Abstract
Aortic aneurysms and dissections (AAD) are common aortic diseases that carry a high risk of mortality. The aortic wall is composed of highly dynamic cell populations and extracellular matrix (ECM). In response to changes in the biomechanical environment, aortic cells and ECM modulate their structure and functions to increase aortic wall strength and meet the hemodynamic demand. Compromise in the structural and functional integrity of aortic components leads to aortic degeneration and biomechanical failure. A better understanding of the molecular pathogenesis of AAD will facilitate the development of effective medications to treat these conditions. Here, we summarize recent findings on AAD published in ATVB. In this issue, we focus on the dynamics of aortic cells and ECM in AAD; in the next issue, we will focus on the role of signaling pathways in AAD.
Keywords: Aortic aneurysms and dissections, smooth muscle cells, extracellular matrix, inflammation, proteoglycans
Subject codes: Aortic Aneurysm, Aortic Dissection
Introduction
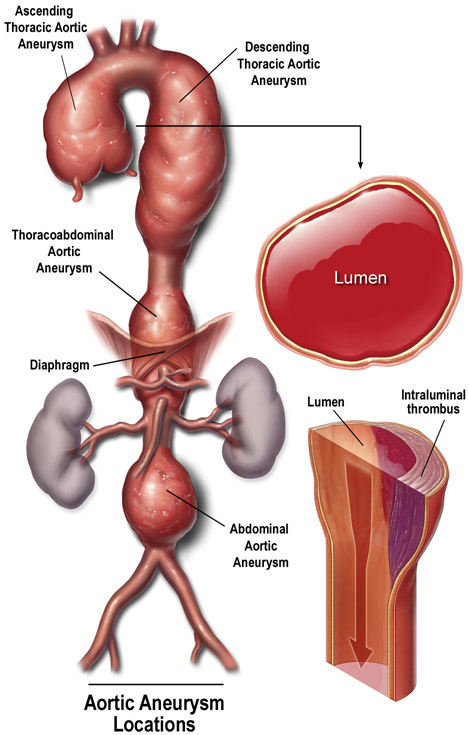
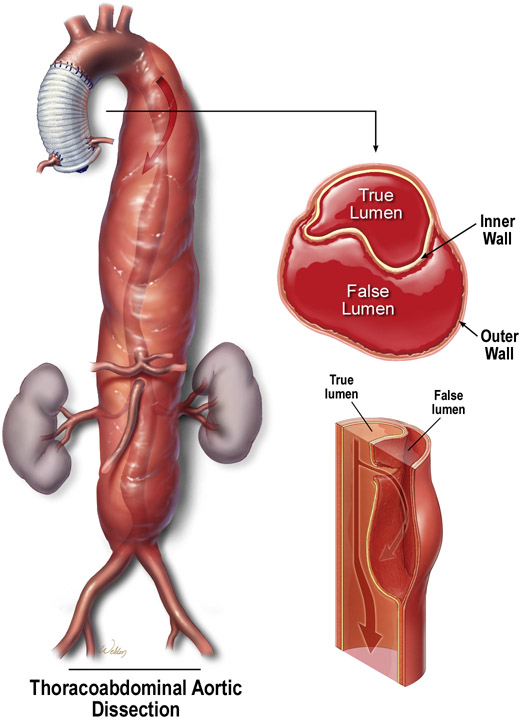
Aortic aneurysms and dissections (AAD) are potentially lethal conditions that cause more than 10,000 deaths in the United States each year. Currently, no medications have been clinically shown to effectively prevent disease progression.1 Aortic aneurysm occurs when the progressive weakening of the aortic wall causes the aorta to enlarge (Figure 1A). Aortic dissection occurs when a tear forms within the aortic wall and causes blood to flow between the laminar layers of the media, thereby separating them and creating a false lumen with a severely weakened outer aortic wall (Figure 1B). From an etiologic standpoint, AAD can be classified as either heritable or sporadic and, depending on the aortic region(s) involved, AAD can be named as either thoracic aortic aneurysm and dissection (TAAD) or abdominal aortic aneurysm (AAA).
Figure 1. Aortic aneurysms and dissections.
(A) Aortic aneurysm occurs when the progressive weakening of the aortic wall causes the aorta to enlarge. (B) Aortic dissection occurs when a tear forms within the aortic wall and causes blood to flow between the laminal layers of the media, thereby creating a false lumen. Used with permission.100
The aortic wall is composed of highly dynamic cell populations and extracellular matrix (ECM) that perform sophisticated biomechanical functions to provide suitable compliance and adequate strength in response to hemodynamic changes. These cellular and extracellular components of the aortic wall undergo dynamic changes during different phases of aortic stress, injury, repair, and remodeling. The dysregulation of these components results in progressive smooth muscle cell (SMC) depletion, ECM destruction, and inflammation, leading to aortic aneurysm, dissection, and rupture. Comprehensively understanding the regulation of aortic cells and ECM will facilitate the development of effective pharmacologic strategies to prevent AAD formation and progression.2
Aortic SMCs in AAD
SMCs are the main cellular component of the aortic wall and play a central role in maintaining aortic functions and homeostasis. In response to increased hemodynamic pressure, aortic SMCs maintain or increase aortic strength by contracting to generate arterial tone and by producing ECM proteins to induce aortic wall remodeling.3 Various factors and types of stress compromise the structural and functional integrity of SMCs, leading to aortic degeneration and, ultimately, biomechanical failure.
