Abstract
Background and aim of the work: Post-operative periprosthetic shoulder fractures incidence is gradually raising due to aging of population and increasing of reverse total shoulder arthroplasty (RTSA). Management of this complication represents a challenge for the orthopedic surgeon. Aim of the present study is to critically review the recent literature about epidemiology, risk factors, diagnosis, management and outcome of post-operative periprosthetic humeral fractures occurring on RTSA. Methods: A systematic search of Embase, Medline and Pubmed was performed by two reviewers who selected the eligible papers favoring studies published in the last ten years. Epidemiology, risk factors, diagnostic features, clinical management and outcome of different techniques were all reviewed. Results: 31 studies including reviews, meta-analysis, case reports, clinical and biomechanical studies were selected. Conclusions: Correct clinical management requires adequate diagnosis and evaluation of risk factors. Conservative treatment is rarely indicated. Locking plate fixation and revision arthroplasty are both valuable treatment methods. Surgical technique should be chosen considering age and functional demand, comorbidities, fracture morphology and location, bone quality and stability of the implant. Given the correct indication all surgical treatment can lead to satisfactory clinical and radiographic results despite a relevant complication rate. (www.actabiomedica.it)
Keywords: periprosthetic shoulder fractures, RTSA, complications, management, humeral fracture
Introduction
Indications for RTSA increased over time from cuff tear arthropathy to include many other conditions that were difficult to treat with anatomical shoulder arthroplasty, such as acute proximal humerus fracture (Neer 3,4), chronic locked dislocation, immunological arthritis (in particular Reumathoid Arthritis, RA), proximal humeral fracture sequelae, failed primary shoulder arthroplasty and tumors (1, 2).
In recent years periprosthetic shoulder fractures became a growing problem due to aging of general population and to the increase in reverse total shoulder arthroplasty (RTSA) implants (3, 4).
RTSA complication rates are in a range between 19% to 68% and include principally scapular notching (50% - 96%), neurological injuries (1%-4%), infections (1%- 15%), instability (2%-31%) and periprosthetic fractures (1%-20%) (5-7).
Fractures include the greater or lesser tuberosity, metaphyseal portion or surgical neck, proximal humeral diaphysis, and the mid- and distal diaphysis. Advanced age and comorbidities often characterizing periprosthetic shoulder fracture patients add to the intrinsic technical difficulty in treating these injuries. Therefore, clinical and surgical management of these lesions can be a challenge for the orthopedic surgeon. Aim of treatment should be early functional recovery and prompt fracture healing minimizing the risk of complications. Aim of the present review is to report a summary of literature evidence about epidemiology, risk factors, diagnosis, management and outcome of post-operative periprosthetic humeral fractures after RTSA.
Methods
Two of the authors (FF and RF) independently reviewed studies by a systematic search of Embase, Medline and Pubmed using various combinations of the terms “periprosthetic, shoulder”, “shoulder arthroplasty, fracture”, “periprosthetic, fracture”, “shoulder arthroplasty, complication”, “RTSA, fracture”, “humeral fracture in RTSA”. The two authors screened the titles and abstracts of the citations identified independently and in duplicate, and acquired the full text of any article that either judged potentially eligible, favoring studies published in the last ten years. Epidemiology, risk factors, diagnostic features, clinical management and outcome of different techniques were all reviewed. Disagreements were resolved by discussion.
Results
A total of 31 studies were selected, including reviews, meta-analysis and clinical series. Case reports and small case series reporting about complications and very uncommon events were also included.
Discussion
Epidemiology
Introduction of reverse shoulder arthroplasty has improved treatment of patients with glenohumeral arthritis or prior failed shoulder arthroplasty associated with rotator cuff disorders in addition to high complex pattern proximal humeral fractures and inveterate dislocation.
Consequently, the utilization of RTSA is increasing, with a reported incidence of 33% in a recent epidemiological study by Schairer et al.(7) in primary shoulder arthroplasty. In USA the use of RTSA was approved by FDA since 2004 with approximately 10.000 RTSA performed in 2007 with a growing number of 30.000 in 2012 (11).
However, periprosthetic humeral fractures are relatively rare and there is limited information in the literature regarding such injuries.
