Abstract
Background
We conducted a pilot study to assess feasibility, on-study retention, trends in natriuretic peptide levels, quality of life (QoL), and safety of a 12-week feeding trial with 1500-mg vs. 3000-mg daily sodium meals in high-risk patients with heart failure (HF).
Methods
Of 196 patients with recent (≤2 weeks) hospitalization for HF, ejection fraction ≤40%, on optimal medical therapy, functionally independent, and able to communicate, 83 (47%) consented to participate. Of these, 27 (age, 62±11 years; 22 men; 20 White; ejection fraction, 26±8%) had 24-h urine sodium ≥3000 mg and agreed to randomly receive either 1500-mg (N=12) or 3000-mg (N=15) sodium meals.
Results
On-study retention at 12 weeks was 77% (82% vs. 73%; P=0.53); 6 patients (2 in 1500-mg, 4 in 3000-mg arm) withdrew before study completion. Food satisfaction questionnaires indicated that both diets were well tolerated. QoL improved in the 1500-mg arm at 12 weeks but did not change in the 3000-mg arm. Average compliance with meals was 52% (based on urinary sodium) and was not significantly different between arms (42% vs. 60%; P=0.25). Study meals reduced 24-h urinary sodium by 137±21 mmol (1500-mg arm) and 82±16 mmol (3000-mg arm), both P<0.001; between-arms difference was 55 mmol (95%CI: 3–107; P=0.037). NT-proBNP was not affected. Hospitalizations and low blood pressure events did not differ significantly between arms. Serum creatinine decreased more (by 0.17 mg/dL, 95%CI: 0.06–0.28; P=0.003) in the 1500-mg arm. Creatinine increases >0.5mg/dL over baseline only occurred in 1 patient in the 3000-mg arm.
Conclusions
Even with prepared meals, investigating optimal dietary sodium in HF comes with challenges, including need for extensive screening, reluctance to participate, and compliance issues. Because both diets reduced urinary sodium without adverse safety or QoL signals, a larger trial, with modifications to improve participation and compliance, would be ethical and feasible.
Clinical Trial Registration
Journal Subject Codes: [110] Heart failure, congestive, [101] Myocardial biology, nutrition
Keywords: Heart failure, dietary sodium, outcomes, clinical trials, pilot studies
Although all heart failure (HF) guidelines emphasize dietary sodium restriction, there is no consensus on the actual level. Recommendations are either nonspecific or suggest a range, e.g. 2000–3000 mg daily, largely based on opinion or observational studies,1–4 underlining the weak database that supports this recommendation.5
Several small studies have examined the impact of sodium intake on clinical outcomes in HF, yielding contradicting results.6–12 A number of single-center studies6–8, 13–15 have actually suggested worse outcomes with strict sodium restriction; however, these trials had significant shortcomings.16, 17 Thus, although it seems reasonable to restrict sodium below 3000 mg daily in patients with HF, it is currently unknown how “low” is appropriate. High sodium intake in HF can cause fluid retention, sympathetic activation, and inflammation.16, 17 On the other hand, neurohormonal activation induced by low sodium intake can potentially harm the failing heart.16, 17 Therefore, the appropriate level of sodium restriction only be addressed through a clinical trial. However, critical knowledge gaps exist in order to properly design a Phase III trial. Although enrolling post-discharge patients after acute HF would select a population at sufficiently high risk to demonstrate an impact of sodium on clinical events with a reasonable sample size,17 there are crucial unknowns in terms of (1) study retention and compliance with provided food; (2) preliminary signals for risk vs. benefit of adequately distinct sodium intakes (1500 vs. 3000 mg/d); (3) eligibility among recently hospitalized patients from a sodium intake perspective (i.e. consuming over 3000 mg daily based on 24-h urine sodium); (4) effects of low dietary sodium on renal function and blood pressure; and (5) appropriate visit schedule, among others.17
To address these critical gaps, we conducted the Prevent Adverse Outcomes in Heart Failure by Limiting Sodium (PROHIBIT Sodium), a clinical trial pilot study ( NCT02467296) to (1) assess what proportion of patients with acute HF and reduced (≤40%) left ventricular ejection fraction (LVEF) would be eligible and willing to participate in a 12-week feeding trial within 2 weeks after hospital discharge and (2) randomize 50 eligible patients to prepared meals with 1500 vs. 3000 mg/d sodium for 12 weeks, followed by a 12-week post-intervention follow-up period, to estimate on-study retention and compliance; trends in outcomes and N-terminal pro-B- type natriuretic peptide (NT-proBNP) levels; diet palatability and quality of life; and safety, by monitoring for adverse events, vital signs, and laboratory assessments throughout the study.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Screening
The rationale and details of the study design have been previously reported.17 Briefly, we asked consecutive patients with HF and LVEF ≤40% (evaluated within 1 year of screening), who were hospitalized at the Stony Brook University Medical Center (Stony Brook, NY) with a primary admission diagnosis of HF, to participate in a 12-week feeding trial followed by 12 weeks follow-up, within 2 weeks after discharge. The study started in January 2015 and was completed (last patient assessment) in July 2018. Patients had to be on optimal guideline-recommended therapy (defined as being on ≥50% of target or maximum tolerated dose of recommended major medication classes i.e., beta blockers, angiotensin-converting inhibitors or angiotensin receptor blockers or sacubitril valsartan, and mineralocorticoid receptor antagonist where indicated), able to consume the research diet, and have systolic blood pressure (SBP) ≥100 mmHg. Patients who were institutionalized or planning to move, had cognitive impairment, were unable to communicate, had severe non-cardiac illness compromising life expectancy within the next 12 months, had any medical or surgical procedure planned in the next 6 months, or participating in any other experimental protocol were not approached (Supplemental Table 1). We originally had planned for pre-screening using a 3-day food record (3DFR) in order to reduce the need for 24-h urine collections. However, that strategy increased logistic complexity (because of multiple patient visits) and thus we modified the protocol to directly assess eligibility among recently hospitalized patients from a sodium intake perspective (i.e. consuming over 3000 mg daily) with 24-h urine collections. Completeness of 24-h urine collections was determined on the basis of volume, with a threshold of ≥250ml.18 The study protocol was approved by the Stony Brook Institutional Review Board. All participants provided written informed consent. The study was terminated early (27 randomized patients vs. 50 planned) because of significantly slower than anticipated study enrollment.
Randomization and Intervention
Eligible patients entered the randomized, double-blind phase, and received food with either 1500 or 3000 mg daily sodium content for 12 weeks, followed by a 12-week surveillance. We opted for a 12-week diet as a reasonable compromise between having enough exposure to diet to demonstrate physiological and potentially clinical effects and keeping the intervention relatively short to maintain study retention, as adherence with provided meals for longer-term periods (e.g. 6 months) is unknown. Meals were prepared by PurFoods, LLC (Ankeny, IA, www.purfoods.com), in a USDA certified kitchen, and were shipped to participants under temperature-controlled conditions. Study coordinators, investigators, and participants were blinded to arm assignment; only an administrative member of the study team and the dietitians responsible for the meals were aware of arm assignment. Meals were individually tailored to each patient to have consistent macronutrients and caloric content throughout the study period to ensure weight maintenance.17 For caloric intake, basal metabolic rate was calculated using indirect calorimetry. Protein intake was adjusted to 0.8 g/kg of body weight. All other nutrients were between 70% and 100% of reference intake. The caloric, fat, protein, and carbohydrate value of meals stayed consistent throughout the trial. To reinforce compliance, participants were explicitly instructed during all interactions to only consume the provided meals. To enhance palatability and compliance, discretionary seasonings (without sodium), but not salt, were allowed. To isolate the effect of sodium intake, patients were instructed to restrict fluids to ≤2L daily. Participants were followed with phone calls and a clinic visit for an additional 12 weeks after the end of the intervention.