SMC Contractile Dysfunction in AAD
Contractile dysfunction in AAD:
The contractile activity of SMCs is fundamental to aortic structure and function.4 The elastin-contractile unit5 (Figure 2) is a unique configuration of elastin fibers, focal adhesions/dense plaques in the SMC plasma membrane, and contractile filaments inside SMCs. Mechanical stimuli are transmitted from elastin fibers, through the focal adhesions/dense plaques in the plasma membranes and anchoring proteins/actin linkage proteins in SMCs, and to the contractile unit, leading to activation of SMC contraction.4 A growing body of evidence has indicated that SMC contractile dysfunction contributes to the development of heritable and sporadic AAD. Defects in the genes encoding the contractile–elastin fiber unit contribute to the pathogenesis of TAAD.6 Loss-of-function mutations in ACTA2 (encoding SMC-specific isoform α-actin),7 MYH11 (encoding SMC-specific myosin heavy chain 11),8,9 and MYLK (encoding myosin light chain kinase [MYLK] that induces SMC contraction)10 have been identified in patients with familial TAAD. Contractile dysfunction is also present in aortic tissues from patients with sporadic AAD. Degradation of SMC contractile proteins in sporadic TAAD tissues11 and reduced expression of SMC contractile proteins in tricuspid aortic valve ascending thoracic aortic aneurysm tissues12 have been reported. By using electric cell-substrate impedance sensing, Bogunovic et al.13 recently showed that contractility was impaired in 23% of aortic tissues from AAA patients.
Figure 2. Elastin-contractile unit.
(A) The lamellar unit is composed of smooth muscle cells (SMCs) sandwiched between two layers of elastic fibers and surrounded by collagen bundles. (B) The elastin-contractile unit is a unique configuration of elastin fibers, focal adhesions/dense plaques in the SMC plasma membrane, and contractile filaments inside the SMCs. Used with permission.100
Mechanisms of contractile dysfunction in sporadic AAD:
While SMC contractile dysfunction in certain forms of heritable AAD can be attributed to specific mutations affecting the contractile unit, questions remain regarding the molecular basis of SMC contractile dysfunction in sporadic diseases. Several factors and pathways have been shown to induce SMC stress and contractile dysfunction in sporadic AAD. For example, the aortic inflammatory response and abnormal activation of the NOD, leucine-rich repeat (LRR), and pyrin domain containing protein 3 (NLRP3) inflammasome in SMCs cause contractile dysfunction.11 NLRP3 activates caspase-1, which directly cleaves and degrades contractile proteins, leading to contractile dysfunction, biomechanical failure, and AAD formation. Blocking inflammasome activation inhibits contractile dysfunction. In contrast, a recent study by Au et al.14 showed that low-density lipoprotein receptor-related protein 1 (LRP1) is important for aortic contractile function. LRP1 directly binds to α2δ−1, a chaperone for voltage-gated Ca2+ channels, and regulates calcium release in response to a ryanodine receptor agonist. LRP1 also induces the expression of proteins involved in actin polymerization. SMC-specific LRP1−/− mice showed compromised aortic contraction and abnormal aortic dilatation.14-16 These findings have indicated that alterations in pathways that modulate aortic SMC contraction may contribute to contractile dysfunction in sporadic disease.
SMC Phenotypes in AAD
Heterogeneity of SMC phenotypes in AAD:
SMCs have the remarkable ability to modulate their phenotypic characteristics. Under normal conditions, most aortic SMCs exhibit a contractile phenotype characterized by an elongated spindle-shaped myocyte morphology, expression of SMC-specific contractile proteins, low production of ECM proteins, and minimal proliferation and migration. Under conditions such as inflammation and injury, contractile SMCs can lose their contractile phenotype,17 a phenomenon termed “phenotypic switch.” The switched SMCs exhibit phenotypes resembling those of mesenchymal stem cells, myofibroblasts, fibroblasts, osteogenic cells, chondrocytes, macrophage-like inflammatory cells, foam cells, or adipocyte-like cells.18-21 This phenotypic switch of SMCs is often observed in aortas of patients with either heritable or sporadic AAD.22-24 However, the phenotypes of SMCs in AAD can vary significantly depending on the type of pathogenesis (e.g., heritable vs. sporadic), focal area (e.g., degenerative vs. compensational proliferative), and disease stage. The most commonly reported phenotypic modulation observed in patients with sporadic TAAD or AAA is the decreased expression of SMC proteins (e.g., SM22-α and SMα-actin) and increased expression of inflammatory proteins (e.g., MMP2 and MMP9). SMC phenotypic switching in AAD has been reviewed comprehensively elsewhere.25
Regulation of SMC phenotypes in AAD:
Because phenotypic changes in SMCs control aortic structure and function, studies have focused on understanding the regulation of SMC phenotypes and on identifying the factors and pathways involved.
Transcriptional control:
The expression of SMC contractile proteins is controlled by transcription factors including myocardin and myocardin-related transcription factors (MRTFs).26,27 Myocardin or MRTFs form a complex with the ubiquitous DNA-binding transcription factor serum response factor (SRF), which binds the CArG motif on SMC genes and serves as a docking platform for the myocardin-mediated transcription of target genes. Pro-inflammatory transcription factors such as Kruppel-like factor 4 (KLF4)28,29 and Forkhead box protein O 4 (FoxO4)30 induce SMC phenotype alterations by interfering with myocardin and MRTF-mediated transcriptional activity, subsequently suppressing the expression of genes encoding SMC contractile proteins.
