Abstract
Background: Individuals with Down syndrome (DS) are at an increased risk of developing thyroid disease, primarily autoimmune, with a lifetime prevalence ranging from 13% to 63%. Unfortunately, there are few studies systematically examining the frequency of thyroid disease in very young children. Aim of the study: The aim of the present study was to investigate the prevalence of different thyroid dysfunctions (TD) in a cohort of infants and children with DS and the growth parameters in subjects with normal versus abnormal thyroid function, followed for 3 years. Patients and methods: All children (n = 102; 48 males and 54 females, aged 2.3±3 years) with the diagnosis of DS who were seen at the General Pediatric Clinic of Hamad General Hospital in Doha (Qatar) from 2014 to 2018 were enrolled in our study. We recorded thyroid function and linear growth parameters [BMI, length/height SDS (Ht-SDS) and weight gain/day] and divided them into 3 groups according to their thyroid function. Group 1: (n = 36 subjects) with normal free T4 (FT4) and TSH; Group 2 (n = 44 subjects) with high TSH >5 and <12 mIU/L, and normal FT4, and Group 3 (n = 22 subjects) with TSH >12 mIU/L and/or FT4 <9 pmol/L. We also compared linear growth parameters in subjects with DS and thyroid dysfunction versus those with normal thyroid function at diagnosis and after treatment with L- thyroxine, for an average of 3 years. Results: In infants with DS (<1 year of age; n = 47, mean age: 5±3.5 months) we documented a higher prevalence of hypothyroidism (HT) (7/47 = 14.9%), both primary (5/47; 10.6%) and secondary (2/47; 4.3%). Subclinical hypothyroidism and positive thyroid antibodies were found in (13/47; 27.7%) and (9/47;19%, respectively). Before treatment with L-thyroxine, DS infants of Group 3 had significantly lower BMI-SDS but were not significantly shorter compared to other two Groups (p= 0.03 and p =0.14, respectively). After an average of 3 years of treatment the BMI- SDS and Ht-SDS did not differ among the treated and not treated infant groups. In the older group (>1 year; n=55; mean age: 5.5±3.3 years) primary HT was detected in 7/55 (12.7%). Subclinical HT was diagnosed in 20/55 (36.4%) and positive thyroid antibodies were found in 26/55 (47.3%). Before treatment with L-thyroxine, using the CDC growth charts for DS, we found that the groups with high TSH (Groups 2 and 3) were significantly shorter and heavier compared to the group with normal TSH (Group 1). After treatment with L-thyroxine, the Ht-SDS and Wt-SDS did not differ between the two groups. The linear growth of those diagnosed early during the first year of life was compared to growth parameters of children who were diagnosed with thyroid dysfunction later in life. Conclusion: Our data provided more evidence to support the findings that L- thyroxine treatment can improve growth of infants and young DS children with high TSH (>5 mIU/L), especially in those with TSH >12 mIU/L. (www.actabiomedica.it)
Keywords: Down syndrome, thyroid function, growth, L- thyroxine treatment, associated morbidities
Introduction
Down syndrome (DS) is one of the most common encountered human chromosomal disorder.
It occurs in ~1 in 700 births in the United States and is associated with a spectrum of physical and cognitive disabilities (1).
Advances in medical care, and increased access to care, have improved health and well-being of individuals with DS in the United States such that life expectancy has risen from 35 years in 1982 to 53 years in 2007 (2, 3).
However, DS subjects are at an increased risk of developing thyroid disease, primarily autoimmune, with a lifetime prevalence ranging from 13% to 63% (4). Beyond the newborn period, the incidence of elevated TSH values in DS increases and has been reported to be as high as 85% of infants under the age of 12 months (5). Sub-clinical hypothyroidism (SH) was the most common thyroid abnormality detected in children with DS by Unachak et al. (6).
Nevertheless, there are few studies systematically examining the frequency of thyroid disease in very young children and the effects of L-thyroxine treatment in subjects with DS with SH (7-11). Recently, it has been shown that the best cut-off level for prediction of persistent hypothyroidism for initial TSH level was 11.6 mIU/L.
The aim of the present study was to investigate the prevalence of different thyroid dysfunctions (TD) in a cohort of infants and children with DS and the growth parameters in DS subjects with normal versus abnormal thyroid function.
