Abstract
Background: Delamination of acetabular articular cartilage is a common progressive abnormality in hips with femoroacetabular impingement. The aim of this study is to compare the effectiveness of two different procedures for the arthroscopic treatment of acetabular delamination: microfractures (MFx) and micro-fragmented autologous adipose tissue transplantation (MATT) technique. Methods: We carried out a controlled retrospective study of 35 patients affected by an acetabular cartilage delamination in femoroacetabular impingement (FAI). In all the selected cases the size of the defect ranged from 1 to 2 cm2, with a mean size of 1.9 cm2 in MFx group and 1.6 cm2 in MATT group (p=0.1). Of these, 18 patients were treated with MFx while 17 patients were treated with MATT. The two groups were similar in terms of clinical, functional and radiological aspects. All the patients were assessed before and after the procedure, for pain and function, with the modified Harris Hip Score (mHHS). The mean preoperative mHHS was 50±5 for MFx group and 53±6 for MATT group (p = 0.245). All the patients were followed-up for two years. Results: The final mHHS was 76±12 in MFx group and 97.1±3 in MATT group (p<0.001). In both groups neither a conversion to total hip arthroplasty nor a revision hip arthroscopy was observed. Conclusions: The results of this study provide proof that MATT technique improves clinical outcomes with a mHH scoring significantly higher than MFx group. (www.actabiomedica.it)
Keywords: hip arthroscopy, cartilage defect, FAI, Lipogems®, microfractures
Introduction
Treatment of hip cartilage disease is challenging and there is no clear algorithm to address this entity. With the growth of surgical skills in hip arthroscopy, cartilage restoration techniques are evolving in a fast and exponential manner (1). Biological and surgical treatments have been proposed to treat these pathologies. Biological treatments include platelet-rich plasma, stem cells or bone marrow aspirate concentration, hyaluronic acid and others (2-6). Surgical treatments include debridement, microfracture, autologous chondrocyte implantation, matrix-induced chondrocyte implantation, autologous matrix-induced chondrogenesis, mosaicplasty, osteochondral allograft transplantation and stem cells, injected or implanted in a matrix (stem cells in membranes/expanded stem cells) (7-20).
Cell-based therapies using either chondrocytes or mesenchymal stem cells (MSCs) represent an appealing alternative strategy. Different protocols of intra-articular MSCs injection have been studied to treat chondral injuries in large animals such as sheep, pigs and horses. According to these interesting preclinical results, support for MSCs-based therapies in clinical practice have been provided (21).
Recently some Authors reported on intra-articular injection of MSCs into the knee for the treatment of focal defect or more generalized cartilage loss in osteoarthritis, showing interesting results regard pain and clinical outcome (22).
The aim of this retrospective study is to compare two kind of treatment for acetabular delamination associated to Femoro-Acetabular Impingement (FAI): microfractures (MFx) and micro-fragmented autologous adipose tissue transplantation (MATT) technique.
The treatment differs in that the MFx technique involves making multiple holes (microfractures) into the subchondral plate at the site of the chondral defect, allowing bone marrow derived pluripotent cells to fill the damaged area. The MATT procedure contrarily, after harvesting the subcutaneous adipose tissue from the perithrocanteric area of the operated hip, incorporates MSC into bioactive units that are injected into the joint to cover the chondral defect, in a single arthroscopic surgical step.
Materials and methods
From 2007 to 2015 we carried out 249 hip arthroscopies in patients where an acetabular delamination was associated with FAI. Chondral defects have been treated with MFx (in 58 cases) or MATT (in 91 cases).
The institutional review board of the involved hospital decided that no ethical approval was necessary as it was thought that for this retrospective study the informed consent of the patient was sufficient. This research has been ethically conducted according to the World Medical Association Declaration of Helsinki.
In order to select two homogeneous groups, to compare outcomes obtained using the two different techniques, we applied stringent inclusion and exclusion criteria.
The inclusion criteria were: patients with symptomatic FAI; acetabular delamination; lesion size from 1 to 2 cm2; less than grade 1 in degenerative radiological changes (Tonnis scale); patients’ age between 18 and 60 years; a minimum follow up of two years.
The exclusion criteria were: osteoarthritis (OA) secondary to hip developmental diseases (developmental hip dysplasia, Perthes disease, slipped capital femoral epiphysis); autoimmune disease; avascular necrosis (AVN); coxa profunda; protrusio acetabuli; any other associated complementary surgical treatment as collagen membranes implantation or fibrin glue and bone marrow stem cells injection.