Signaling pathways:
Transforming growth factor (TGF)-β signaling is a key regulatory pathway for maintaining aortic contractile phenotype and function. TGF-β signaling promotes SMC gene expression by inducing SRF expression and enhancing its binding to the CArG boxes of target genes. A recent study showed that mice with the postnatal disruption of the gene encoding TGF-β receptor 2 (Tgfbr2) exhibited deficiency in contractility and elasticity in the ascending and proximal descending thoracic aorta.31 Furthermore, SMCs lacking Smad3 switched from a contractile phenotype to a synthetic phenotype.32 LRP1 has also been identified as an important regulator for maintaining SMC contractile phenotype. Proteomics analysis of smLRP1−/− mice revealed the downregulation of proteins involved in actin polymerization and contraction. LRP1 regulates calcium release and the expression of proteins involved in actin polymerization and contractility in the aortic wall.14 In addition to signaling pathways, endoplasmic reticulum (ER) stress and autophagy regulate the phenotype of SMCs in the aortic wall.33 Whereas ER stress promotes the switch of SMCs to an inflammatory phenotype, autophagy maintains the SMC contractile phenotype. The deletion of the gene encoding autophagy protein 5 (Atg5) in SMCs enhances ER stress–induced SMC inflammation and death, leading to increased aortic pathology.33
MicroRNAs (miRNAs):
MiRNAs have been shown to play an important role in SMC phenotypic modulation. MiRNAs such as miR-143/145 enhance the contractile phenotype of SMCs, whereas miRNAs such as miR-21, miR-221, and miR-222 promote SMC proliferation.34,35 A role for the pro-inflammatory miR-33 in promoting SMC phenotypic changes in AAA has also been reported.36 The abundance of miR-33 is increased in the diseased aortic wall. MiR-33 suppresses the activation of pro-inflammatory Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK). MiR-33 deletion attenuated AAA formation in angiotensin II (AngII)-induced and calcium chloride–induced mouse models of AAA. The attenuation of AAA in these models was associated with reduced monocyte chemoattractant protein-1 (MCP-1) mRNA abundance in SMCs and reduced matrix metalloproteinase 9 (MMP9) mRNA abundance in macrophages.36 Interestingly, miR-21, which is known to promote the proliferation, migration, de-differentiation, and phenotypic switch of SMCs,37 has been shown to protect the aorta against TAAD formation. Deleting miR-21 in Smad3-deficient mice (Smad3+/−:miR-21−/−) further suppressed canonical TGF-β signaling, reduced SMC gene expression, and increased inflammatory gene expression in SMCs, leading to enhanced aortic dilation and rupture after AngII infusion. miR-21 stimulates TGF-β signaling through the suppression of SMAD7, an inhibitory SMAD for canonical TGF-β signaling. Silencing Smad7 prevented AngII-induced TAAD formation in Smad3+/−:miR-21−/− mice.32 These studies highlight the diverse and complex roles and regulation of SMC phenotypic switching in aortic homeostasis.
Epigenetic regulation:
SMC phenotypes are also controlled by chromatin remodeling.38,39 Epigenetic control of SMC phenotypes has been observed in AAD. For example, ubiquitin-like containing PHD and RING finger domains 1 (UHRF1) promotes SMC phenotypic switching during AAD development by epigenetically suppressing the expression of genes involved in cell-cycle inhibition and differentiation.40 In contrast, Rho guanine nucleotide exchange factor 18 (ARHGAP18) epigenetically prevents SMC phenotypic switching and protects the aortic wall. Arhgap18 deficiency in mice results in an induction of permissive histone modification (H3K4me3) and a reduction of repressive histone modification (H3K27me3) at Mmp2 and Tnfα promoters in SMCs, leading to a proteolytic and pro-inflammatory SMC phenotype and TAA development.41 A role for the DNA methylation of SMC genes in SMC phenotypic changes has also been recognized. In de-differentiated SMCs, downregulation of DNA demethylase Ten–eleven translocation-2 (TET2), increased DNA methylation, and decreased chromatin accessibility at SMC contractile gene promoters have been reported.42 Consistent with these findings, the downregulation of SM22α expression was found to be associated with increased DNA methylation of the SM22α gene promoter in AAA tissues.43
SMC clonal expansion in AAD:
The recent development of multicolor reporter lineage tracing systems allows for the detection of SMC clonal expansion in the vascular wall during development of cardiovascular disease.44 Using the multicolor lineage tracing of SMCs in a mouse model of AngII-induced AAD, Clement et al.33 showed that a subset of medial SMCs underwent clonal expansion in the adventitia and borders of the false channel during AAD development. The clonally expanded SMCs underwent phenotypic switching, shown by the downregulation of SMC differentiation markers and the upregulation of phagocytic markers.33 This finding suggests that SMC clonality is an important mechanism underlying the expansion of SMC subpopulations during aortic remodeling.
Other Cell Types in AAD
Endothelial Dysfunction in AAD
Endothelial cells and endothelial cell–derived factors play a fundamental role in regulating SMC functions, providing barrier functions, and preventing inflammation and thrombosis. Endothelial dysfunction has been shown to initiate vascular disease in various cardiovascular conditions.45,46 Evidence has indicated that intact endothelial function plays a pivotal role in protecting the aorta against AAD formation. A recent study showed that overproduction of NO by the induction of inducible nitric oxide synthase (iNOS) contributes to AAD development.47 Although the effects of endothelial dysfunction in AAD development have been increasingly recognized, the molecular mechanisms involved remain to be understood. Interestingly, a recent study by Pan et al.48 showed that NO overproduction and the subsequent S-nitrosylation of plastin-3 (an actin-binding protein) induced endothelial barrier dysfunction and exacerbated TAD. The authors showed that protein S-nitrosylation, particularly plastin-3 S-nitrosylation, was increased in aortic tissues from TAD patients. In cultured endothelial cells, AngII-induced plastin-3 S-nitrosylation via an iNOS-dependent pathway. S-nitrosylation of plastin-3 increased its association with plectin and cofilin to form a plastin-3/plectin/cofilin complex, which weakened adherens junction formation and increased cell permeability. Administering an endothelial-specific de-nitrosylated form of plastin-3 partially mitigated the AngII-induced disruption of cell junctions and TAD development in ApoE−/− mice. Furthermore, inhibition of iNOS attenuated plastin-3 S-nitrosylation and plastin-3/plectin/cofilin complex formation, resulting in improved endothelial barrier function.48 This study highlights the importance of endothelial barrier dysfunction in TAD formation and provides a novel mechanism linking NO overproduction and endothelial barrier dysfunction.