Patients and methods
DS infants and children (n = 102; 48 males and 54 females; aged 2.3±3 years) followed at the General Pediatric Clinic of Hamad General Hospital of Doha (Qatar), from 1-1-2014 to 1-1-2018, were enrolled in the study. Thyroid tests, including free thyroxine (FT4), thyroid stimulating hormone (TSH), anti TPO antibodies (thyroperoxidase), and growth parameters [weight, height, BMI, lenght/height SDS (Ht-SDS)] were assessed and registered in all subjects.
The diagnosis of thyroid dysfunctions were based on the following criteria:
Primary hypothyroidism (HT) is diagnosed when FT4 was <9 pmol/L, and TSH is >5 mIU/mL
Subclinical hypothyroidism (SH) was diagnosed when FT4 is normal, and TSH is >5 mIU/mL and <12 mIU/L .
Central hypothyroidism (CH) was diagnosed when FT4 is <9 pmol/l and basal TSH is low or normal.
Infants and children with DS were divided into 3 groups according to their thyroid function. Group1: n = 36 infants /children with normal FT4 and TSH; Group 2: n = 44 infants/children with high TSH >5 and <12 mIU/L, and normal FT4; Group 3: n = 22 infants/ children with TSH >12 mIU/L and/or FT4 below the lowest normal range (<9 pmol/L).
Children with high TSH >5 mIU/l and /or FT4 <9 pmol/L were treated with L-thyroxine.
Linear growth parameters in infant and children with DS and thyroid dysfunction were compared, at diagnosis and after treatment with L- thyroxine for an average of 3 years, to DS infants/children with normal thyroid function .
Growth charts for DS subjects were used to assess growth parameters (12).The longitudinal linear growth of patients diagnosed with thyroid dysfunction, during the first year of life, was also compared to linear growth of those diagnosed with thyroid dysfunction later in life.
FT4 was measured by radioimmunoassay, and TSH was measured by immune-radiometric assay using kits purchased from Genprice Inc, Santa Clara, CA 95054. The inter-assay and intra-assay coefficients of variations of FT4 were 6.6%, and 3.9%, respectively, and those of TSH were 6.1%, and 2.4%, respectively. The normal range for FT4 was 9.5 -17.5 pmol/L and TSH was 0.25-4.5 mIU/L.
Student- t test was used to compare the growth and lab variables when the data was normally distributed and the Mann-Whitney U test was used when the data were not normally distributed. Linear correlation was used to investigate possible relations between different variables. Significance was accepted when p was <0.05.
The study was approved by the Institutional Review Board of Hamad Medical Centre, Doha, Qatar.
Results
The prevalence of thyroid disorders in 102 infants and children with DS is shown in table 1. Congenital hypothyroidism was diagnosed in 3/102 children by neonatal screening program. The prevalence of thyroid dysfunction, both HT and SH, did not differ significantly between DS infants <1 year of age and >1 year of age. The prevalence of positive thyroid antibodies (TPO) was significantly higher in the older group of DS subjects. Central hypothyroidism was diagnosed in 2/47 infants with DS but not in the older group. The prevalence of HT (low FT4 and/or high TSH) was higher in females compared to males (29 versus 18 subjects). Primary hypothyroidism occurred in 7 out of 68 of DS children with congenital heart disease (CHD) and in 5 out of 46 DS children without CHD.
Table 1.
Prevalence of thyroid dysfunction in infants and children with Down Syndrome (DS) and associated disorders
| DS <1 yr | DS >1 yr | Total | P value | |
| Number | 47 | 55 | 102 | |
| Age (years); mean and SD | 0.5 ±0.3 | 5.5 ±3.5 | 2.3 ±3 | |
| Primary hypothyroidism (low FT4 pmol/L and/or TSH >12 mIU/L) | 5/47 (10.6%) | 7/55 (12.7%) | 12/102 (11.8%) | 0.74 |
| Central hypothyroidism | 2/47 (4.4%) | 0 | 2/102 (1.96%) | 0.12 |
| TSH >5 and <12 mIU/L (subclinical hypothyroidism) | 13/47 (27.7%) | 20/55 (36.4%) | 33/102 (32.4%) | 0.34 |
| Positive anti thyroid antibodies (TPO) | 9/47 (19%) | 26/55 (47.3%) | 35/102 (34.3% | 0.003 |
| Type 1 diabetes mellitus | 1/47 (2%) | 1/55 (1.8%) | 2/102 (1.96%) | 0.91 |
| Congenital Heart Disease (CHD) | 36/47 (76.6%) | 26/55 (47.3%) | 62/102 (60.8%) | 0.0025 |
Thyroid function did not differ between the two groups of DS subjects with and without CHD (FT4 = 13.8±3.2 and 13.6±4.8 pmol/L, respectively) .