In determining criteria used to select the patients our objective was to obtain two homogeneous groups in terms of age, clinical aspects and degree, area and localization of chondral lesion.
Patients who strictly met inclusion and exclusion criteria were 35, reviewed retrospectively and divided in two groups (Table 1):
Table 1.
Characteristics of patients enrolled
| MFx | MATT | |
| n° | 18 (6 F/12M) | 17 (8 F/9 M) |
| Mean age | 36±13 (range 19-59) | 35±9 (range 22-54) |
| Acetabular chondral lesion (cm2) | 1.9 ± 0.3 (range 1-2) | 1.6 ± 0.5 (range 1-2) |
| FAI | 13 Cam 4 Pincer 1 combined | 12 Cam 2 Pincer 3 combined |
- A) 18 patients treated with arthroscopic MFx (12 male, 6 female) with a mean age at surgery of 36±13 years (range 19-59) and a mean lesion size of 1.9±0.3 cm2 (range 1-2 cm2); in this group the FAI classified as Cam in 13 cases, Pincer in four cases and combined in one case.
- B) 17 patients were treated with arthroscopic MATT (9 male, 8 female) with a mean age at surgery of 35±9 years (range 22-54) and a mean lesion size of 1.6±0.5 cm2 (range 1-2 cm2); in this group the FAI classified as Cam in 12 cases, Pincer in two cases and combined in three cases.
All patients took standard antero-posterior (AP) X-ray of the pelvis, AP view and cross-table view of the affected hip preoperatively; an MRI (Magnetic Resonance Imaging) and e CT (Cumputed Tomography)-scan with a 3D reconstruction of the affected hip was performed to diagnose the labral injury, chondral defects, bone deformities and other intra and extra articular pathologies.
The modified Harris Hip Score (mHHS) was administered to all patients before surgery (T0) and postoperatively at six months (T1), one year (T2) and at final two years follow-up (T3).
Surgical technique
All hip arthroscopies were performed by the same operator (AF). The patient was positioned in lateral decubitus, under combined anesthesia (spinal anesthesia and deep sedation). The affected hip was prepared and draped in the usual sterile fashion, and traction was applied to open the joint space. A first supra-throcanteric and a second anterior-supra-para-throcanteric portal were used. The first portal was performed under image amplifier guidance, while the second under direct arthroscopic visualization. A 70° arthroscope was used. To treat FAI deformities was performed an arthroscopic femoral head and/or acetabular rim resection, and any detached labrum was reattached to the acetabular rim with suture anchors while any fibrillar deterioration of the labrum was treated with radiofrequencies. Acetabular delamination was detected and localized by using an arthroscopic probe. The wave or carpet sign was defined as the major arthroscopic evidence of the delaminated area. Any detachment of the chondral fibrous mantel from the subchondral bone was assessed. In cases where the subchondral bone was exposed, the chondral defect was classified as a flap lesion and not as a delamination. The delaminated area was topographically localized according to the mapping system illustrated in Fig. 1.
Figure 1.
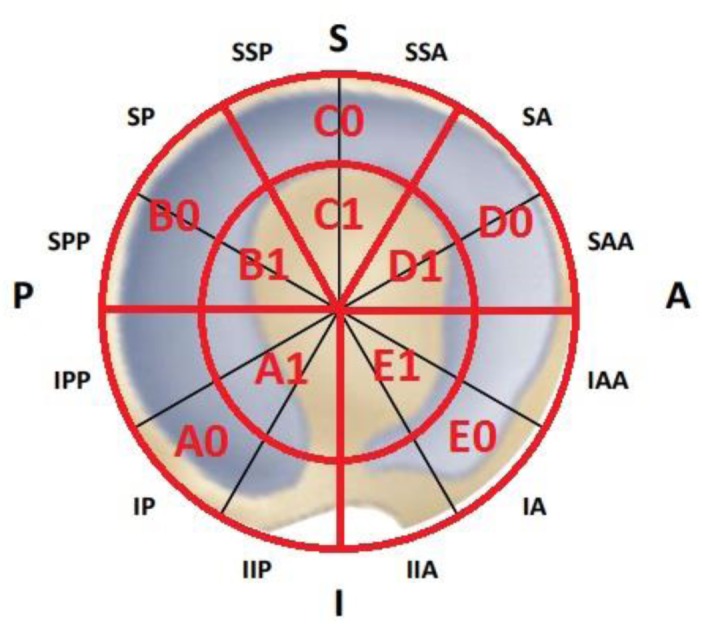
Acetabular topographic localization mapping system
MFxs were arthroscopically performed using a 30° or 60° degree angled awl, carefully inserted in between the subchondral bone and the fibrous chondral layer. The subchondral surface was therefore traumatized or scratched by hammering the awl, in order to allow the bleeding. Maximum care was taken not to interrupt the delaminated layer (Fig. 2).