Study Endpoints
The primary endpoint was on-study retention, defined as the proportion of patients remaining on the study in the absence of clinical or safety events, and adherence, through patient diaries and 4-weekly 24-h urine collections. The secondary endpoint was the composite of mortality and hospitalization. Patients and caregivers were asked to report any interim event at any institution to the study team during regular encounters. Additional endpoints were changes in NT-pro-BNP levels and quality of life, quantified with the Kansas City Cardiomyopathy Questionnaire (KCCQ) and questionnaires related to satisfaction with the provided meals.
Safety endpoints included (1) SBP drop >20mmHg for those with baseline >120mmHg, >10mmHg for baseline 100–120mmHg, and any SBP <100mmHg with symptoms, at any visit; and (2) serum creatinine increase >0.5mg over baseline at any visit. For patients meeting these criteria, medical therapy was adjusted, and patients were re-evaluated in 1 week. Patients with persistent findings despite adjustments or those with SBP <90 mmHg were withdrawn.
Statistical Analysis
On-study retention (primary endpoint) was calculated according to the Kaplan-Meier principle, i.e. patients meeting a serious clinical event not permitting continuation of the intervention (e.g. death, need for mechanical circulatory support) or safety event were censored as on-study at the time of the event. Because food diaries were inconsistently completed, we relied on sodium excretion to quantify adherence, which was a prespecified secondary method to assess adherence.17 In studies with fixed sodium intake, 90% of ingested sodium was excreted in the urine with a ±15% variation.19, 20 We thus considered values outside 1150 to 1550 mg for the 1500 mg/d sodium arm and 2300 to 3100 mg for the 3000 mg/d sodium arm, as evidence of nonadherence. We used mixed-effects models with a random intercept at the patient level and included the baseline measurements, to model continuous efficacy and safety endpoints (urinary sodium, biomarkers, and questionnaire scores) that were measured longitudinally. The changes from baseline within and between groups have been estimated using marginal effects in full factorial models with time (discrete time points) and arm interaction in the model. All analyses were performed with STATA version 14.2 (StataCorp LLC, College Station, Texas).
RESULTS
Screening and Eligibility
Between January 2015 and December 2017, we approached 196 patients who fulfilled the initial eligibility criteria (within 2 weeks of discharge after admission for HF, LVEF ≤40% within 1 year, on optimal medical therapy, SBP≥100 mmHg, functionally independent, able to communicate). Of these, 83 (47%) consented to participate; the remaining patients either declined (N=69) or did not respond (N=23). Eventually, 27 of 83 (33%) were randomized; the remaining patients either failed screening (mostly because of urine sodium) or declined to enter the feeding phase. Figure 1 summarizes the study screening and randomization process and study follow-up. The median time from discharge to study enrollment was 4.5 days (interquartile range, 0 to 19 days).
Figure 1.
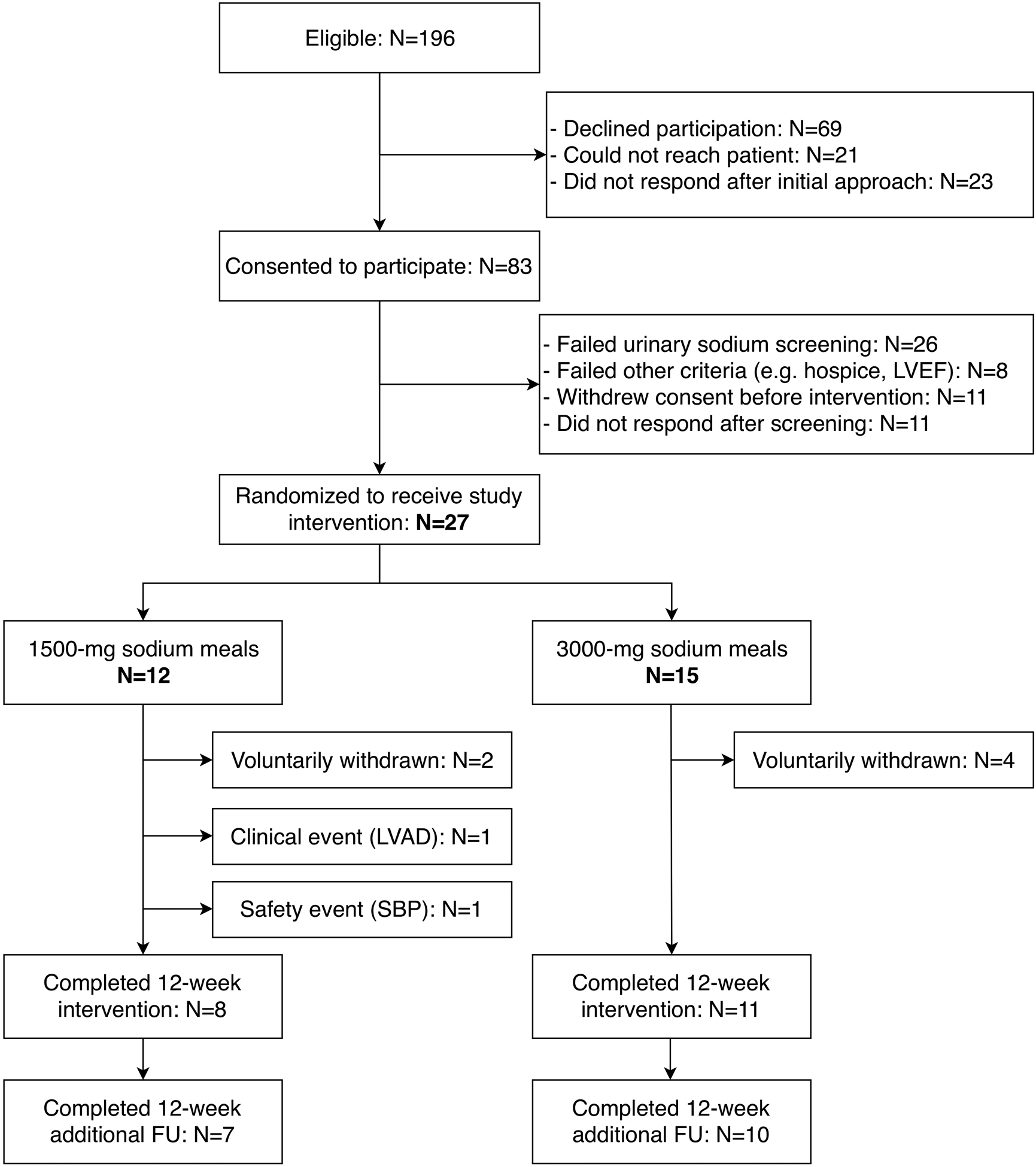
Patient population and follow-up
Patient Characteristics and Study Retention
Table 1 summarizes the characteristics of the 27 patients who were randomized to receive either 1500-mg (N=12) or 3000-mg (N=15) sodium meals. Average daily caloric content of the provided meals was 2142±299 kcals in the 1500-mg and 2323±337 kcals in the 3000-mg arm (P=0.17). Two patients in the 1500-mg and 4 in the 3000-mg arm refused to continue consuming study meals before completing the 12-week intervention. Two participants in the 1500-mg arm were withdrawn because of clinical indications: 1 received left ventricular assist device and 1 met the SBP safety endpoint. All voluntary withdrawals occurred ≤4 weeks. One patient in each arm was lost to follow-up after the 12-week intervention. All others, including those who discontinued the meals, were followed up to 24 weeks. The on-study retention (Kaplan-Meier estimate, censoring for 2 clinically indicated withdrawals as per analysis plan) was 77% (95%CI: 56%–89%) at 12 weeks and did not differ significantly between the 1500-mg (82%) and the 3000-mg (73%) arm (log-rank test; P=0.53), Figure 2.