Table 2 shows the TSH and FT4 values before and after treatment in infants and children with DS, and the mean dose of L- thyroxine used during the follow up period (~3 years). After treatment with L- thyroxine the TSH and FT4 levels did not differ significantly between the two groups of DS infants and children (treated with L- thyroxine versus normal thyroid function).
Table 2.
Thyroid function (Mean ± SD) in 2 groups of Down Syndrome (DS) subjects before and after treatment with L- thyroxine versus DS subjects with normal thyroid function
| Groups | Age (1) yr | TSH (1) mIU/L | FT4 (1) pmol/L | L-thyroxineμg/day | Age (2) yr | FT4 (2) mIU/L | TSH (2) pmol/L | |
| TSH >12 mIU/L or FT4 <9 pmol/L | Mean | 3.86 | 50.75 | 10.93 | 34.38 | 7.73 | 13.85 | 6.05 |
| Treated | SD | 5.63 | 38.80 | 3.47 | 16.93 | 5.52 | 2.14 | 4.00 |
| TSH >5 and <12 mIU/L | Mean | 2.88 | 9.50 | 13.88 | 29.32 | 8.88 | 14.29 | 6.14 |
| Treated | SD | 2.70 | 4.83 | 4.13 | 9.34 | 5.04 | 4.00 | 3.80 |
| Normal thyroid function | Mean | 1.64 | 4.80 | 15.16 | NT | 4.15 | 14.16 | 5.77 |
| SD | 2.03 | 2.06 | 3.33 | NT | 2.81 | 2.45 | 2.73 | |
| p value | 0.077 | 0.000035 | 0.0032 | 0.65 | 0.0008 | 0.973 | 0.45 |
Abbreviations: 1 = before treatment, 2 = after treatment with L-thyroxine
Before treatment with L-thyroxine, DS infants of Group 3 had significantly lower BMI-SDS but were not significantly shorter compared to other two Groups of DS subjects (p = 0.03 and p = 0.14, respectively). After an average of 3 years of treatment with L-thyroxine the BMI-SDS and Ht-SDS did not differ among the treated and not treated infant groups (Table 3).
Table 3.
Anthropometric data (Mean± SD) in the 2 groups of infants (<1 year) with Down Syndrome (DS) before and after treatment with L-thyroxine compared to DS of Group 1 (normal thyroid function)
| Groups | Age (1) (yr) | Ht-SDS (1) | BMI-SDS (1) | Age (2) (yr) | HT-SDS (2) | BMI-SDS (2) | Delta Lenght-SDS | Delta BMI-SDS | |
| TSH >5 and <12 mIU/L | Mean | 0.48 | -1.46 | -1.04 | 4.4* | -1.99 | 0.43 | -0.53 | 1.43 |
| Treated | SDS | 0.38 | 1.28 | 1.38 | 4.25 | 0.81 | 1.14 | 1.16 | 1.63 |
| TSH >12 mIU/L | Mean | 0.29 | -2.43 | -2.77* | 4.4* | -2.24 | 0.69 | 0.20 | 3.46# |
| Treated | SDS | 0.31 | 1.70 | 1.76 | 4.69 | 0.74 | 0.75 | 1.72 | 2.33 |
| TSH <5 mIU/L | Mean | 0.54 | -1.75 | -0.99 | 2.5* | -1.82 | 0.33 | -0.07 | 1.32 |
| Not Treated | SDS | 0.39 | 2.51 | 1.33 | 1.00 | 1.53 | 1.62 | 1.43 | 2.14 |
Abbreviations: 1 = before treatment, 2 = after treatment with L-thyroxine .*p <0.05 Wilcoxon test before vs after; # p <0.05 ANOVA test between the 3 groups.