Fibroblasts in AAD
Fibroblasts are abundant in the aortic adventitia and are believed to be crucial in maintaining the structure and integrity of the aortic wall. Compelling evidence has indicated that fibroblast abnormalities contribute to AAD.49-51 Proposed mechanisms include the contribution of abnormal fibroblasts to inflammation, the disruption of ECM, and the impairment of cell-cell interactions. In two recent studies, it was reported that a cytokine-like protein dikkopf-3 (DKK3) transdifferentiated fibroblasts into endothelial cells52 or SMCs.53 In a screen of transcription factors, Mycod, Mef2C, and Gata6 were found to orchestrate the reprogramming of fibroblasts into SM-like cells.54 These studies have provided new platforms for understanding whether and how fibroblasts and other cell types interact to promote AAD.
Myofibroblasts in AAD
Myofibroblasts are specialized cells that have a more contractile phenotype and produce more ECM proteins than fibroblasts. They can be derived from multiple cell types, such as mesenchymal cells, fibroblasts, SMCs, and endothelial cells.55,56 Myofibroblasts are activated in response to mechanical stress57,58 and tissue injury,59 and contribute to tissue repair and fibrotic remodeling.56,60-63 Recent studies have drawn attention to a possible role for myofibroblasts in aortic diseases.64 Activated myofibroblasts have been found in human TAA65 and AAA66 tissues and in AAD tissues in several animal models.58,59 Several signaling pathways and factors, including TGF-β, Ang II, connective tissue growth factor (CTGF), platelet-derived growth factor, wingless/INT (WNT), and yes-associated protein 1 (YAP), have been linked to the activation and maintenance of myofibroblasts and fibrotic remodeling.62 TGF-β signaling is a key regulator of myofibroblast activation. Fibroblasts explanted from patients with TGFBR2 mutations failed to transform into mature myofibroblasts in response to TGF-β,22 suggesting that deficient TGF-β signaling can result in impaired myofibroblast activation in AAD. AngII signaling is also involved in myofibroblast activation in pressure-induced aortic remodeling. Antagonizing AngII signaling with losartan was shown to partially prevent pressure-induced myofibroblast activation, collagen accumulation, adventitial hyperplasia, and aortic dilatation.58 Despite recent advances, our understanding of aortic myofibroblasts in aortic homeostasis is limited. The precise roles of myofibroblasts in aortic structure and function, as well as the regulation of myofibroblast activation and inactivation during different stages of AAD development remain to be understood.
Inflammatory Cells in AAD
Inflammatory cells actively participate in tissue injury, repair, and remodeling. A variety of inflammatory cells including lymphocytes, macrophages, mast cells, and neutrophils have been detected in media and adventitia of TAAD and AAA tissues.67,68 Recently, several inflammatory factors such as chemokine family with sequence similarity 3, member D (FAM3D),69 serum amyloid A (SAA),70 CD40L,71 interleukin (IL)-1β72 and tumor necrosis factor (TNF)-α73 have been shown to contribute to aortic inflammation and AAD development. These inflammatory factors are upregulated in AAA tissues, and their deficiency in mice prevents AAA development in different disease models. Inflammatory factors promote aortic inflammation and AAA through different mechanisms. For example, IL-1β promotes AAA by inducing neutrophil extracellular trap formation.72 In addition to pro-inflammatory factors, several anti-inflammatory factors such as IL-10,74 IL-12p40,75 and IL-3376 have been shown to have protective roles against AAD formation. Treating mice with IL-10 or IL-33 reduces AAA formation, and IL12p40 deficiency in mice increases aortic enlargement.75 These factors repress aortic inflammation and protect the aortic wall by increasing CD4+Foxp3+ regulatory T cells, reducing CD8+/granzyme B+ cytotoxic T cells, and inducing alternatively activated macrophages.74,76
The ECM in AAD
The ECM is a dynamic noncellular component of the aorta that provides structural support and controls the plasticity and strength of the aortic wall. The ECM also actively regulates cellular functions by mediating matrix and cell attachments, transducing biomechanical signals in cells, and controlling signaling in a spatially patterned fashion. The integrity of the ECM is critical for maintaining aortic homeostasis. The abnormal production and degradation of ECM components including proteoglycans contributes to aortic destruction and dysfunction.77-79 Recent studies published in ATVB have provided novel insight into proteoglycan metabolism and AAD.