Table 1.
Clinical Characteristics of Randomized Participants (N=27)
| Characteristic | Total (N=27) | 1500 mg (N=12) | 3000 mg (N=15) |
|---|---|---|---|
| Age, years | 62.0 ± 10.5 | 59.5 ± 9.1 | 63.9 ± 11.4 |
| Male, N %) | 22 (81) | 9 (75) | 13 (87) |
| Race, N (%) | |||
| Caucasian | 20 (74) | 9 (75) | 11 (73) |
| African-American | 4 (15) | 2 (17) | 2 (13) |
| Other | 3 (11) | 1 (8) | 2 (13) |
| Recent (≤1 year) LVEF, % | 26.1 ± 8.2 | 24.9 ± 9.3 | 27.2 ± 7.3 |
| History of coronary artery disease, * N (%) | 14 (52) | 6 (50) | 8 (53) |
| Hypertension, N (%) | 20 (74) | 9 (75) | 11 (73.4) |
| Diabetes mellitus, N (%) | 11 (41) | 6 (50) | 5 (33) |
| Atrial fibrillation, N (%) | 9 (33) | 4 (33) | 5 (33) |
| Chronic kidney disease (stage 1–3), N (%) | 3 (11) | 2 (17) | 1 (7) |
| Active smoker, N (%) | 5 (19) | 2 (17) | 3 (20) |
| Dyslipidemia, N (%) | 16 (59) | 7 (58) | 9 (60) |
| Sleep Apnea, N (%) | 4 (15) | 3 (25) | 1 (7) |
| Body mass index, kg/m2 | 32.5 ± 6.5 | 30.9 ± 6.2 | 33.8 ± 6.5 |
| Systolic blood pressure, mm Hg | 118 ± 14 | 114 ± 13 | 121 ± 16 |
| Heart rate, beats/min | 75 ± 12 | 67 ± 11 | 81 ± 9 |
| Glucose, mg/dL | 139 ± 52 | 142 ± 60 | 137 ± 47 |
| Sodium, mmol/L | 139 ± 3.7 | 138 ± 4.6 | 139 ± 2.8 |
| Potassium, mmol/L | 4.5 ± 0.5 | 4.7 ± 0.6 | 4.4 ± 0.4 |
| Creatinine, mg/dL | 1.1 (0.9 – 1.3) | 1.2 (1.1 – 1.6) | 1.0 (0.8 – 1.3) |
| Blood urea nitrogen, mg/dL | 23 (16 – 27) | 26 (10 – 29) | 22 (15 – 25) |
| NT-proBNP, pg/mL | 971 (250 – 1800) | 824 (186 – 1600) | 971 (334 – 1800) |
| 24-h urinary sodium, mmol | 202 (155 – 232) | 198 (151 – 276) | 202 (180 – 224) |
| Therapy, N (%) | |||
| ACEi or ARB | 17 (63) | 8 (67) | 9 (60) |
| Sacubitril valsartan | 7 (26) | 2 (17) | 5 (33) |
| Mineralocorticoid receptor antagonist | 21 (78) | 10 (83) | 11 (73) |
| Beta blocker | 27 (100) | 12 (100) | 15 (100) |
| Loop diuretic | 24 (89) | 11 (92) | 13 (87) |
| Aspirin | 18 (67) | 10 (83) | 8 (53) |
| ADP inhibitors | 5 (19) | 1 (8) | 4 (27) |
| Warfarin | 3 (11) | 3 (25) | 0 (0) |
| Newer anticoagulants | 7 (26) | 1 (8) | 6 (40) |
| Implantable cardioverter defibrillator | 10 (37) | 7 (58) † | 3 (20) |
Defined as history of myocardial infarction or percutaneous or surgical revascularization.
One patient had a combined defibrillator-resynchronization device. Values for continuous variables represent mean ± standard deviation or median (25th – 75th percentile). ACEi: angiotensin-converting enzyme inhibitor; ADP: ARB: angiotensin receptor blocker; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-B-type natriuretic peptide
Figure 2.
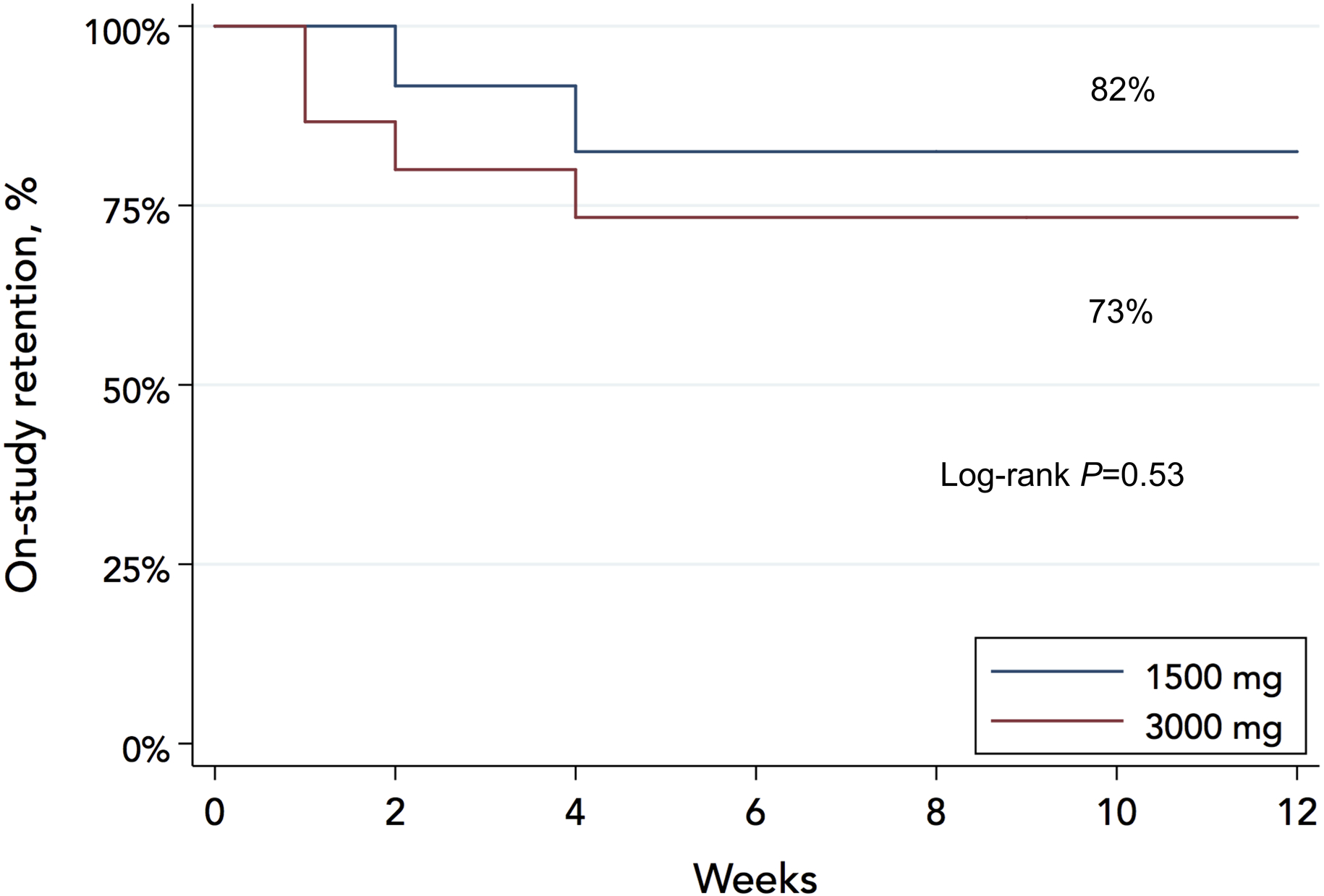
On-study retention rates
Compliance
The study intervention reduced 24-h urinary sodium significantly in both arms, Figure 3. In the 1500-mg arm, 24-h sodium decreased from (mean ± standard error) 208±20 mmol at baseline to time-average 71±9 mmol while on study meals; a 137±21 mmol reduction (P<0.001). In the 3000-mg arm, 24-h sodium decreased from 211±18 mmol at baseline to time-average 129±13 mmol while on study meals; an 82±16 mmol reduction (P<0.001). The 1500-mg diet reduced 24-h sodium by 55 mmol (95%CI: 3 to 107) more than the 3000-mg diet (P=0.037 for the between-arms difference in change). Sodium excretion rebounded in both arms after the intervention period as evident from the 14- and 24-week assessments but remained below baseline in the 3000-mg arm, Figure 3. Based on pre-specified limits for 24-h urinary sodium excretion, compliance with provided meals was 52% on average among patients retained in the study, with numerically higher compliance in the 3000-mg vs the 1500-mg arm (60% vs. 42%); however, this difference was not statistically significant (18%; 95%CI −13% to 49%; P=0.25), Figure 4. We excluded 1 (4-week follow-up) 24-h urine collection in the 3000-mg arm from analysis because of inadequate volume (<250 ml).
