eastern Sicily, Calabria, Puglia and Liguria, and new areas (e.g. metropolitan area of Milan) emerged as a consequence of imported cases from high risk countries (Figure 3).
Figure 3.
Distribution of leprosy cases in Italy, 1947 and in 1980 (9,11)
On this regard, also the geographical origin of patients has radically changed: while in earlier reports the largest proportion of cases had a South American origin (36.1% for 1920 - 1980), last decades were characterized by a raising share of cases from Asian (Bangladesh, India, Pakistan, Philippines, Sri Lanka) and African countries (Cameron, Egypt, Nigeria, Senegal, Sierra Leone, Sudan, Tanzania) (5,9,12).
The changing demographics of leprosy was associated with a main shift in the clinical characteristics: while up to 1980 the majority of patients were lepromatous ones, borderline leprosy is nowadays the most frequently reported (5,9,11), with a share of highly infectious multibacillary leprosy ranging from 17.4% to 51.4% (5,28,32,43,46). Even in individual reports, not only borderline-borderline leprosy accounted for 26.1% cases, but considering also borderline-tuberculoid and borderline-lepromatous the total share climbed to 39.0%. However, as the Ridley and Jopling classification was introduced only in 1966, historical comparisons should be cautiously performed (15).
Accurate data on latency were retrieved by individual cases, and 52.2% of them reported a delay between earlier symptoms and final diagnosis that ranged between 2 and 20 years, even for multibacillary ones (28,32,43,46).
Discussion
Our comprehensive review suggests that leprosy, once endemic in Italy, is nowadays a sporadically reported disease, that mainly affects subjects who were born or who spent several years in high-risk areas. Despite the potential public health relevance, our results should be carefully interpreted for several reasons.
Firstly, not only more recent reports are of heterogeneous quality and apparently inconsistent, but most of the available data have been only partially published in grey literature, without any external validation (25,26). In fact, a comparison of official data with available reports suggests that a significant share of cases has remained unknown to the National Authorities (28,37,38).
Secondly, actual figures for Hansen’s disease are intrinsically inaccurate (3): not only a diagnosis of leprosy is generally difficult in initial stages, but the interplay between social and religious stigma, lack of access to appropriate healthcare services, unfamiliarity of Western medical professionals with a rare disease, diffusely hinder or at least delay appropriate diagnosis and treatment (4,5). Actually, the majority of individual cases we collected were appropriately diagnosed and treated only after several years (29,31,39-41,43,45). As accurate data collection on index cases was irregularly reported, and some of such patients are possibly unknown to the National Registry, we may guess whether the collection of personal history, analysis of familiarity, and identification of possible contacts had been appropriately performed (28,37,38). As a consequence, it is reasonable that a significant number of contact cases still remains unnoticed. More precisely as many refugees and illegal migrants actually come from highly endemicity areas, being frequently forced to living environments that facilitate the spreading of pathogens as M. leprae, it is possible that the ratio between notified cases and actual cases may range between 2 and 10 to 1, with around 40 to 50 new cases by year (2,5,22,23,47,6,7,16-21).
Third, it should be stressed that evidence drawn from individual reports is inherently biased, as cases characterized by a difficult diagnosis, or severe clinical involvement, are more likely to be published. In other words, the alarming share of patients who have received a very late diagnosis, even in multibacillary leprosy, may be largely overestimated (4,5).
Conclusions
In summary, our data reflect the need and importance of shedding light on this ancient but not vanished disease. As knowledge gaps of medical professionals may contribute to the unsatisfactory reporting rates we identified, teaching programs for medical specialties more likely to get in touch with possible cases (i.e. not only dermatologists and neurologists, but also general practitioners, pediatricians, and occupational physicians) are highly in need (29,44,45,48-50). Similarly, paramedical and social professionals that may interact with cases occurring in migrants and refugees should recall that a leprosy case remains possible even in the 21st century, addressing the suspected cases to an appropriate medical referral as soon as possible (2,4,5). As leprosy is a treatable infectious disease, and an untreated multibacillary patient can release more than 10,000,000 bacilli per day, which can survive for 4-5 weeks in the Italian climate, early identification and treatment of new cases is a public health priority that should not be forgotten.