In the older group of DS subjects (>1 year; n = 55; mean age: 5.5±3.3 years) HT was detected in 7/55 (12.7%). Subclinical HT was diagnosed in 20/55 (36.4%) and positive thyroid antibodies were found in 26/55 (47.3%). Before treatment with L-thyroxine, using the CDC growth charts for DS (8), we found that the groups with high TSH (Groups 2 and 3) were significantly shorter and heavier compared to the DS group with normal TSH (Group 1). After treatment with L-thyroxine, the Ht-SDS and Wt-SDS did not differ between the two groups (Tables 4 and 5).
Table 4.
Anthropometric data (Mean± SD) in 2 groups of children with Down Syndrome (DS) ( >1 yr) with high TSH before and after treatment with L-thyroxine compared to DS subjects with normal thyroid function
| Groups | Age-1 (yr) | Ht-SDS (1) | BMI-SDS (1) | Age-2 (yr) | Ht-SDS (2) | BMI-SDS (2) | Δ Ht-SDS | Δ BMI-SDS | |
| TSH >12 mIU/L or FT4 <9 pmol/L | Mean | 3.86 | -2.15 | -0.77 | 7.73 | -2.39 | 0.66* | -0.24 | 1.43 |
| Treated | SD | 5.63 | 1.46 | 2.63 | 5.52 | 1.01 | 1.69 | 1.32 | 2.49 |
| TSH >5 and <12 mIU/L | Mean | 2.88 | -1.57 | 0.38 | 8.88 | -2.18 | 1.37* | -0.62 | 1.23 |
| Treated | SD | 2.70 | 1.04 | 1.82 | 5.04 | 1.27 | 1.98 | 1.09 | 3.25 |
| Normal thyroid function | Mean | 1.64 | -1.90 | -0.13 | 4.65 | -2.08 | 0.59* | -0.17 | 0.69 |
| SD | 2.03 | 1.36 | 1.68 | 2.81 | 0.93 | 1.48 | 0.97 | 1.41 | |
| P value | 0.08 | 0.1182 | 0.089 | <0.00 | 0.47 | 0.29 | 0.21 | 0.28 |
Abbreviations: 1 = before treatment, 2 = after treatment with L-thyroxine
Table 5.
Anthropometric data (Mean ± SD), using Down Syndrome (DS) growth curves, in infants and children with DS with high TSH before and after treatment compared to DS subjects with normal thyroid function (Group 1)
| Age-1 | Ht-SDS (1) | Weight-SDS (1) | Age -2 | Ht-SDS (2) | Weight-SDS (2) | |
| Treated group | 3.8 | -1.85 | 1.05 | 8.3 | -0.8 | # 0.95 |
| N =47 | 2.4 | 0.9 | 0.7 | 3.8 | 0.42 | 0.6 |
| Untreated group | 2.5 | -1.5 | 0.6 | 4.7 | -0.7 # | 1.03 # |
| N= 55 | 2.9 | 0.81 | 0.4 | 3.3 | 0.38 | 0.55 |
| P value between the 2 groups | 0.038* | 0.013* | 0.02* | 0.01* | 0.08 | 0.32 |
Abbreviations: 1 = before treatment, 2 = after treatment with L-thyroxine; #p<0.05 t test before vs after; *p <0.05 between 2 groups
The dose of L- thyroxine did not correlate with the height growth velocity in the treated group (r = 0.08; p = 0.61). The change in Ht-SDS was correlated with the level of FT4 (r = 0.32; p = 0.05) (Figure 1).
Figure 1.