Figure 3.
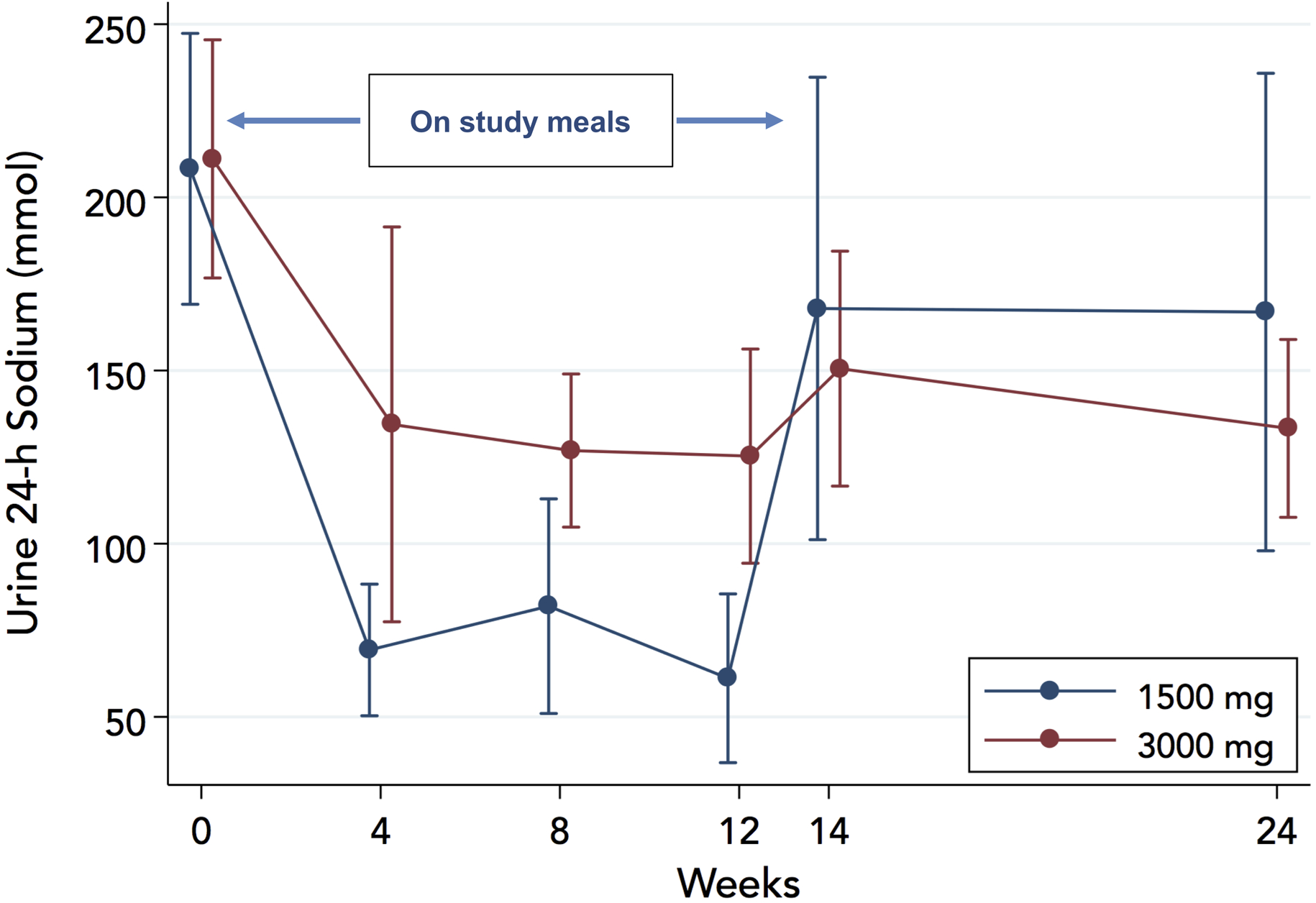
Effect of study diets on 24-h urinary sodium excretion
Figure 4.

Compliance with provided diet based on 24-h urinary sodium excretion
Clinical Outcomes and Natriuretic Peptide Levels
Nine patients experienced a total of 24 hospitalizations; 5 patients (8 events) in the 1500-mg and 4 patients (16 events) in the 3000-mg arm. Table 2 presents the distribution of events. There were no deaths; 1 hospitalization led to left ventricular assist device evaluation. The protocol-defined Kaplan-Meier event rate at 24 weeks was 33% and did not differ significantly between arms (42% in 1500-mg vs. 27% in 3000-mg; log-rank P=0.50). However, the study was not powered to detect differences in clinical event rates between arms.
Table 2.
Summary of Per-protocol Defined Events (N=19)
| Outcome | Total (N=27) | 1500 mg (N=12) | 3000 mg (N=15) |
|---|---|---|---|
| Death | 0 | 0 | 0 |
| Left ventricular assist device | 1 | 1 | 0 |
| Heart transplant | 0 | 0 | 0 |
| Serious adverse events* | 2 | 1 | 1 |
| All cause hospitalizations | |||
| Total N of hospitalizations | 24 | 8 | 16† |
| Patients with hospitalizations | 9 | 5 | 4 |
| Cardiovascular hospitalizations | |||
| Total N of CV hospitalizations | 20 | 7 | 13 † |
| Patients with CV hospitalizations | 9 | 5 | 4 |
| Heart failure (HF) hospitalizations | |||
| Total N of HF hospitalizations | 9 | 2 | 7 † |
| Patients with HF hospitalizations | 4 | 1 | 3 |
Systolic blood pressure drop <90 mmHg in the 1500mg arm and creatine increase >0.5 mg/dL in the 3000-mg arm
1 patient had 10 encounters; 8 for cardiovascular reasons and 4 specifically for heart failure
Log10-transformed NT-proBNP did not change significantly in either arm and was not affected differentially by study diets (P=0.70 and P=0.69 for between-groups differential change at 12 weeks and 24 weeks, respectively), Figure 5.