Disclosures.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. Ethics approval was not required for this review. The facts, conclusions, and opinions stated in the article represent the authors’ research, conclusions, and opinions and are believed to be substantiated, accurate, valid, and reliable. However, as this article includes the results of personal researches of the Authors, presenting correspondent, personal conclusions and opinions, parent employers are not forced in any way to endorse or share its content and its potential implications.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Norman FF, Fanciulli C, Pérez-Molina JA, Monge-Maillo B, López-Vélez R. Imported and autochthonous leprosy presenting in Madrid (1989-2015): A case series and review of the literature. Travel Med Infect Dis. 2016;14(4):331–349. doi: 10.1016/j.tmaid.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Global leprosy update, 2017: reducing the disease burden due to leprosy. Wkly Epidemiol Rec. 2018;93(35):445–456. [Google Scholar]
- 3.Salgado CG, Barreto JG, da Silva MB, Goulart IMB, Barreto JA, de Medeiros Junior NF, et al. Are leprosy case numbers reliable. Lancet Infect Dis. 2018;18(2):135–137. doi: 10.1016/S1473-3099(18)30012-4. [DOI] [PubMed] [Google Scholar]
- 4.Schreuder PAM, Noto S, Richardus JH. Epidemiologic trends of leprosy for the 21st century. Clin Dermatol. 2016;34(1):24–31. doi: 10.1016/j.clindermatol.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Massone C, Brunasso AMG, Noto S, Campbell TM, Clapasson A, Nunzi E. Imported leprosy in Italy. J Eur Acad Dermatology Venereol. 2012;26(8):999–1006. doi: 10.1111/j.1468-3083.2011.04201.x. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global leprosy update, 2016: accelerating reduction of disease burden. Wkly Epidemiol Rec. 2017;92(35):501–520. [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) Global leprosy update, 2015: time for action, accountability and inclusion. World Heal Organ Wkly Epidemiol Rec. 2016;91(35):405–420. [PubMed] [Google Scholar]
- 8.Abeje T, Negera E, Kebede E, Hailu T, Hassen I, Lema T, et al. Performance of general health workers in leprosy control activities at public health facilities in Amhara and Oromia States, Ethiopia. BMC Health Serv Res. 2016;16(1):122. doi: 10.1186/s12913-016-1329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greco D, Galanti MR. Leprosy in Italy. Int J Lepr Other Mycobact Dis. 1983;51(4):495–499. [PubMed] [Google Scholar]
- 10.Traversa E. L’etat actuel de lutte contre la lepre en italie. Int J Lepr. 1953;21(4):463–465. [PubMed] [Google Scholar]
- 11.Terni M, Signorini FL. The present situation of leprosy in Italy. Int J Lepr. 1950;18(4):519–523. [PubMed] [Google Scholar]
- 12.Greco D, Galanti MR, Moro ML. La lebbra oggi in Italia. Epidemiol Prev. 1984;21/22:19–24. [Google Scholar]
- 13.Zammarchi L, Vellere I, Stella L, Bartalesi F, Strohmeyer M, Bartoloni A. Spectrum and burden of neglected tropical diseases observed in an infectious and tropical diseases unit in Florence, Italy (2000-2015) Intern Emerg Med. 2017;12(4):467–477. doi: 10.1007/s11739-016-1597-1. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e100097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridley DS, Jopling WH. Classification of Leprosy According to Immunity. Int J Lepr. 1966;34(3):255–73. [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Global leprosy: update on the 2012 situation. Wkly Epidemiol Rec. 2013;88(35):365–380. [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) Global leprosy update, 2013; reducing disease burden. Wkly Epidemiol Rec. 2014;89(36):389–400. [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Global leprosy situation, 2012. Wkly Epidemiol Rec. 2012;87(34):317–328. [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) Leprosy update, 2011. Wkly Epidemiol Rec. 2011;36(86):389–400. [Google Scholar]