Proteoglycans in AAD
Normal proteoglycan function in the vascular wall:
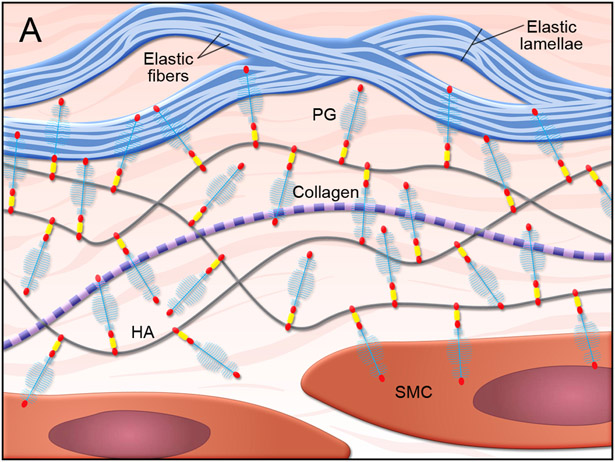
Proteoglycans in the ECM are important for maintaining vascular structure and function.80 In the normal vasculature (Figure 3),81 proteoglycans such as versican form large aggregates with hyaluronan to create compressive and reversible structures that can smooth pressure waves and avoid vessel deformation.82-85 Normal proteoglycan aggregates also pressurize the intralamellar spaces and promote the connection between SMCs and elastic laminae in the arterial wall to facilitate mechanosensing.85 In addition, proteoglycans maintain aortic hemostasis by regulating elastic fiber assembly86,87 and SMC proliferation.83,88
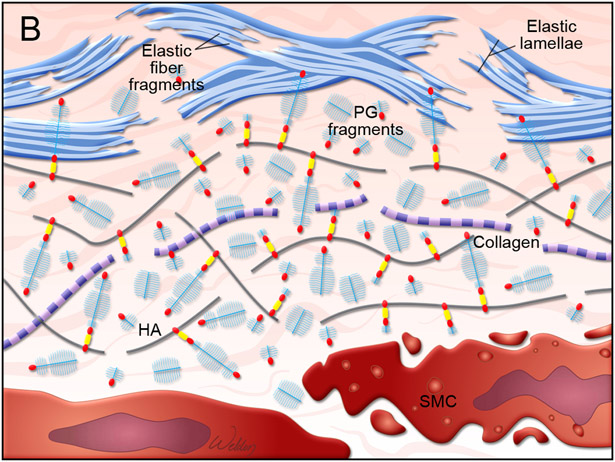
Figure 3. Proteoglycan metabolism in the aortic wall.
(A) Illustration of normal aortic wall with intact proteoglycans (PG), such as aggrecans, attached to hyaluronan (HA) and healthy smooth muscle cells (SMC). (B) Illustration of degenerative aortic wall with accumulated proteoglycan (e.g., aggrecan) fragments, elastin fiber fragmentation, and SMC death.81
Abnormal proteoglycan accumulation and its destructive role in AAD:
Proteoglycan content is minimal in normal vascular tissue but significantly increases in diseased vascular tissue.80 Abnormal proteoglycan accumulation, characterized by a bluish-green color in Alcian blue staining, has been detected frequently in the degenerative aortic media of TAAD tissues, a phenomenon termed mucoid ECM accumulation89 and similar to the outdated concept of “cystic medial necrosis.”90 Increased levels of proteoglycans and glycopeptides including aggrecan and versican have been detected in ascending aortic tissues from TAAD patients, including those with Marfan syndrome.91,92 Abnormal accumulation of proteoglycans can be destructive to aortic structure and function. Recently, studies performed by using computational simulations have indicated that an abnormal increase in proteoglycan aggregate mass in the aorta increases intralamellar swelling pressure, disrupts cell-matrix interactions, and delaminates the microstructure of the aortic wall, compromising aortic mechanosensing and structural integrity.85,93,94
Proteoglycan metabolism in AAD:
An imbalance between proteoglycan production and catabolism may disturb the integrity of aortic structure and function.80,85,93,94 Understanding the regulation of proteoglycan production, degradation, and catabolism and the dynamics between proteoglycan metabolism and other components of the aortic wall is critically important. Proteoglycans are cleaved by MMPs and a disintegrin-like and metalloprotease domain with thrombospondin-type motifs (ADAMTSs). The ADAMTS family of extracellular proteinases has been shown to be involved in ECM turnover.95 Several recent studies have indicated an important role for ADAMTS-5 in proteoglycan catabolism and aortic protection.96-98 Adamts5-deficient (Adamts5−/−) mice displayed abnormalities in the ascending aorta that were associated with aggrecan accumulation, indicating the importance of ADAMTS-5–mediated aggrecan cleavage for normal aortic development.96 Mice lacking the catalytic domain of ADAMTS-5 (Adamts5Δcat) showed thoracic aortic dilatation97 and exacerbated ascending aortic enlargement when challenged with AngII.98 Aortas from Adamts5Δcat mice showed decreased levels of ADAMTS-specific versican cleavage product, increased levels of versican,98 and increased accumulation of aggrecan.97 These findings have suggested that the ADAMTS-5–mediated cleavage and turnover of aggrecan and versican are critical for aortic morphogenesis during development and for the maintenance of aortic integrity throughout adulthood.
Future studies are needed to understand the dynamic roles of proteoglycan metabolism in aortic inflammation, injury, repair, and remodeling; to identify the factors and pathways that stimulate proteoglycan production; to comprehensively understand the effects and regulation of proteoglycan cleavage, degradation, and turnover; and to determine the specific roles of ADAMTS-1,47 ADAMTS-5,96-98 and ADAMTS-499 in proteoglycan metabolism.
Summary
Although our understanding of the dynamics of cell populations and ECM in aortic physiology and pathology has improved, key questions and challenges remain. A future goal in the field is to characterize the diverse cell populations of the aorta by evaluating their dynamic changes and specific functions in different phases of aortic adaptation, injury, repair, and remodeling. Another goal is to understand the genetic and epigenetic regulation of aortic cell clonal expansion and phenotypic transitions. In the next issue of this Recent Highlights series, we will cover the topic of recent advancements made toward understanding how signaling pathways and other contributors play a role in AAD.
Acknowledgments
We gratefully acknowledge Nicole Stancel, PhD, ELS(D), of Scientific Publications at the Texas Heart Institute, for editorial support, and Scott Weldon, MA, FAMI, from the Division of Cardiothoracic Surgery, Baylor College of Medicine, for creating the illustrations.