Figure 5.
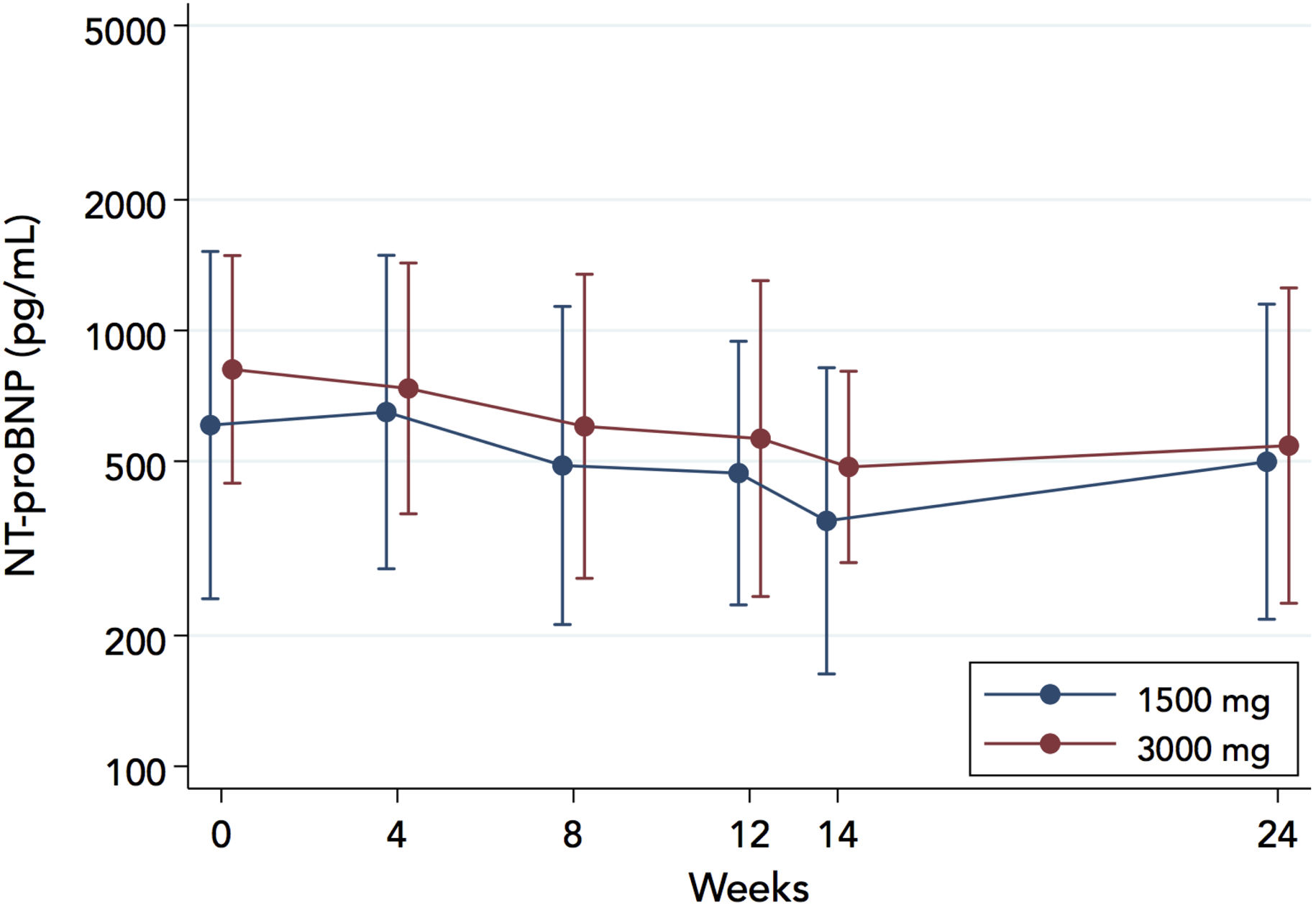
Effect of study diets on NT-proBNP concentrations
Food Satisfaction and Quality of Life
Patient satisfaction with the provided meals was recorded with a food satisfaction questionnaire (Appendix). In a Likert scale from 1 (least acceptable) to 5 (highly acceptable), the average rating of appearance, taste, and quantity of food was 3.8±0.2 points in both arms (P=0.98 for the difference). The average rating improved over time (3.6 [95% CI 3.1–4.1] at 4 weeks; 3.8 [95% CI 3.5–4.2] at 8 weeks; 4.1 [95% CI 3.8–4.4] at 12 weeks P=0.019 for overall change), without difference between groups (P=0.98 for time-arm interaction). When asked to rate their hunger at various times during the day in a Likert scale from 0 (not hungry) to 3 (always hungry), patients responded “a little hungry” or “not hungry” most of the time (73% in the 1500-mg and 79% in the 3000-mg arm, P=0.64 for the difference). These responses did not change significantly over time. Supplemental Table 2 summarizes the food satisfaction questionnaire responses.
The overall and the clinical KCCQ summary scores improved in the 1500-mg arm but did not change in the 3000-mg arm, Supplemental Figure 1. By 12 weeks, the overall KCCQ score improved by 12±2 units in the 1500-mg (P<0.001) and 1±2 units in the 3000-mg arm (P=0.82) over baseline. The difference in change (12 units in favor of the 1500-mg arm; 95%CI: 6 to 18) was significant (P<0.001). Similarly, the clinical KCCQ score improved by 12±2 units in the 1500-mg (P=0.001) and worsened by −2±2 units in the 3000-mg arm (P=0.29). The difference in change (14 units in favor of the 1500-mg arm; 95%CI: 8 to 20) was significant (P<0.001).
Effects on Loop Diuretics and Body Weight
At baseline, 11 (92%) and 13 (87%) of patients in the 1500-mg and 3000-mg arms, respectively, were on loop diuretics. Loop diuretic use was reduced by 33% (P<0.001) in the 1500-mg and by 30% (P=0.004) in the 3000-mg arm on average vs. baseline during the entire follow-up period (between-groups difference 3%; 95%CI: −24% to 29%; P=0.86 for interaction of time with arm), Supplemental Figure 2. The average loop diuretic dose (in furosemide equivalent) at baseline was 50 and 52 mg in the 1500-mg and 3000-mg arms, respectively, and did not change significantly in either arm (−1±1 mg in the 1500-mg; P=0.32, and +3±11 mg in the 3000-mg arm; P=0.80) on average during the entire follow-up. The between-groups difference in change was −4 mg (95%CI: −26 to 18; P=0.70 for interaction of time with arm), Supplemental Figure 3.
Baseline weight at baseline was 91 and 106 kg in the 1500-mg and 3000-mg arms, respectively, and did not change significantly in either arm (−0.3±3.0 kg in the 1500-mg; P=0.92, and +0.4±1.4 kg in the 3000-mg arm; P=0.79) on average during the entire follow-up. The between-groups difference in change was −0.7 kg (95%CI: −7.2 to 5.9; P=0.84 for interaction of time with arm), Supplemental Figure 4.