Nonetheless, these fractures became a growing problem in recent years. The reasons of this trend probably reside both in aging of the general population, with several comorbidities including osteoporosis and higher risk of fall to the ground and in the growing number of RTSA (11).
The reported incidence in the USA reaches between 0,6 to 3% for RTSA, changing to 1,6 to 2,3% if all shoulder arthroplasties are considered. A retrospective cohort study using the data from the Mayo Clinic Medical Center Total Joint Registry (1976-2008) identified a postoperative humeral fractures rate of 0.9% (8-10).
Risk Factors
Risk factors can be divided in patient related and implant related. The main patient related risk factor for periprosthetic shoulder fracture is advanced age, particularly because of its association with higher risk of fall to the ground and with osteoporosis, which may both be considered as independent risk factors. Nonetheless, in the last years mean age of periprosthetic fracture patients notably raised, with a reported mean age of 80 years in 2018, resulting higher than the reported mean age in 1994 (71 years) (12, 13). Medical conditions associated to ambulation instability and/or to higher risk of fall as cardiac and neurologic pathologies may all be considered as risk factors. Chronic use of osteopenia inducing drugs such as corticosteroids or any other medical condition affecting bone quality may also be identified as risk factor. Moreover, bone quality is a critical factor to be considered for conservative treatment versus surgical treatment.
Other medical conditions as diabetes may act both as risk factor for fracture and as factors negatively affecting outcome. Diabetic patients may indeed be considered at risk for both periprosthetic fracture because of risk of fall due to hypoglycemia episodes and postoperative infections. Moreover, the same patients may be at risk for unfavorable outcome and complications because of immunological, vascular and neurologic peripheral compromise.
Epidemiologic data identify rheumatoid arthritis as a major risk factor, associated to about half of all the periprosthetic shoulder fracture cases described in the literature (14, 15).
The implant related risk factors include revision surgery, over-reaming or using an oversized broach in the humeral component preparation, humeral deformity, and excessive soft tissues tightness coming from errors in bone cuts or components size (16).
Diagnosis
In most cases periprosthetic shoulder fractures diagnosis is straightforward, based on clinical suspicion that should always arise in case of trauma occurring to a prosthetic joint.
Radiographic evaluation is important to identify potential component loosening. A Grashey view of the glenohumeral joint and a true axillary view to assess for humeral head subluxation and glenoid component loosening should be better obtained. Images should include orthogonal views of the fracture.
A CT scan is usually diagnostic in doubt cases (classification type) and might be useful for preop planning.
Classification
Fractures can be first of all divided in intraoperative fractures (59%) and postoperative fractures (41%) (17).
The first classification was developed by Wright and Cofield and was a classification system simply based on the location of the fracture relative to the tip of the humeral prosthesis. This classification was originally created for post-operative fractures and it is limited to those occurring near the tip of the humeral stem. Type A fractures occur at tip with proximal extension greater than 1/3 of the stem length. Type B fracture also occur about the stem tip, with less proximal extension. Type C fractures occur distal to stem tip and are considered humeral shaft fractures (18). Nonoperative treatment is often limited to type A and well aligned type C fractures.
Later Campbell et al. (19) defined four categories related to the fracture site: (A) tuberosities region; (B) metaphyseal portion or surgical neck; (C) proximal humeral diaphysis; and (D) mid- and distal humeral diaphysis. This type of classification results more adequate for intra-operative fractures.
Groh et al. (20) distinguished Type I fractures as occurring proximal to the tip of the prosthesis; Type II extending from the proximal part of the humeral shaft to beyond the distal tip of the stem; and Type III as fractures lying distal to the tip of the prosthesis.
In 2018 Kirchkoff et al (21) developed a more complex classification including three subclassifications: location of the fractures (acromial, glenoidal and humeral), type of fractures (tuberosities, spiral, oblique, distal) and implant stability (stable, loose). They also proposed a simple algorithm with these three classification subtypes to suggest the treatment (ORIF vs conservative or revision).
Treatment
Correct indication for treatment of these complex lesions can differ case by case. The variables influencing the decisional process are many: general health status and functional demand of the patient, fracture location and morphology, bone quality, and implant stability.