Sources of Funding
Our research activities are supported by grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (HL131980, HL143359, HL127111, HL133723, HL139748, HL134731, and HL107326) and from the American Heart Association (AHA) Vascular Diseases Strategically Focused Research Networks (SFRN) (AHA18SFRN33960114). The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA. Dr. LeMaire’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine.
Nonstandard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- AAD
aneurysms and dissections
- ADAMTS
a disintegrin-like and metalloprotease domain with thrombospondin-type motifs
- ARB
AT1 receptor blocker
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- ERK1/2
extracellular signal-regulated kinase
- ILK
integrin-linked kinase
- JNK
Jun N-terminal kinase
- MasR
Mas receptor
- MLCK
myosin light chain kinase
- MRTF
myocardin-related transcription factors
- MYLK
myosin light chain kinase
- SMC
smooth muscle cell
- SRF
serum response factor
- TAAD
thoracic aortic aneurysms and dissections
- TAC
transverse aortic constriction
- VEGF
vascular endothelial growth factor
- WNT
wingless/INT
Footnotes
Disclosures
The authors have no potential conflicts of interest to disclose.
Contributor Information
Ying H. Shen, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX; Department of Cardiovascular Surgery, Texas Heart Institute, Houston, TX
Scott A. LeMaire, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX; Department of Cardiovascular Surgery, Texas Heart Institute, Houston, TX
Nancy R. Webb, Department of Pharmacology and Nutritional Sciences, University of Kentucky, Lexington, KY
Lisa A. Cassis, Department of Pharmacology and Nutritional Sciences, University of Kentucky, Lexington, KY
Alan Daugherty, Department of Physiology and Saha Cardiovascular Research Center, University of Kentucky, Lexington, KY.
Hong S. Lu, Department of Physiology and Saha Cardiovascular Research Center, University of Kentucky, Lexington, KY
References
- 1.Milewicz DM, Ramirez F. Therapies for thoracic aortic aneurysms and acute aortic dissections. Arterioscler Thromb Vasc Biol. 2019:39:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis FM, Daugherty A, Lu HS. Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol. 2019:39:e83–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty R, Saddouk FZ, Carrao AC, Krause DS, Greif DM, Martin KA. Promoters to study vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2019:39:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res. 2015:116:1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest. 1993:68:89–99. [PubMed] [Google Scholar]
- 6.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008:9:283–302. [DOI] [PubMed] [Google Scholar]
- 7.Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007:39:1488–1493. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006:38:343–349. [DOI] [PubMed] [Google Scholar]
- 9.Pannu H, Tran-Fadulu V, Papke CL, et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007:16:2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Guo DC, Cao J, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet. 2010:87:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Ren P, Zheng Y, Zhang L, Xu G, Xie W, Lloyd EE, Zhang S, Zhang Q, Curci JA, Coselli JS, Milewicz DM, Shen YH, LeMaire SA. NLRP3 (nucleotide oligomerization domain-like receptor family, pyrin domain containing 3)-caspase-1 inflammasome degrades contractile proteins: implications for aortic biomechanical dysfunction and aneurysm and dissection formation. Arterioscler Thromb Vasc Biol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern C, Scharinger B, Tuerkcan A, et al. Strong signs for a weak wall in tricuspid aortic valve associated aneurysms and a role for osteopontin in bicuspid aortic valve associated aneurysms. Int J Mol Sci. 2019:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogunovic N, Meekel JP, Micha D, Blankensteijn JD, Hordijk PL, Yeung KK. Impaired smooth muscle cell contractility as a novel concept of abdominal aortic aneurysm pathophysiology. Sci Rep. 2019:9:6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au DT, Ying Z, Hernandez-Ochoa EO, Fondrie WE, Hampton B, Migliorini M, Galisteo R, Schneider MF, Daugherty A, Rateri DL, Strickland DK, Muratoglu SC. LRP1 (Low-density lipoprotein receptor-related protein 1) regulates smooth muscle contractility by modulating Ca(2+) signaling and expression of cytoskeleton-related proteins. Arterioscler Thromb Vasc Biol. 2018:38:2651–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonias SL. Mechanisms by which LRP1 (low-density lipoprotein receptor-related protein-1) maintains arterial integrity. Arterioscler Thromb Vasc Biol. 2018:38:2548–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis FM, Rateri DL, Balakrishnan A, Howatt DA, Strickland DK, Muratoglu SC, Haggerty CM, Fornwalt BK, Cassis LA, Daugherty A. Smooth muscle cell deletion of low-density lipoprotein receptor-related protein 1 augments angiotensin II-induced superior mesenteric arterial and ascending aortic aneurysms. Arterioscler Thromb Vasc Biol. 2015:35:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi N, Chen SY. Smooth muscle cell differentiation: Model systems, regulatory mechanisms, and vascular diseases. J Cell Physiol. 2016:231:777–787. [DOI] [PubMed] [Google Scholar]
- 18.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006:26:2696–2702. [DOI] [PubMed] [Google Scholar]
- 19.Choi HY, Rahmani M, Wong BW, Allahverdian S, McManus BM, Pickering JG, Chan T, Francis GA. ATP-binding cassette transporter A1 expression and apolipoprotein A-I binding are impaired in intima-type arterial smooth muscle cells. Circulation. 2009:119:3223–3231. [DOI] [PubMed] [Google Scholar]
- 20.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014:129:1551–1559. [DOI] [PubMed] [Google Scholar]