Figure 2.
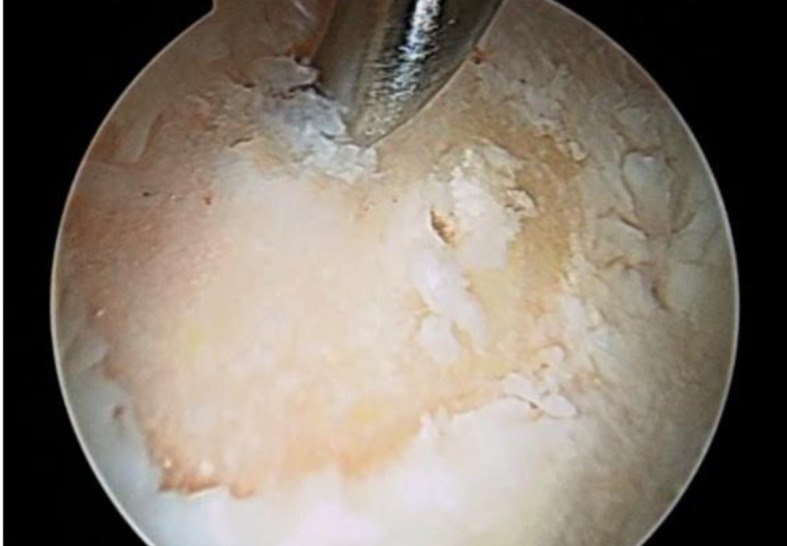
Intra-operative image of MFx. Subchondral bone was perforated by hammering the awl to allow bleeding
The MATT procedure was as well performed as a single arthroscopic surgical step. About 40 ml of subcutaneous adipose tissue was harvested from the perithrocanteric area of the operated hip by manual liposuction. Mild mechanical forces were applied using a completely closed system (Lipogems®), avoiding enzymes, additives, and other manipulations. The harvested tissue was therefore manually microfragmented in order to remove blood and oil residuals and washed with saline solution. The product obtained (the transplant), of about 7 ml, incorporates MSC into bioactive units (23). One needle was inserted in between the subchondral bone and the fibrous chondral layer and the transplant injected directly in the delaminated area, after removing the arthroscopic fluids from the joint (Fig. 3).
Figure 3.
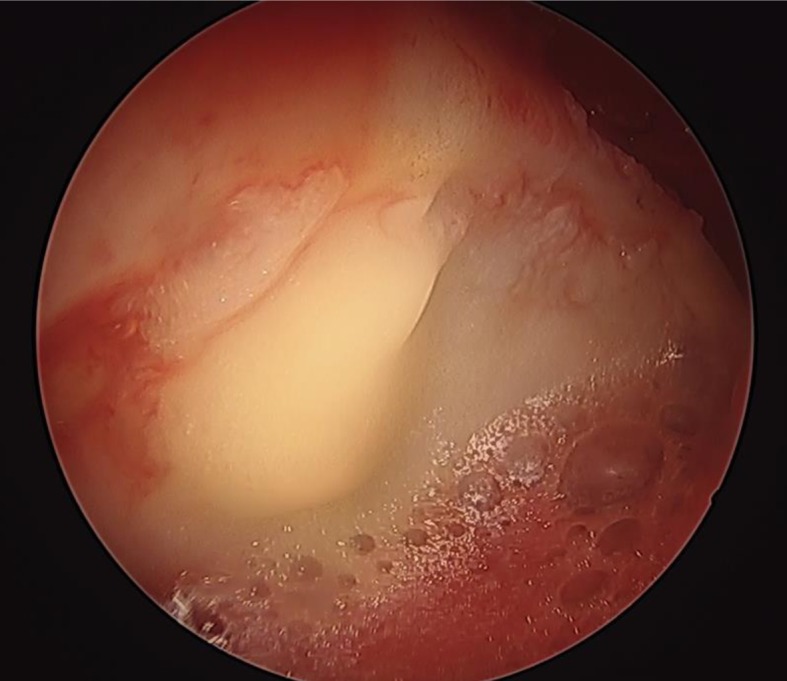
Intra-operative image of MATT. Lipogems® injected directly in the delaminated area by a needle after water suction
Postoperative and Rehabilitation Protocol
Postoperatively the patients in both groups followed the same rehabilitation program.