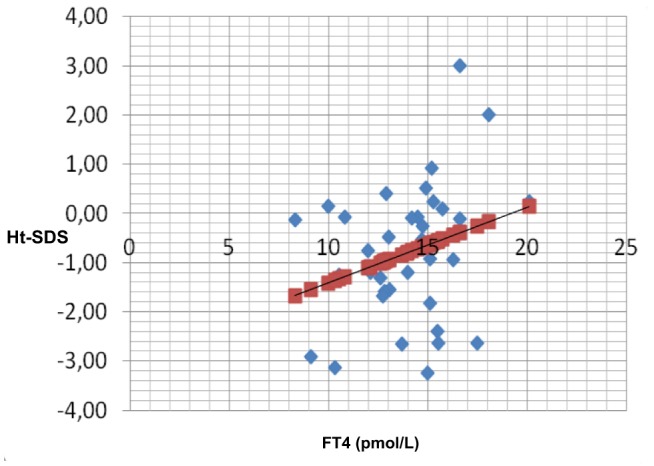
Correlation between FT4 and change in Ht-SDS (r = 0.32; p = 0.05)
At first examination (age = 2.3±2.5 years), 16 out of 102 DS children (15.7%) were overweight (BMI-SDS >1.5) and 17 out of 102 (16.7%) were underweight (BMI-SDS <-1.5). At age of 5±3.1 years, 33/102 DS children were overweight (BMI-SDS >1.5) and 7 /102 were underweight.
There was no significant difference in the thyroid function or linear growth in DS children with CHD when compared to those without CHD (Table 6).
Table 6.
Linear growth and thyroid function in children with Down Syndrome (DS) with (n = 62) and without (n = 40) congenital heart disease (CHD).
| Age (yr) (1) | Ht-SDS (1) | BMI-SDS (1) | TSH mIU/L | FT4 pmol/l | Age (yr) (2) | Ht-SDS (2) | BMI SDS (2) | |
| CHD | ||||||||
| Mean | 2.43 | -1.95 | -0.15 | 8.57 | 13.76 | 5.5 | -2.13 | 0.90 |
| SD | 3.09 | 1.70 | 1.95 | 12.7 | 3.24 | 3.7 | 1.21 | 1.47 |
| No CHD | ||||||||
| Mean | 3.37 | -1.70 | 0.22 | 13.5 | 13.6 | 7.6 | -2.00 | 0.96 |
| SD | 3.90 | 0.94 | 1.72 | 16 | 4.78 | 5.7 | 0.91 | 1.02 |
Abbreviations: 1 = before treatment, 2 = after treatment with L-thyroxine
Discussion
The increased prevalence of hypothyroidism in patients with DS has been well documented (4,6-11). The female:male ratio of approximately 3:2 (13). In our young patients with DS (n = 102) the prevalence of thyroid abnormalities (low FT4 and/or high TSH) was 47/102 (46%) with a female to male ratio of 29/18 (1.6:1).
In our patients, congenital hypothyroidism (CH) was diagnosed in 3/47 (prevalence of 1/141 compared to the incidence in our newborn screening of 1: 2150) Ultrasonography did not show athyrosis or ectopic gland in any of the patients.
The incidence of CH in newborn without DS recorded by the neonatal screening in Qatar, during the same periods of study, was 1/2150. These data confirm the higher prevalence of CH in neonates with DS compared to normal population of newborns. Fort et al. (14) found that CH was about 28 times more common among infants with DS than in the general population with an incidence of 1% (0.7% permanent and 0.3% transient congenital hypothyroidism) detected by newborn screening.The authors concluded that DS are at high risk for CH and should have careful follow-up to prevent further deterioration of their mental development or growth (14).
During the first year of life two of our DS infants developed HT and in 2 a CeH was diagnosed. In the following years, primary HT was diagnosed in 12.5% and SH in 36.4% of 55 DS children followed in our clinic. The older group had also a significant higher prevalence of thyroid antibodies and 4 out of the 7 DS children with positive thyroid antibodies developed primary HT. Therefore, it would seem that thyroid autoimmunity could have a clinical impact in DS from early age. This is in contrast to a previous observation reporting a gradual increase in the concentrations of thyroid autoantibodies from the age of 8 years (15).
These findings support the view of an increased autoimmune aggression with age. A genetic predisposition and a propensity to acquire autoimmune disorders seem to be possible factors, though their causal relation remains unclear (16). The occurrence of type 1 diabetes mellitus with positive anti-GAD antibodies in two of our hypothyroid children with DS and positive anti-thyroid antibodies is in favor of this view.