Surgical experience of the treating surgeon should also be considered (22-23).
Aim of surgical treatment should be functional recovery with respect to pre-injury activity level, minimizing complications. Healing is generally considered when the patient fully recovers activities of daily living without pain, associated with radiographic healing.
Athwal et al. (16) reported that the first treatment for periprosthetic humeral fractures (PHF) begins with prevention and that special care should be taken in patients with documented risk factors (osteopenia, RA, revision surgery, etc.) to avoid increasing stress on the humerus.
Treatment modalities for periprosthetic shoulder fractures range from conservative treatment to ORIF (open reduction internal fixation) and revision arthroplasty. In case of non-displaced or minimal displaced fractures with transverse or spiroid morphology conservative treatment may be indicated: splint mobilization in neutral rotation or abduction is preferable to avoid diaphyseal rotational malunion (24).
In displaced periprosthetic fractures conservative treatment may be indicated only in low functional requirements patients or in the presence of severe comorbidities (21).
Osteosynthesis can be done using various approaches: anterior shoulder approach (deltopectoral approach), posterior or lateral approach. The latter two enable visualization and protection of radial nerve, with different advantages and indications related mostly to fracture location. In particular, posterior approach is generally preferred for more distal fractures and deltopectoral or antero-lateral approaches for proximal/mid third fractures. Identification of the radial nerve is always needed to allow its protection when diaphyseal cerclages have to be performed.
Fixation can be done with plates and screws, plates and cerclages, and plates and screws associated with cerclages. Locked screws promote rotational control, and cerclage increases the stability of the construct at the stem level.
Angelini et al. (25) suggest cerclage wires to function as a temporary tool for reduction during surgery and that when correctly applied the damage to bone blood supply is less than expected. Cameron et al. (26) reported that when treating unstable diaphyseal periprosthetic humeral fractures with well-fixed components, a heavy plate with proximal cerclage wires and distal screws is the treatment of choice (Figure 1). The plate should overlap the tip of the prosthesis by two cortical diameters to avoid the creation of a stress riser.
Figure 1.
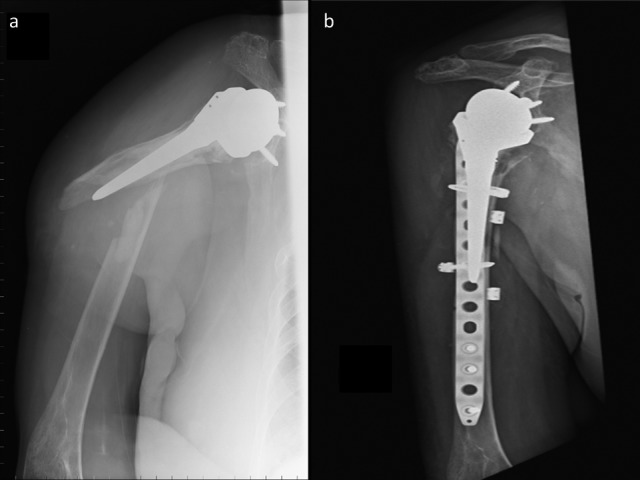
A 78-year-old woman, right-handed, fell to the ground 10 years after cemented RTSA implantation for right shoulder cuff tear arthropathy. a) pre-op radiograph showing periprosthetic humeral fracture. b) post-operative radiograph showing fracture reduction and fixation with 4.5mm 14 holes locking plate with cerclages and screws. Clinical healing with full activities of daily living recovery was documented at 18 months
There seem to be no significant differences in the rate of periprosthetic humeral fractures between uncemented RTSA and cemented RTSA (27).
Based on the experience with Vancouver B3 hip fractures, Thes et al (28) described an internal fixation technique for periprosthetic humeral fractures in patients with severe osteoporosis and bone loss. The authors describe a technique where the fracture is surrounded by two hemicylinder of tibial allografts, placed around the humerus to create a “sarcophagus” system. The allograft was as long as possible for optimal mechanical stability, without creating impingement with the glenoid and the elbow. Final fixation of the allograft is obtained with two cerclage wires.