Safety Endpoints
Study meals had no significant effect on SBP. The marginal effect until week 12 was −3.7±3.5 mmHg in the 1500-mg arm (P=0.29) and −3.5±3.0 mmHg in the 3000-mg arm (P=0.24); the 1500- vs 3000-mg arm difference in change was −0.2 mmHg (95%CI: −9.1 to 8.8; P=0.97), Figure 6. The results were similar when the entire follow-up (24 weeks) was taken into account (−5.0±3.8 mmHg in the 1500-mg arm, P=0.19; −0.8±3.4 mmHg in the 3000-mg arm, P=0.80; between-groups difference in change −4.2mmHg [95%CI: −14.2 to 5.8], P=0.41). Seven patients in each arm met the prespecified SBP thresholds for adjustment of therapy; however, only 1 (in the 1500 mg arm) had to be withdrawn from the study because of persistently low SBP.
Figure 6.
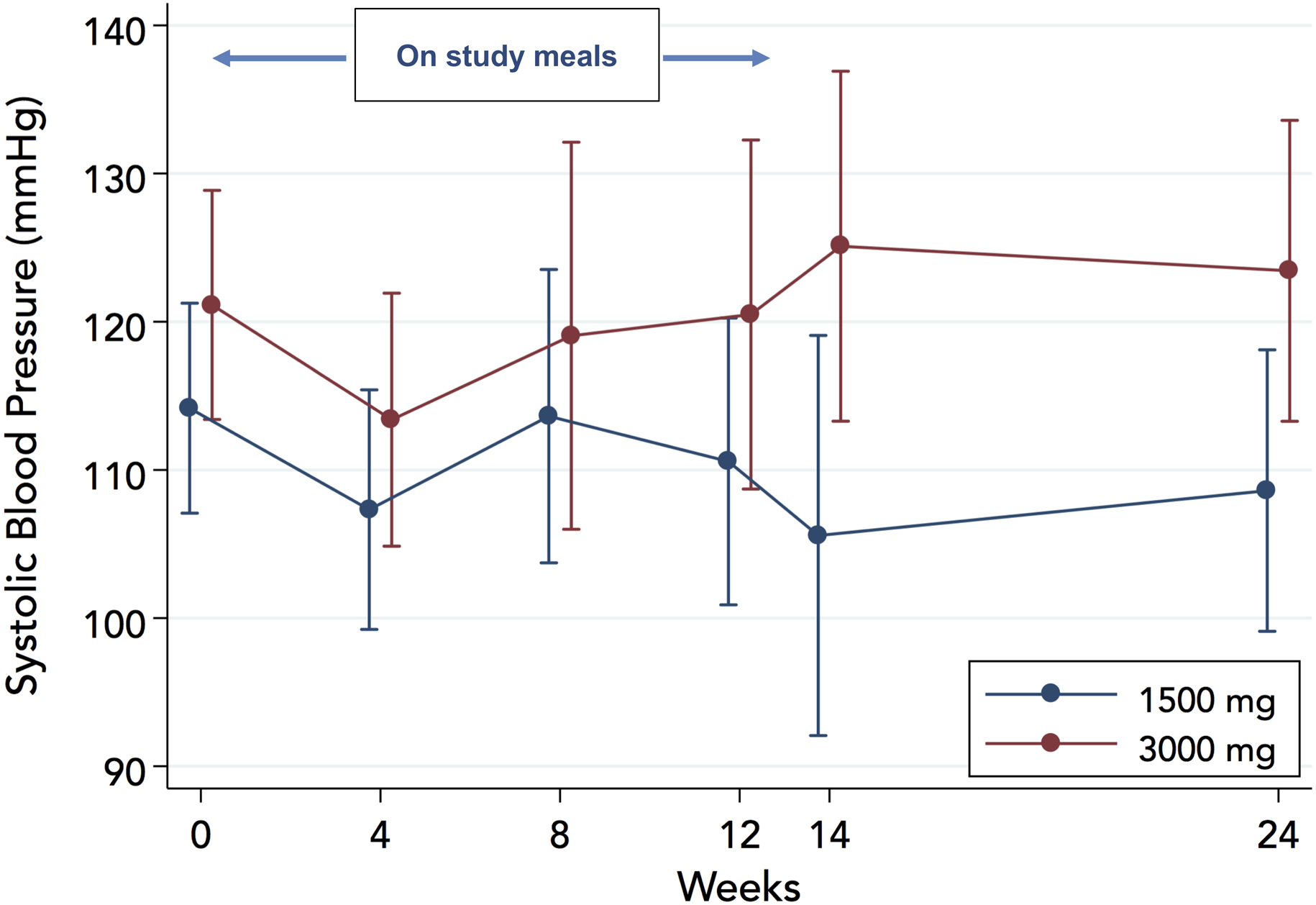
Effect of study diets on systolic blood pressure
Serum creatinine decreased in the 1500-mg arm while on study meals (−0.16±0.05 mg/dL, P=0.001) and remained unchanged in the 3000-mg arm (0.00±0.03 mg/dL; P=0.94), Figure 7. The between-groups difference in change was significant (0.16 mg/dL; 95%CI: 0.05 to 0.26: P=0.005). Results were similar when the entire follow-up (24 weeks) was taken into account (−0.15±0.05 mg/dL in the 1500-mg arm, P=0.001; 0.02±0.03 mg/dL in the 3000-mg arm, P=0.55; 0.17 mg/dL, 95%CI: 0.06 to 0.28; P=0.003 for the between-groups difference in change). No patient discontinued the study as a result of worsening renal function, although 1 patient in the 3000-mg arm met the worsening renal function endpoint (creatinine increase >0.5mg/dL over baseline) at the final (24-week) visit.
Figure 7.
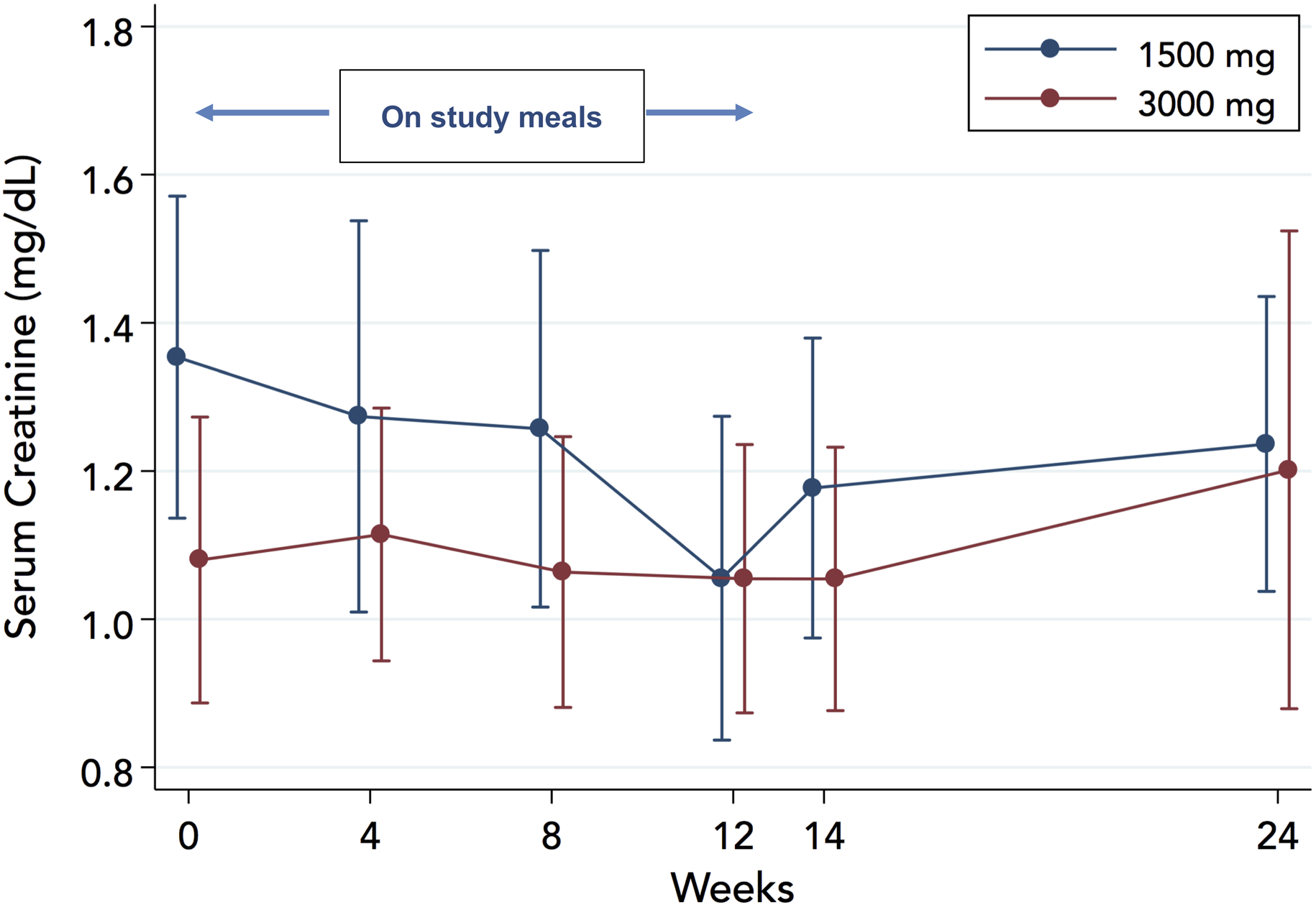
Effect of study diets on serum creatinine
DISCUSSION
In this pilot clinical trial, our findings suggest that a large-scale clinical trial with prepared meals in patients with HF with reduced ejection fraction (HFrEF) to assess the effect of dietary sodium on HF outcomes would be met with significant challenges, especially in post-discharge patients. Only a small fraction of patients admitted with HF eventually participated in the randomized part of the study, as relatively few were both willing and sodium-eligible and many patients withdrew before the feeding part. After 3 years of recruitment efforts, we opted to terminate the study early, as we felt that the unfavorable enrollment trends would not change. Three quarters of the patients were retained throughout the study, but only half remained compliant with the provided food as evident from the serial 24-h urinary collections. On the positive side, patients tolerated both the 1500-mg and the 3000-mg sodium diet well, without any safety signals in terms of clinical events or safety surrogate endpoints. The provided food did not adversely affect quality of life – and food satisfaction improved over time. Several findings merit further discussion.
Even with the presumed convenience of delivered food, patient willingness to participate in a feeding trial is limited. Also, despite the notion of a large pool of eligible patients with HFrEF and high dietary sodium content, there is probably a carryover effect in post-discharge patients from the admission period, during which patients consume less sodium. Although our design provided for a 2-week window to mitigate this carryover effect, we may have not succeeded in this. Therefore, many patients do not meet the dietary sodium threshold to be ethically included a study with a high-sodium diet arm. When all eligibility criteria are applied, the pool of participants narrows significantly. Also, willingness to participate declined between the immediate post-discharge period, when screening took place, and the start of the actual feeding period. A potential explanation is that, as patients become more independent after the immediate, vulnerable post-discharge period, willingness to compromise in terms of food selection diminishes. Of note, in the recently reported GOURMET-HF pilot study, which delivered sodium-restricted (1500-mg) meals for 4 weeks to post-discharge patients (n=33) to compare KCCQ changes vs. usual care (n=33), the investigators had to relax the age entry criterion because of slower-than-expected enrollment despite the lack of urinary sodium requirements.21 In that study, KCCQ improved significantly in both arms by 4 weeks, highlighting the highly dynamic nature of the immediate post-discharge period in patients with acute HF.21