- 21.Lomashvili KA, Wang X, O’Neill WC. Role of local versus systemic vitamin D receptors in vascular calcification. Arterioscler Thromb Vasc Biol. 2014:34:146–151. [DOI] [PubMed] [Google Scholar]
- 22.Inamoto S, Kwartler CS, Lafont AL, et al. TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections. Cardiovasc Res. 2010:88:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009:138:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branchetti E, Poggio P, Sainger R, Shang E, Grau JB, Jackson BM, Lai EK, Parmacek MS, Gorman RC, Gorman JH, Bavaria JE, Ferrari G. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc Res. 2013:100:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, Reutelingsperger C, Jacobs M, Mees B, Schurgers L. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2019:39:1351–1368. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003:100:7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miano JM. Myocardin in biology and disease. J Biomed Res. 2015:29:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmon M, Gomez D, Greene E, Shankman L, Owens GK. Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22alpha promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res. 2012:111:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmon M, Johnston WF, Woo A, Pope NH, Su G, Upchurch GR Jr., Owens GK, Ailawadi G. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013:128:S163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao G, Fu Y, Cai Z, Yu F, Gong Z, Dai R, Hu Y, Zeng L, Xu Q, Kong W. Unspliced XBP1 confers VSMC homeostasis and prevents aortic aneurysm formation via FoxO4 interaction. Circ Res. 2017:121:1331–1345. [DOI] [PubMed] [Google Scholar]
- 31.Ferruzzi J, Murtada SI, Li G, Jiao Y, Uman S, Ting MY, Tellides G, Humphrey JD. Pharmacologically improved contractility protects against aortic dissection in mice with disrupted transforming growth factor-beta signaling despite compromised extracellular matrix properties. Arterioscler Thromb Vasc Biol. 2016:36:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Yue Z, Wu J, Chen J, Wang S, Wu J, Ren L, Zhang A, Deng P, Wang K, Wu C, Ding X, Ye P, Xia J. MicroRNA-21 knockout exacerbates angiotensin II-induced thoracic aortic aneurysm and dissection in mice with abnormal transforming growth factor-beta-SMAD3 signaling. Arterioscler Thromb Vasc Biol. 2018:38:1086–1101. [DOI] [PubMed] [Google Scholar]
- 33.Clement M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, Taleb S, Jorgensen HF, Mallat Z. Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler Thromb Vasc Biol. 2019:39:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leeper NJ, Maegdefessel L. Non-coding RNAs: key regulators of smooth muscle cell fate in vascular disease. Cardiovasc Res. 2018:114:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018:114:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakao T, Horie T, Baba O, et al. Genetic ablation of microRNA-33 attenuates inflammation and abdominal aortic aneurysm formation via several anti-inflammatory pathways. Arterioscler Thromb Vasc Biol. 2017:37:2161–2170. [DOI] [PubMed] [Google Scholar]
- 37.Yang G, Pei Y, Cao Q, Wang R. MicroRNA-21 represses human cystathionine gamma-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J Cell Physiol. 2012:227:3192–3200. [DOI] [PubMed] [Google Scholar]
- 38.McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007:100:1428–1441. [DOI] [PubMed] [Google Scholar]
- 39.Gomez D, Swiatlowska P, Owens GK. Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler Thromb Vasc Biol. 2015:35:2508–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elia L, Kunderfranco P, Carullo P, Vacchiano M, Farina FM, Hall IF, Mantero S, Panico C, Papait R, Condorelli G, Quintavalle M. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest. 2018:128:2473–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R, Lo L, Lay AJ, et al. ARHGAP18 protects against thoracic aortic aneurysm formation by mitigating the synthetic and proinflammatory smooth muscle cell phenotype. Circ Res. 2017:121:512–524. [DOI] [PubMed] [Google Scholar]
- 42.Liu R, Jin Y, Tang W, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. TET2 is a Master Regulator of Smooth Muscle Cell Plasticity. Circulation. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong L, He X, Si X, Wang H, Li B, Hu Y, Li M, Chen X, Liao W, Liao Y, Bin J. SM22alpha (smooth muscle 22alpha) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-kappaB (nuclear factor-kappaB). Arterioscler Thromb Vasc Biol. 2019:39:e10–e25. [DOI] [PubMed] [Google Scholar]
- 44.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jorgensen HF. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016:119:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payne S, De Val S, Neal A. Endothelial-specific Cre mouse models. Arterioscler Thromb Vasc Biol. 2018:38:2550–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang LJ, Xiao F, Kong LM, Wang DN, Li HY, Wei YG, Tan C, Zhao H, Zhang T, Cao GQ, Zhang K, Wei YQ, Yang HS, Zhang W. Intermedin enlarges the vascular lumen by inducing the quiescent endothelial cell proliferation. Arterioscler Thromb Vasc Biol. 2018:38:398–413. [DOI] [PubMed] [Google Scholar]
- 47.Oller J, Mendez-Barbero N, Ruiz EJ, et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017:23:200–212. [DOI] [PubMed] [Google Scholar]
- 48.Pan L, Lin Z, Tang X, Tian J, Zheng Q, Jing J, Xie L, Chen H, Lu Q, Wang H, Li Q, Han Y, Ji Y. S-nitrosylation of plastin-3 exacerbates thoracic aortic dissection formation via endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119313440. [DOI] [PubMed] [Google Scholar]