- 20.World Health Organization (WHO) Global Leprosy Situation, 2010. Wkly Epidemiol Rec. 2010;85(35):337–348. [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) Global leprosy situation, 2009. Wkly Epidemiol Rec. 2009;84(33):405–420. [Google Scholar]
- 22.World Health Organization (WHO) Global leprosy situation. Wkly Epidemiol Rec. 2007;82(25):225–232. [PubMed] [Google Scholar]
- 23.Istituto Superiore di Sanità. La Lebbra in Italia. Boll Epidemiol Naz. 1981;11:7. [Google Scholar]
- 24.Nunzi E, Clapasson A, Noto S. La Lebbra Oggi. Scuola Follereau AIFO Bologna. 2007 avalaible from: www.aifo.it. (accessed 12/06/2019) [Google Scholar]
- 25.Noto S. Epidemiologia della Lebbra. Scuola Follereau AIFO Bologna. 2006 Avalaible from: http://www.liber-rebil.it/wp-content/uploads/2012/01/lebbra-.pdf. (accessed 12/06/2019) [Google Scholar]
- 26.Istituto Nazionale di Statistica (ISTAT) Rome: 2004. Le notifiche di malattie infettive in Italia 2000-2001. avalaible from: https://ebiblio.istat.it/digibib/Informazioni/RER0151658InformazioniN7_2004Notifiche_malattie_infettive_Italia_20002001.pdf . [Google Scholar]
- 27.World Health Organization (WHO) Global leprosy update, 2014: need for early case detection. Wkly Epidemiol Rec. 2015;90(36):461–476. [PubMed] [Google Scholar]
- 28.Marotta M, Dallolio L, Toni G, Toni F, Leoni E. A case of imported leprosy in Italy: Implications for surveillance by Public Health Services of Local Health Authorities. Travel Med Infect Dis. 2019 doi: 10.1016/j.tmaid.2019.04.002. in press. [DOI] [PubMed] [Google Scholar]
- 29.Maritati M, Contini C. A Case of Leprosy in Italy: A Multifaceted Disease Which Continues to Challenge Medical Doctors. J Immigr Minor Heal. 2016;18(2):490–3. doi: 10.1007/s10903-015-0223-z. [DOI] [PubMed] [Google Scholar]
- 30.Filippetti R. Lebbra semiborderline : un caso clinico. Atti del XVII Congr Naz AIDA, Riccione 1-4 Oct 2008. Available from: www.dermatologialegale.it/docs/atti_xvii_aida/lebbrasemiborder.html. (accessed 12/06/2019) [Google Scholar]
- 31.Fiallo P, Cabiddu F, Clapasson A, Parodi A. Lichen scrofulosorum caused by Mycobacterium leprae: First report. Int J Dermatol. 2014;53:1244–8. doi: 10.1111/ijd.12504. [DOI] [PubMed] [Google Scholar]
- 32.Bongiorno MR, Pistone G, Noto S, Aricò M. Tuberculoid leprosy and Type 1 lepra reaction. Travel Med Infect Dis. 2008;6(5):311–4. doi: 10.1016/j.tmaid.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Fiallo P, Nunzi E, Bisighini G, Vaccari G. Leprosy in an italian tourist visiting the tropics. Trans R Soc Trop Med Hyg. 1993;87(6):675. doi: 10.1016/0035-9203(93)90287-z. [DOI] [PubMed] [Google Scholar]
- 34.Passarini B, Bandini P, Filacchione C, Lehmann J, Varotti C. Lebbra: inquadramento patologico e descrizione di un caso clinico. G Ital Dermatol Venereol. 2001;136(1):55–8. [Google Scholar]
- 35.Visco-Comandini U, Longo B, Cuzzi T, Paglia MG, Antonucci G. Tuberculoid Leprosy in a Patient with AIDS: A Manifestation of Immune Restoration Syndrome. Scand J Infect Dis. 2004;36(11-12):881–3. doi: 10.1080/00365540410025357. [DOI] [PubMed] [Google Scholar]