Although the study met the predefined threshold for retention, compliance was modest. Approximately half of the patients consumed the provided food exclusively, with a propensity for lower compliance in the 1500-mg sodium arm. Although we explicitly encouraged all patients to adhere to the provided diet, and allowances were made for non-sodium taste-enhancing items, many patients probably consumed sodium-containing items outside the study diet. Adherence is an important component of dietary intervention, but is difficult to maintain even with coordinated efforts.10, 22 On the positive side, satisfaction with food was acceptable (approximately 4 points out of 5) and improving over time. However, this may also represent selection bias, as patients who did not continue (all before or at 4 weeks) may have done so because of food palatability.
Both diets reduced 24-h urinary sodium substantially. Reduced dietary sodium did not affect NT-proBNP and clinical event rates did not differ significantly between arms, although our study was not powered for clinical events. Numerically more events in the 1500-mg arm were not accompanied by NT-proBNP worsening. Therefore, it is unlikely that the diets caused HF worsening. We did not observe significant effects on SBP; only 1 patient had to be withdrawn because of low SBP, not an unusual finding in HFrEF. Similarly, there were no safety signals for renal function, as only 1 patient developed worsening creatinine several weeks after the active intervention. Similar safety signals with detailed biomarker and clinical data have been reported for a 1500-mg sodium diet (4 weeks) by the GOURMET-HF investigators. Therefore21, we can reasonably conclude that the range of dietary sodium studied in the current pilot trial poses no safety concern. This is not a theoretical concern, as observational work has suggested that sodium restriction may be associated with worse outcomes in HF.23
Providing prepared meals takes away the uncertainty associated with e.g. adherence to modification of lifestyle and cooking habits but brings a host of new challenges. The ongoing SODIUM-HF trial uses intensive counseling to achieve a 1500-mg sodium diet in the active arm (vs. usual care), as the planned sample (N=1000) and multi-national nature of the study makes prepared meals prohibitive.24 The less prescriptive, pragmatic approach helps enrollment rates; however, the actual level of dietary sodium achieved will have to rely on food records, highlighting the trade-offs between feasibility and scientific rigor in dietary intervention studies. Of note, trials with prepared meals are only suitable to assess efficacy, i.e. prove whether a highly sodium-restricted diet (as currently recommended) has any true physiological effects on the course of HF and provide evidence for this common but weakly supported recommendation. However, real-world effectiveness of any dietary strategy would be highly dependent upon factors unrelated to sodium content, including barriers related to age and social support.
Our study has several limitations. First, we only included patients with HFrEF, based on previously outlined rationale,17 and therefore any findings do not apply to the population with HF and preserved ejection fraction. Second, the levels of dietary sodium intake selected for this study (1500 and 3000 mg daily) were arbitrary and based on the need for adequate separation and ethical considerations. Although we did not observe any adverse safety signals, separation of sodium content, especially with partial compliance, may have not been adequate to observe an impact e.g. on NT-proBNP levels. However, our 24-h urinary collection analysis indicated an average of approximately 1000-mg lower sodium intake in the 1500-mg arm. Considering the ongoing debate for the appropriateness of the 2300- vs. the 1500-mg daily sodium intake target, a 800-mg difference, the degree of sodium intake separation achieved in our pilot study seems reasonable. Third, non-Whites and women were not adequately represented in our study and therefore, our conclusions may not apply to these HFrEF subpopulations. Fourth, as patients may have already been rehospitalized by the 2-week enrollment window, we have probably underestimated the rate of the composite of mortality and hospitalization by 12 weeks in the target population. Fifth, evaluating adherence to study diet was challenging as food diaries were inconsistently completed and therefore, we relied on sodium excretion to quantify adherence, which may not be a reproducible method. In addition, completeness of 24-h urinary collections was determined on volume alone, which is an insensitive method.25 Finally, our sample size was smaller than planned and therefore we may have missed important effects on clinical variables and outcomes.