Revision surgery should always be considered when humeral component loosening is detected. Described radiographic signs of loosening are the presence of a radiolucent line measuring >2 mm in three or more zones around the perimeter of the stem or when a change in the relative position on the stem is found on serial radiographs (29). In these cases, analyzing previous radiographs is crucial for treatment planning.
In post-operative management, all patients should be immobilized in a shoulder sling. Shoulder flexion and abduction should be limited to 90° for six weeks post-operatively. Clinical and radiological controls should be performed at six weeks, 12 weeks and 12 months after operative treatment. Periprosthetic humeral fractures healed in literature reports at a mean time of 18 weeks (range 16-20) with a non-union rate of about 13% (30).
The overall complication rate is reported to be between 20% to 40%. Non-union or malunion are especially associated with conservative treatment. Loss of shoulder motion is the primary reason for an unsatisfactory result (based on the Neer criteria).
Other complications included neurapraxias ( axillary nerve or radial nerves, 6-25% reported), frozen shoulder, and superficial infection (30, 31).
Clinical outcomes are usually evaluated using a visual analog scale (VAS) for pain, American Shoulder and Elbow Surgeon (ASES) score, and subjective shoulder value (SSV). Active ROM (range of motion) is normally evaluated in terms of forward flexion, abduction, and external rotation with the arm at the side and internal rotation with the arm at the back. Radiological outcomes should be assessed by serial plain radiographs.
Conclusions
Periprosthetic shoulder fractures are a growing clinical problem. Correct clinical management requires adequate diagnosis and evaluation of risk factors. Conservative treatment is rarely indicated. Locking plate fixation, cerclages and revision arthroplasty are all valuable treatment methods. Surgical technique should be chosen considering age and functional demand, comorbidities, fracture morphology and type, bone quality and stability of the implant. Given a correct indication all surgical treatment can lead to satisfactory clinical and radiographic results despite a relevant complication rate.
Compliance with ethical standards
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Austin Zmistowski B, Chang ES, Williams GR., Jr Is reverse shoulder arthroplasty a reasonable alternative for revision arthroplasty. Clin Orthop Relat Res. 2011;469:2531–7. doi: 10.1007/s11999-010-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: A systematic review. J Bone Joint Surg [Br] 2012;94:577–83. doi: 10.1302/0301-620X.94B5.27596. [DOI] [PubMed] [Google Scholar]
- 3.Frankle M, Siegal S, Pupello D, et al. The Reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg [Am] 2005;87:1697–705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 4.Werner CM, steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and socket total shoulder prosthesis. J Bone Joint Surg [Am] 2005;87:1476–86. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 5.Affonso J, Nicholson GP, Frankle Ma, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157–68. [PubMed] [Google Scholar]
- 6.Wierks C, skolasky rl, Ji JH, Mcfarland Eg. Reverse total shoulder replacement. Intraoperative and early postoperative complications. Clin Orthop Relat Res. 2009;467:225–34. doi: 10.1007/s11999-008-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schairer WW, Nwachukwu BU, Lyman S, et al. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24:91–7. doi: 10.1016/j.jse.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388–94. doi: 10.1016/j.jse.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 9.García-Fernández C, Lópiz-Morales Y, Rodríguez A, López-Durán L, Martínez FM. Periprosthetic humeral fractures associated with reverse total shoulder arthroplasty: incidence and management. Int Orthop. 2015;39(10):1965–9. doi: 10.1007/s00264-015-2972-7. [DOI] [PubMed] [Google Scholar]