- 36.Mozzillo R, Colasanti P, Cordedda M, Zanchini R, Berruti V, Spanò G, et al. Leprematous leprosy. A case report. G Ital Dermatol Venereol. 2006;141(6):541–4. [PubMed] [Google Scholar]
- 37.Cusini M, Barabino G, Benardon S. A case of autochthonous leprosy in an elderly Italian patient leaving in Milan province with peculiar clinical presentation. J Am Acad Dermatol. 2017;76(6):AB3. [Google Scholar]
- 38.Beltrame A, Barabino G, Cicciò C, Badona Monteiro G, Cavalchini A, Carbognin G, et al. Magnetic resonance imaging in pure neural leprosy. Int J Infect Dis. 2017;60:42–3. doi: 10.1016/j.ijid.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Liguori R, Terlizzi R, Giannoccaro MP, Amati A, Foschini MP, Parodi A, et al. An inflammatory myopathy unmasks a case of leprosy in an Italian patient. J Neurol. 2015;262(9):2179–81. doi: 10.1007/s00415-015-7864-7. [DOI] [PubMed] [Google Scholar]
- 40.Massone C, Clapasson A, Nunzi E. Borderline lepromatous leprosy in an Italian man. Am J Trop Med Hyg. 2013;88(2):211. doi: 10.4269/ajtmh.12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piras MA, Are R, Figoni M, Salis M, Caddeo A, Fiori M, et al. Archives of leprosy mailing list. Archives of Leprosy Mailing List. 2011 Available from: http://english.aifo.it/leprosy/mailing_list/2011/210511-2.htm . [Google Scholar]
- 42.Simeoni S, Puccetti A, Tinazzi E, Codella OM, Sorleto M, Patuzzo G, et al. Leprosy initially misdiagnosed as sarcoidosis, adult-onset still disease, or autoinflammatory disease. J Clin Rheumatol. 2011;17(8):432–5. doi: 10.1097/RHU.0b013e31823a55e5. [DOI] [PubMed] [Google Scholar]
- 43.Massone C, Nunzi E, Cerroni L. Histopathologic Diagnosis of Leprosy in a Nonendemic Area. Am J Dermatopathol. 2010;32(4):417–9. doi: 10.1097/DAD.0b013e3181bb0cda. [DOI] [PubMed] [Google Scholar]
- 44.Giacomet V, Vigano A, Fabiano V, Antinori S, Longhi E, Zuccotti G. Leprosy: A disease not to be forgotten in the era of globalization. Pediatr Int. 2010;52(5):849–50. doi: 10.1111/j.1442-200X.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 45.Rongioletti F, Gallo R, Cozzani E, Parodi A. Leprosy: A diagnostic trap for dermatopathologists in nonendemic area. Am J Dermatopathol. 2009;31(6):607–10. doi: 10.1097/DAD.0b013e3181a105a1. [DOI] [PubMed] [Google Scholar]
- 46.Aridon P, Ragonese P, Mazzola MA, Terruso V, Palermo A, D’Amelio M, et al. Leprosy: Report of a case with severe peripheral neuropathy. Neurol Sci. 2010;31(1):75–7. doi: 10.1007/s10072-009-0152-5. [DOI] [PubMed] [Google Scholar]
- 47.Odone A, Riccò M, Morandi M, Borrini BM, Pasquarella C, Signorelli C. Epidemiology of tuberculosis in a low-incidence Italian region with high immigration rates: differences between not Italy-born and Italy-born TB cases. 2011. BMC Public Health. 2011;11:376. doi: 10.1186/1471-2458-11-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manzoli L, Sotgiu G, Magnavita N, Durando P, Barchitta M, Carducci A, et al. Evidence-based approach for continuous improvement of occupational health. Epidemiol Prev. 2015;39(4S1):81–5. [PubMed] [Google Scholar]
- 49.Veronesi L, Virdis R, Bizzoco S, Colucci ME, Affanni P, Paganuzzi F, et al. Vaccination status and prevalence of enteric viruses in internationally adopted children. The case of Parma, Italy. Acta Biomed. 2011;82(208):13–49. [PubMed] [Google Scholar]
- 50.Riccò M, Garbarino S, Bragazzi NL. Migrant Workers from the Eastern-Mediterranean Region and Occupational Injuries: A Retrospective Database-Based Analysis from North-Eastern Italy. Int J Env Res Public Heal. 2019;16:673. doi: 10.3390/ijerph16040673. [DOI] [PMC free article] [PubMed] [Google Scholar]