Isotonic and isometric quadriceps and gluteus contractions started the first postoperative day as well as continuous passive motion, from 0°-40° to 0°-90° of flexion with a daily increase of 10°. Walking was allowed with the aid of two crutches with partial weight-bearing (30% of body weight) on the operated leg for three weeks. From post-operative day two, patients started gym bike up to speed “0” 15 minutes three times a day, and active exercises to regain the full range of motion. Swimming freestyle or on the back was allowed after two weeks. At four weeks post-op, walking with the aid of one crutch opposite to the treated leg was allowed for seven days, then normal walking thereafter.
Resumption of normal work activities became possible 2/4 weeks after surgery. Impact sport activities could resume at three months post-op and complete return to sport activities was allowed six months after surgery.
Statistical analysis
The data from this study was analyzed through the use of the SPSS statistics software program (SPSS Inc. Chicago, USA). A t test was used for normally distributed, continuous variables. The Mann-Whitney U test was applied for continuous variables, which were not normally distributed.
A p value <0.05 was considered statistically significant. Descriptive statistics are presented as mean (±SD).
Results
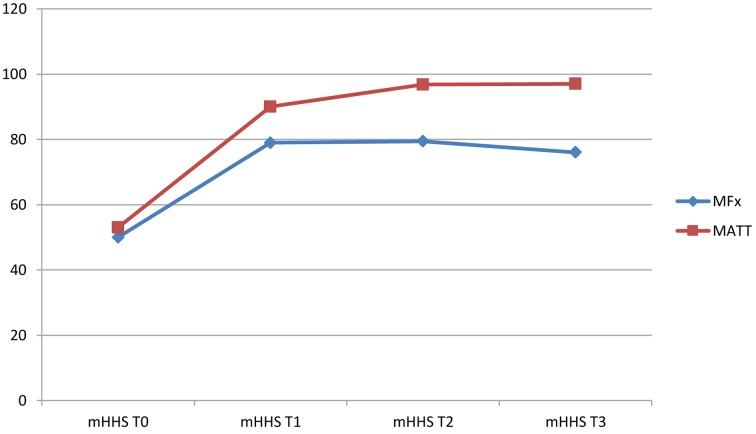
The median preoperative (T0) mHHS was 50±5 for MFx group and 53±6 for MATT group. Both increased at six months (T1) to 79±7 for MFx and 90±13 for MATT, and at one year (T2) to 79.4±11 for MFx and 96.8±3 for MATT.
At follow-up of two years (T3) we observed a progressive reduction of functionality in MFx group with a mHHS of 76±12 while in the MATT group mHHS score increased to 97.1±3.
In both groups neither a conversion to total hip arthroplasty nor a revision hip arthroscopy was observed. No major complications or infections were reported in both groups.
Differences in outcomes between the two groups were significant at T1 (p=.003), T2 and T3 (p<0.001) while there are no statistically significant differences at T0 (p=.245) (Table 2; Fig. 4)
Table 2.
Results
| mHHS | MFx | MATT | p |
| T0 | 50±5 | 53±6 | .245 |
| T1 | 79±7 | 90±13 | .003 |
| T2 | 79.4±11 | 96.8±3 | <0.001 |
| T3 | 76±12 | 97.1±3 | <0.001 |
A p-value <0.05 was considered statistically significant.
Figure 4.
Evolution of clinical and functional scoring (mHHS) in two treatment groups (MFx Vs MATT)
Discussion
This is the first study to compare clinical outcomes of using MFx and MATT in treatment of FAI-induced early acetabular chondral lesion (24).
We know that when compromised cartilage is associated with FAI, treatment which targets both pathological processes gives a better outcome (25), but anyway very few data are present in literature about chondral lesions of the hip and their treatment, and even less showing differences between two or more kinds of treatment (8).