We treated with L- thyroxine DS infants and children with TSH >5 mIU/L and /or FT4 <9 pmol/L. Doses of L thyroxine required to keep TSH = or <5 mIU/L and FT4 in the normal range, did not differ significantly between those with HT and with SH. The linear correlation between FT4 levels and growth parameters expressed in Ht-SDS suggests also a positive effect of treatment on the linear growth. Our data also put in evidence that treatment with L-thyroxine in DS children with high TSH prevented also an excessive weight gain, that was observed in DS children with normal thyroid function (Group 1).
These data support the findings of Trotsenburg et al. (10) who demonstrated that L-thyroxine replacement in the first two years of life improve the growth in young infants with DS, and the Marchal et al. observations (9). The Authors showed that T4-treated children tended to be taller compared to placebo treated children with DS and high TSH.
Congenital heart defects (CHD) are present in patients with DS in approximately 40-50% of cases (17-20). Among the most common cardiac defects are atrioventricular septal defect (AVSD), representing approximately 45% of cases, and ventricular septal defect (VSD), representing 20-30% (17, 20).
In our study 62/102 (60.7%) had CHD. No significant difference was observed in the thyroid function or linear growth in DS children with CHD when compared to those without CHD. In support to our findings, Mıhçı E et al.(35) reported no significant relationship between CHD and TSH and FT4 levels (21).
Conclusion
Our data provided more evidence to support the hypothesis that L-thyroxine treatment can improve growth of infants and young children with DS presenting a TSH >5 mIU/L and especially in those with TSH >12 mIU/L. On treatment of these children, FT4 levels increased and this was associated with better linear growth. The onset of hypothyroidism may be associated with symptoms and clinical findings that are subtle and attributed to the underlying disorder. Therefore we recommend, according to guidelines for children with DS, to review the results of the newborn thyroid function screening, and to repeat thyroid function tests at the age of 6 months and 12 months, and then annually (22). An early treatment is also recommended for those with high TSH or low FT4 levels.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A. National Birth Defects Prevention Network. National Birth Defects Prevention Network. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Thase ME. Longevity and mortality in Down’s syndrome. J Ment Defic Res. 1982;26:177–192. doi: 10.1111/j.1365-2788.1982.tb00144.x. [PubMed: 6217345] [DOI] [PubMed] [Google Scholar]
- 3.Presson AP, Partyka G, Jensen KM, Devine OJ, Rasmussen SA, McCabe LL, McCabe ER. Current estimate of Down syndrome population prevalence in the United States. J Pediatr. 2013;163:1163–1168. doi: 10.1016/j.jpeds.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy O, Worley G, Lee MM, Chaing S, Mackey J, Crissman B, Kishnani PS. Hypothyroidism in Down syndrome: screening guidelines and testing methodology. Am J Med Genet A. 2004;124A:436–437. doi: 10.1002/ajmg.a.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharav T, Collins R, Baab P. Growth studies in infants and children with Down’s syndrome and elevated levels of thyrotropin. Am J Dis Child. 1988;142:1302–1306. doi: 10.1001/archpedi.1988.02150120056040. [DOI] [PubMed] [Google Scholar]
- 6.Unachak K, Tanpaiboon P, Pongprot Y, Sittivangkul R, Silvilairat S, Dejkhamron P, Sudasna J. Thyroid functions in children with Down’s syndrome. J Med Assoc Thai. 2008;91:56–61. [PubMed] [Google Scholar]
- 7.Pierce MJ, LaFranchi SH, Pinter JD. Characterization of Thyroid Abnormalities in a Large Cohort of Children with Down Syndrome. Horm Res Paediatr. 2017;87:170–178. doi: 10.1159/000457952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwaveling-Soonawala N, Witteveen ME, Marchal JP, Klouwer FCC, Ikelaar NA, Smets AMJB, van Rijn RR, Endert E, Fliers E, van Trotsenburg ASP. Early thyroxine treatment in Down syndrome and thyroid function later in life. Eur J Endocrinol. 2017;176:505–513. doi: 10.1530/EJE-16-0858. [DOI] [PubMed] [Google Scholar]
- 9.Marchal JP, Maurice-Stam H, Ikelaar NA, Klouwer FC, Verhorstert KW, Witteveen ME, Houtzager BA, Grootenhuis MA, van Trotsenburg AS. Effects of early thyroxine treatment on development and growth at age 10.7 years: follow-up of a randomized placebo-controlled trial in children with Down’s syndrome. J Clin Endocrinol Metab. 2014;99:E2722–2729. doi: 10.1210/jc.2014-2849. [DOI] [PubMed] [Google Scholar]
- 10.van Trotsenburg AS, Vulsma T, van Santen HM, Cheung W, de Vijlder JJ. Lower neonatal screening thyroxine concentrations in Down syndrome newborns. J Clin Endocrinol Metab. 2003;88:1512–1515. doi: 10.1210/jc.2002-021303. [DOI] [PubMed] [Google Scholar]
- 11.Sankar HV, Anjukrishna K, Riaz I. Thyroid Stimulating Hormone Level at Diagnosis as a Predictor of Persistent Subclinical Hypothyroidism in Children with Down Syndrome. Indian Pediatr. 2018;55:576–578. [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health. [Accessed March 20, 2015] The CDC growth charts for children with special health care needs. Issues regarding the use of condition-specific growth charts. Available at: http://depts.washington.edu/growth/cshcn/text/page6b.htm .