- 10.Singh JA, Sperling J, Schleck C, Harmsen W, Cofield R. Periprosthetic fractures associated with primary total shoulder arthroplasty and primary humeral head replacement. J Bone Joint Surg Am. 2012;94:1777–1785. doi: 10.2106/JBJS.J.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Wise Bl, Zhang Y, szabo rM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–54. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 12.Mineo GV, Accetta R, Franceschini M, et al. Management of shoulder periprosthetic fractures: our institutional experience and review of the literature. Injury. 2013;44(S1):S82–85. doi: 10.1016/S0020-1383(13)70018-4. [DOI] [PubMed] [Google Scholar]
- 13.Greiner S, Stein V, Scheibel M. Periprosthetic humeral fractures after shoulder and elbow arthroplasty. Acta Chir Orthop Traumatol Cechoslov. 2011;78(6):490–500. [PubMed] [Google Scholar]
- 14.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and non reconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10:17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 15.Ekelund A, Nyberg R. Can reverse shoulder arthroplasty be used with few complications in rheumatoid arthritis. Clin Orthop Relat Res. 2011;469:2483–8. doi: 10.1007/s11999-010-1654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Athwal GS, Sperling JW, Rispoli DM, et al. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am. 2009;91:594–603. doi: 10.2106/JBJS.H.00439. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. JBJS Am. 2004;86:6809. doi: 10.2106/00004623-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Wright TW, Cofield RH. Humeral fractures after shoulder arthroplasty. J. Bone Joint Surg. Am. 1995 131 Sep;77(9):1340–1346. doi: 10.2106/00004623-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Campbell JT, Moore RS, Iannotti JP, et al. Periprosthetic humeral fractures: mechanisms of fracture and treatment options. J Shoulder Elbow Surg. 1998;7:406–413. doi: 10.1016/s1058-2746(98)90033-7. [DOI] [PubMed] [Google Scholar]
- 20.Groh GI, Heckman MM, Wirth MA, et al. Treatment of fractures adjacent to humeral prostheses. J Shoulder Elbow Surg. 2008;17:85–89. doi: 10.1016/j.jse.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff Chlodwig, Marc Beirer, Ulrich Brunner, Arne Buchholz, Peter Biberthaler. Moritz Crönlein - Validation of a new classification for periprosthetic shoulder fractures - SICOT aisbl 2018. doi: 10.1007/s00264-018-3774-5. [DOI] [PubMed] [Google Scholar]
- 22.Chul-Hyun Cho, MD, Kwang-Soon Song, MD, Tae-Won Koo, MD. Clinical Outcomes and Complications during the Learning Curve for Reverse Total Shoulder Arthroplasty: An Analysis of the First 40 Cases. Clinics in Orthopedic Surgery. 2017;9:213–217. doi: 10.4055/cios.2017.9.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempton LB, Ankerson E, Wiater JM. A complication based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496–504. doi: 10.1007/s11999-011-1811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boileau, Complication and revision of reverse total shoulder arthroplasty Orthopedic & Traumatology Surgery & Research. 102(2016):S33–S43. doi: 10.1016/j.otsr.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Angelini A, Battiato C. Past and present of the use of cerclage wires in orthopedics. Eur J Orthop Surg Traumatol. 2015;25:623–635. doi: 10.1007/s00590-014-1520-2. [DOI] [PubMed] [Google Scholar]
- 26.Cameron B, Iannottu JP. Periprosthetic of the humerus and scapula: management and prevention. Orthop Clin N Am. 1999;30:305–318. doi: 10.1016/s0030-5898(05)70085-7. [DOI] [PubMed] [Google Scholar]
- 27.King JJ, Farmer KW, Struk AM, Wright TW. Uncemented versus cemented humeral stem fixation in reverse shoulder arthroplasty. Int Orthop. 2015;39:291–298. doi: 10.1007/s00264-014-2593-6. [DOI] [PubMed] [Google Scholar]
- 28.Thes Andre, Shahnaz Klouche, Marine de Tienda, Thomas Bauer, Philippe Hardy. Cortical onlay strut allograft with cerclage wiring of periprosthetic fractures of the humerus without stem loosening: technique and preliminary results. Technical Note • Shoulder - Arthroplasty Springer-Verlag France. 2017 doi: 10.1007/s00590-017-1961-5. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Sotelo J, Wright TW, O’Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16:180–187. doi: 10.1054/arth.2001.20905. [DOI] [PubMed] [Google Scholar]
- 30.Wierks C, Skolasky RL, Ji JH, McFarland EG. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res. 2009;467(1):225–234. doi: 10.1007/s11999-008-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd AD, Jr, Thornhill TS, Barnes CL. Fractures adjacent to humeral prostheses. J Bone Joint Surg Am. 1992;74(10):1498–1504. [PubMed] [Google Scholar]