Conclusion
Even with use of prepared meals, an efficacy study to assess optimal dietary sodium in HF is associated with significant challenges. These include need for extensive screening, overcoming reluctance to participate in a feeding study, on-study retention, and compliance with meals. However, both 1500-mg and 3000-mg diets reduced urinary sodium without any adverse safety signal and while maintaining quality of life, indicating that a larger efficacy trial, potentially with modifications to improve patient willingness to participate and compliance, would be ethical and feasible despite the challenges.
Supplementary Material
CLINICAL PERSPECTIVE.
What’s new?
This pilot study gauged the proportion of post-discharge patients with heart failure who exceed the 3000-mg dietary sodium threshold and are willing to participate in a short-term feeding trial and the retention of these patients in a 12-week feeding trial and their compliance with provided meals.
In addition, the study assessed the short-term impact of a 1500-mg vs. 3000-mg sodium diet on natriuretic peptide levels, quality of life, and safety endpoints, including systolic blood pressure and renal function, and trends on clinical endpoints in these patients.
There was no adverse safety or quality of life signal with the provided diets
What are the clinical implications?
Recruiting patients with heart failure for a feeding trial is challenging, even with use of prepared meals, which makes investigating optimal dietary sodium in heart failure problematic.
There is need for extensive screening, as patients may be reluctant to participate in a feeding trial, and compliance with prepared meals is challenging.
However, both the 1500-mg and 3000-mg sodium diets were well tolerated with good food satisfaction and maintained quality of life (in fact, the 1500-mg diet improved quality of life at 12 weeks), without safety concerns.
Therefore, a larger trial, with modifications to improve participation and compliance, would be appropriate.
Funding Sources
This work was supported by a National Heart, Lung, and Blood Institute grant (R34 HL119773).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- 3DFR
3-day food record
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LVEF
left ventricular ejection fraction
- NT-proBNP
N-terminal pro-B- type natriuretic peptide
- PROHIBIT
Prevent Adverse Outcomes in Heart Failure by Limiting Sodium
- SBP
systolic blood pressure
Footnotes
Conflict of Interest Disclosures
APK has received research support from the National Heart, Lung, and Blood Institute, the American Heart Association, the American Society of Echocardiography, the Centers od Disease Control and Prevention, and Critical Diagnostics
JB has received research support from the National Institutes of Health, the Patient Centered Outcomes Research Institute, and the European Union. He serves on the speaker bureau for Novartis, Janssen, and NovoNordisk. He serves as a consultant and/or on steering committee, clinical events committee, or data safety monitoring boards for Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, BerlinCures, Boehringer Ingelheim, Bristol Myers Squib, Cardiocell, CVRx, G3 Pharmaceutical, Innolife, Janssen, Lantheus, LinaNova, Luitpold, Medtronic, Merck, Relypsa, Roche, Sanofi, StealthPeptide, SC Pharma, V-Wave Limited, Vifor, and ZS Pharma.
LP, VVG, SBD, and HS has no relevant disclosures.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852 [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33:1342–1433 [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. , Group ESCSD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200 [DOI] [PubMed] [Google Scholar]
- 4.Real J, Cowles E, Wierzbicki AS, Guideline C. Chronic heart failure in adults: summary of updated NICE guidance. BMJ. 2018;362:k3646. [DOI] [PubMed] [Google Scholar]
- 5.Aronow WS, Shamliyan TA. Dietary Sodium Interventions to Prevent Hospitalization and Readmission in Adults with Congestive Heart Failure. Am J Med. 2018;131:365–370 e361 [DOI] [PubMed] [Google Scholar]
- 6.Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114:221–230 [DOI] [PubMed] [Google Scholar]
- 7.Paterna S, Parrinello G, Cannizzaro S, Fasullo S, Torres D, Sarullo FM, Di Pasquale P. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103:93–102 [DOI] [PubMed] [Google Scholar]
- 8.Parrinello G, Di Pasquale P, Licata G, Torres D, Giammanco M, Fasullo S, Mezzero M, Paterna S. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J Card Fail. 2009;15:864–873 [DOI] [PubMed] [Google Scholar]
- 9.Arcand J, Floras JS, Azevedo E, Mak S, Newton GE, Allard JP. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: the confounding effect of loop diuretics. Am J Clin Nutr. 2011;93:535–541 [DOI] [PubMed] [Google Scholar]
- 10.Lennie TA, Song EK, Wu JR, Chung ML, Dunbar SB, Pressler SJ, Moser DK. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J Card Fail. 2011;17:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son YJ, Lee Y, Song EK. Adherence to a sodium-restricted diet is associated with lower symptom burden and longer cardiac event-free survival in patients with heart failure. J Clin Nurs. 2011;20:3029–3038 [DOI] [PubMed] [Google Scholar]
- 12.Song EK, Moser DK, Dunbar SB, Pressler SJ, Lennie TA. Dietary sodium restriction below 2 g per day predicted shorter event-free survival in patients with mild heart failure. Eur J Cardiovasc Nurs. 2014;13:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, Argano C, Tuttolomondo A, Paterna S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145:459–466 [DOI] [PubMed] [Google Scholar]
- 14.Paterna S, Di Pasquale P, Parrinello G, Fornaciari E, Di Gaudio F, Fasullo S, Giammanco M, Sarullo FM, Licata G. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure: a double-blind study. J Am Coll Cardiol. 2005;45:1997–2003 [DOI] [PubMed] [Google Scholar]
- 15.Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, Terrazzino G, Maringhini G, Ganci F, Scalzo S, et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci. 2011;342:27–37 [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Dunbar SB, Reilly CM, Sands JM, Fonarow GC, Jessup M, Gheorghiade M, Yancy C, Butler J. Dietary sodium intake in heart failure. Circulation. 2012;126:479–485 [DOI] [PubMed] [Google Scholar]
- 17.Butler J, Papadimitriou L, Georgiopoulou V, Skopicki H, Dunbar S, Kalogeropoulos A. Comparing Sodium Intake Strategies in Heart Failure: Rationale and Design of the Prevent Adverse Outcomes in Heart Failure by Limiting Sodium (PROHIBIT) Study. Circ Heart Fail. 2015;8:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, Ueshima H, Zhou BF, Group IR. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). J Hum Hypertens. 2003;17:591–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension. 1982;4:805–808 [DOI] [PubMed] [Google Scholar]
- 20.Luft FC, Sloan RS, Fineberg NS, Free AH. The utility of overnight urine collections in assessing compliance with a low sodium intake diet. JAMA. 1983;249:1764–1768 [PubMed] [Google Scholar]
- 21.Hummel SL, Karmally W, Gillespie BW, Helmke S, Teruya S, Wells J, Trumble E, Jimenez O, Marolt C, Wessler JD, et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ Heart Fail. 2018;11:e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunbar SB, Clark PC, Reilly CM, Gary RA, Smith A, McCarty F, Higgins M, Grossniklaus D, Kaslow N, Frediani J, et al. A trial of family partnership and education interventions in heart failure. J Card Fail. 2013;19:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin MF, Richardson D, Powell LH. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC Heart Fail. 2016;4:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colin-Ramirez E, Ezekowitz JA, investigators S-H. Rationale and design of the Study of Dietary Intervention Under 100 MMOL in Heart Failure (SODIUM-HF). Am Heart J. 2018;205:87–96 [DOI] [PubMed] [Google Scholar]
- 25.John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJ, Patel SM. Accuracy and Usefulness of Select Methods for Assessing Complete Collection of 24-Hour Urine: A Systematic Review. J Clin Hypertens (Greenwich). 2016;18:456–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