Several arthroscopic techniques have been used to treat hip chondral lesions, as an example the use of stem cells has been reported in preclinical studies (26), but only few studies report valuable outcomes (7). Therefore comprehensive evidence-based guidelines for the treatment of chondral lesions of the hip remain to be defined (27-29).
MFx is still the treatment of choice for small chondral defects of the acetabulum and femoral head (30). Satisfactory clinical results after MFx for lesions in the hip, including those in athletes, have been recently reported (31). Although MFx has been shown to be effective in management of lesion measuring less than 2 cm2 in the knee, deterioration in the function of the knee in 47% and 80% of patients after a mean of two years has been reported in an evidence-based systematic analysis assessing a total of 3122 patients (7 to 1200) (32). In the hip also the worst results were recorded in cases with chondral defect equal to or greater than 3 cm2 (8, 33).
These are the reasons why we strictly respected the inclusion criteria illustrated, enrolling exclusively patients with chondral defect equal to or inferior than 2 cm2, with no statistically significant differences in lesion size in the two treated groups (p=0.1).
In our study MATT appears to give more satisfactory results. This technique exploits the regenerative potential of mesenchymal progenitor cells deriving from adipose tissue. In addition the microfragmentation of adipose tissue provides bio-active units able to supply the regenerating with a proper microenvironment which support cells adhesion, growth and differentiation (23).
Our results demonstrate a better outcome of MATT technique compared with microfractures at a short-median follow up. This demonstrates as well an intrinsic lack of biological effectiveness in the microfractures technique, that must be carefully considered in our treatment options, especially in athletes or very young and active patients.
Our results are exclusively based on clinical data and this must be considered as a limitation. Postoperative MRI evaluation of the chondral defects could have been helpful in clarifying results, as well as a histological evaluation.
In our opinion this study also has two major limitations: firstly it was a retrospective observational not randomized study, secondly our clinical outcomes were evaluated using only one validated score system (mHHS).
Conclusion
The results of this study provide proof that MATT therapy improve clinical outcomes. The MATT group showed durable improvement at follow up of two years, with a scoring significantly better than MFx group, in which was observed a sensible reduction of the clinical scoring also.
However further studies involving even more patients with larger follow-up and others clinical and functional scores may be necessary.
The work was done at COF Lanzo Hospital, Ortopedia 1, Lanzo d’Intelvi (Co) 22024, Italy
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Chahla J, LaPrade R, Mardones R, et al. Biological Therapies for Cartilage Lesions in the Hip: A New Horizon. Orthopeadics. 2016;39:e715–e723. doi: 10.3928/01477447-20160623-01. [DOI] [PubMed] [Google Scholar]
- 2.Hsu WK, Mishra A, Rodeo SR, et al. Platelet rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739–748. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 3.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 4.Migliore A, Granata M, Tormenta S, et al. Hip viscosupplementation under ultra-sound guidance reduces NSAID consumption in symptomatic hip osteoarthritis patients in a long follow-up: data from Italian registry. Eur Rev Med Pharmacol Sci. 2011;15(1):2534. [PubMed] [Google Scholar]
- 5.Kawakami Y, Matsuo K, Murata M, et al. Expression of angiotensin II receptor-1 in human articular chondrocytes. Arthritis. 2012;2012:648537. doi: 10.1155/2012/648537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Current Reviews in Musc Med. 2012;5(3):244–253. doi: 10.1007/s12178-012-9134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana A, Bistolfi A, Crova M, Rosso F, Massazza G, et al. Arthroscopic Treatment of Hip Chondral Defects: autologous chondrocyte transplantation versus simple debridement-A Pilot Study. Arthroscopy. 2012;28(3):322–9. doi: 10.1016/j.arthro.2011.08.304. [DOI] [PubMed] [Google Scholar]
- 9.Haviv B, Singh PJ, Takla A, O’Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. JBJS. 2010;92:629–33. doi: 10.1302/0301-620X.92B5.23667. [DOI] [PubMed] [Google Scholar]