- 13.Gruñeiro de Papendieck L, Chiesa A, Bastida MG, Alonso G, Finkielstain G, Heinrich JJ. Thyroid dysfunction and high thyroid stimulating hormone levels in children with Down’s syndrome. J Pediatr Endocrinol Metab. 2002;15:1543–1548. doi: 10.1515/jpem.2002.15.9.1543. [DOI] [PubMed] [Google Scholar]
- 14.Fort P, Lifshitz F, Bellisario R, Davis J, Lanes R, Pugliese M, Richman R, Post EM, David R. Abnormalities of thyroid function in infants with Down syndrome. J Pediatr. 1984;104:545–549. doi: 10.1016/s0022-3476(84)80544-2. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson B, Gustafsson J, Hedov G, Ivarsson SA, Annerén G. Thyroid dysfunction in Down’s syndrome: relation to age and thyroid autoimmunity. Arch Dis Child. 1998;79:242–245. doi: 10.1136/adc.79.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iughetti L, Lucaccioni L, Fugetto F, Mason A, Predieri B. Thyroid function in Down syndrome. Expert Rev Endocrinol Metab. 2015;10:525–532. doi: 10.1586/17446651.2015.1063995. [DOI] [PubMed] [Google Scholar]
- 17.Plaiasu V. Down syndrome: genetics and cardiogenetics. Maedica (Buchar) 2017;12(3):208–13. [PMC free article] [PubMed] [Google Scholar]
- 18.Lal PS, Chavan B, Devendran VR, Varghese R, Murmu UC, Kumar RS. Surgical outcome of congenital heart disease in Down’s syndrome. Asian Cardiovasc Thorac Ann. 2013;21(2):166–9. doi: 10.1177/0218492312450701. [DOI] [PubMed] [Google Scholar]
- 19.Santos FCGB, Croti UA, Marchi CH, Murakami AN, Brachine JDP, Borim BC, Finoti RG, Godoy MF. Surgical Treatment for Congenital Heart Defects in Down Syndrome Patients. Braz J Cardiovasc Surg. 2019;34:1–7. doi: 10.21470/1678-9741-2018-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergstrom S, Carr H, Petersson G, Stephansson O, Bonamy AK, Dahlstrom A, et al. Trends in congenital heart defects in infants with Down syndrome. Pediatrics. 2016;138(1) doi: 10.1542/peds.2016-0123. pii: e20160123. [DOI] [PubMed] [Google Scholar]
- 21.Mıhçı E, Akçurin G, Eren E, Kardelen F, Akçurin S, Keser I, Ertuğ H. Evaluation of congenital heart diseases and thyroid abnormalities in children with Down syndrome. Anadolu Kardiyol Derg. 2010;10:440–445. doi: 10.5152/akd.2010.143. [DOI] [PubMed] [Google Scholar]
- 22.Cunniff C, Frias JL, Kaye C, Moeschler JB, Panny SR, Trotter TL, de la Cruz F, Hanson JW, Lloyd-Puryear M, Moore CA, Williams J, Hoyme HE, Bull MJ, Cohen WI, Desposito F, Pletcher BA, Roizen N, Wappner R, Hall LA. American Academy of Pediatrics, Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2001;107:442–450. [Google Scholar]