- 10.Horisberger M, Brunner A, Herzog RF. Arthroscopic treatment of femoral acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy. 2010;26:623–9. doi: 10.1016/j.arthro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Nam D, Shindle MK, Buly RL, Kelly BT, Lorich DG. Traumatic osteochondral injury of the femoral head treated by mosaicplasty: a report of two cases. HSS J. 2010;6:228–34. doi: 10.1007/s11420-010-9159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in acetabular chondral defects. Arthroscopy. 2008;24:46–50. doi: 10.1016/j.arthro.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya JK, Martin RL, Lesniak BP. Arthroscopic repair of delaminated acetabular articular cartilage in femoroacetabular impingement. Orthopedics. 2009:32. doi: 10.3928/01477447-20090728-44. [DOI] [PubMed] [Google Scholar]
- 14.Akimau P, Bhosale A, Harrison PE. Autologous chondrocyte implantation with bone grafting for osteochondral defect due to posttraumatic osteonecrosis of the hip-a case report. Acta Orthop. 2006;77:333–6. doi: 10.1080/17453670610046208. [DOI] [PubMed] [Google Scholar]
- 15.Hart R, Janecek M, Visna P, Bucek P, Kocis J. Mosaicplasty for the treatment of femoral head defect after incorrect resorbable screw insertion. Arthroscopy. 2003;19:E1–5. doi: 10.1016/j.arthro.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Sotereanos NG, DeMeo PJ, Hughes TB, Bargiotas K, Wohlrab D. Autogenous osteochondral transfer in the femoral head after osteonecrosis. Orthopedics. 2008;31:177. doi: 10.3928/01477447-20080201-33. [DOI] [PubMed] [Google Scholar]
- 17.Krych AJ, Lorich DG, Kelly BT. Treatment of focal osteochondral defects of the acetabulum with osteochondral allograft transplantation. Orthopedics. 2011;34:e307–11. doi: 10.3928/01477447-20110526-24. [DOI] [PubMed] [Google Scholar]
- 18.Van Stralen RA, Haverkamp D, Van Bergen CJ, Eijer H. Partial resurfacing with varus osteotomy for an osteochondral defect of the femoral head. Hip Int. 2009;19:67–70. doi: 10.1177/112070000901900113. [DOI] [PubMed] [Google Scholar]
- 19.Stafford GH, Bunn JR, Villar RN. Arthroscopic repair of delaminated acetabular articular cartilage using fibrin adhesive. Results at one to three years. Hip Int. 2011;21:744–50. doi: 10.5301/HIP.2011.8843. [DOI] [PubMed] [Google Scholar]
- 20.Tzaveas AP, Villar RN. Arthroscopic repair of acetabular chondral delamination with fibrin adhesive. Hip Int. 2010;20:115–9. doi: 10.1177/112070001002000117. [DOI] [PubMed] [Google Scholar]
- 21.Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Research & Therapy. 2014;16:432–451. doi: 10.1186/s13075-014-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitag J, Ford J, Bates D, et al. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open. 2015;5:e009332. doi: 10.1136/bmjopen-2015-009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–77. doi: 10.3727/096368912X657855. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo Mardones, et al. Cell therapy for cartilage defects of the hip. MLTJ. 2016;6(3):361–366. doi: 10.11138/mltj/2016.6.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis HB, Briggs KK, Philippon MJ. Innovation in hip arthroscopy: is hip arthritis preventable in the athlete. BJSM. 2011;45:253–8. doi: 10.1136/bjsm.2010.082529. [DOI] [PubMed] [Google Scholar]
- 26.Pers YM, Rackwitz L, Ferreira R, et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. STEM CELLS Trans Med. 5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubbs AJ, Potter HG. Section VII: chondral lesions. JBJS Am. 2009;91(Suppl 1):119. doi: 10.2106/JBJS.H.01452. [DOI] [PubMed] [Google Scholar]
- 28.Girard J, Roumazeille T, Sakr M, Migaud H. Osteochondral mosaicplasty of the femoral head. Hip Int. 2011;21:542–8. doi: 10.5301/HIP.2011.8659. [DOI] [PubMed] [Google Scholar]
- 29.Meyers MH. Resurfacing of the femoral head with fresh osteochondral allografts. Long-term results. Clin Orthop Relat Res. 1985;197:111–4. [PubMed] [Google Scholar]
- 30.Rodrigo Mardones, Catalina Larrain; Cartilage restoration technique of the hip. JHPS. 2016;3(1):30–36. doi: 10.1093/jhps/hnv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25:327–35. doi: 10.1016/j.csm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical Efficacy of the Microfracture Technique for Articular Cartilage Repair in the Knee: An Evidence-Based Systematic Analysis. The Am J Sports Med. 2009;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 33.Glick JM. Hip arthroscopy using the lateral approach. Instr Course Lect. 1988;37:223–231. [PubMed] [Google Scholar]