- 49.Suh JH, Yoon JS, Kim HW, Jo KH. Adventitial fibroblast abormality in thoracic aortic aneurysms and aortic dissections. Korean J Thorac Cardiovasc Surg. 2011:44:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009:119:3637–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast angiotensin II type 1a receptors contribute to angiotensin II-induced medial hyperplasia in the ascending aorta. Arterioscler Thromb Vasc Biol. 2015:35:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen T, Karamariti E, Hong X, Deng J, Wu Y, Gu W, Simpson R, Wong MM, Yu B, Hu Y, Qu A, Xu Q, Zhang L. DKK3 (Dikkopf-3) transdifferentiates fibroblasts into functional endothelial cells-brief report. Arterioscler Thromb Vasc Biol. 2019:39:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karamariti E, Zhai C, Yu B, et al. DKK3 (Dickkopf 3) alters atherosclerotic plaque phenotype involving vascular progenitor and fibroblast differentiation into smooth muscle cells. Arterioscler Thromb Vasc Biol. 2018:38:425–437. [DOI] [PubMed] [Google Scholar]
- 54.Hirai H, Yang B, Garcia-Barrio MT, Rom O, Ma PX, Zhang J, Chen YE. Direct reprogramming of fibroblasts into smooth muscle-like cells with defined transcription factors-brief report. Arterioscler Thromb Vasc Biol. 2018:38:2191–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013:19:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016. [DOI] [PubMed] [Google Scholar]
- 57.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2016:93:133–142. [DOI] [PubMed] [Google Scholar]
- 58.Kuang SQ, Geng L, Prakash SK, Cao JM, Guo S, Villamizar C, Kwartler CS, Peters AM, Brasier AR, Milewicz DM. Aortic remodeling after transverse aortic constriction in mice is attenuated with AT1 receptor blockade. Arterioscler Thromb Vasc Biol. 2013:33:2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones JA, Beck C, Barbour JR, Zavadzkas JA, Mukherjee R, Spinale FG, Ikonomidis JS. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast-mediated vascular remodeling. Am J Pathol. 2009:175:1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramkisoensing AA, de Vries AA, Atsma DE, Schalij MJ, Pijnappels DA. Interaction between myofibroblasts and stem cells in the fibrotic heart: balancing between deterioration and regeneration. Cardiovasc Res. 2014:102:224–231. [DOI] [PubMed] [Google Scholar]
- 61.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015:116:1269–1276. [DOI] [PubMed] [Google Scholar]
- 62.Piersma B, Bank RA, Boersema M. Signaling in fibrosis: TGF-beta, WNT, and YAP/TAZ converge. Front Med (Lausanne). 2015:2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ Res. 2016:119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res. 2010:88:395–405. [DOI] [PubMed] [Google Scholar]
- 65.Forte A, Della Corte A, Grossi M, Bancone C, Maiello C, Galderisi U, Cipollaro M. Differential expression of proteins related to smooth muscle cells and myofibroblasts in human thoracic aortic aneurysm. Histol Histopathol. 2013:28:795–803. [DOI] [PubMed] [Google Scholar]
- 66.Sakata N, Nabeshima K, Iwasaki H, Tashiro T, Uesugi N, Nakashima O, Ito H, Kawanami T, Furuya K, Kojima M. Possible involvement of myofibroblast in the development of inflammatory aortic aneurysm. Pathol Res Pract. 2007:203:21–29. [DOI] [PubMed] [Google Scholar]
- 67.Dale MA, Ruhlman MK, Baxter BT. Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol. 2015:35:1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piacentini L, Werba JP, Bono E, Saccu C, Tremoli E, Spirito R, Colombo GI. Genome-wide expression profiling unveils autoimmune response signatures in the perivascular adipose tissue of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2019:39:237–249. [DOI] [PubMed] [Google Scholar]
- 69.He L, Fu Y, Deng J, et al. Deficiency of FAM3D (family with sequence similarity 3, member D), a novel chemokine, attenuates neutrophil recruitment and ameliorates abdominal aortic aneurysm development. Arterioscler Thromb Vasc Biol. 2018:38:1616–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Webb NR, De Beer MC, Wroblewski JM, Ji A, Bailey W, Shridas P, Charnigo RJ, Noffsinger VP, Witta J, Howatt DA, Balakrishnan A, Rateri DL, Daugherty A, De Beer FC. Deficiency of Endogenous Acute-Phase Serum Amyloid A Protects apoE−/− Mice From Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arterioscler Thromb Vasc Biol. 2015:35:11056–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusters PJH, Seijkens TTP, Beckers L, Lievens D, Winkels H, de Waard V, Duijvestijn A, Lindquist Liljeqvist M, Roy J, Daugherty A, Newby A, Gerdes N, Lutgens E. CD40L deficiency protects against aneurysm formation. Arterioscler Thromb Vasc Biol. 2018:38:1076–1085. [DOI] [PubMed] [Google Scholar]
- 72.Meher AK, Spinosa M, Davis JP, Pope N, Laubach VE, Su G, Serbulea V, Leitinger N, Ailawadi G, Upchurch GR Jr. Novel role of IL (Interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018:38:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batra R, Suh MK, Carson JS, Dale MA, Meisinger TM, Fitzgerald M, Opperman PJ, Luo J, Pipinos II, Xiong W, Baxter BT. IL-1beta (Interleukin-1beta) and TNF-alpha (tumor necrosis factor-alpha) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler Thromb Vasc Biol. 2018:38:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adam M, Kooreman NG, Jagger A, et al. Systemic upregulation of IL-10 (Interleukin-10) using a nonimmunogenic vector reduces growth and rate of dissecting abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2018:38:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma N, Dev R, Belenchia AM, Aroor AR, Whaley-Connell A, Pulakat L, Hans CP. Deficiency of IL12p40 (interleukin 12 p40) promotes ang II (angiotensin II)-induced abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2019:39:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Xia N, Wen S, et al. IL (Interleukin)-33 suppresses abdominal aortic aneurysm by enhancing regulatory T-cell expansion and activity. Arterioscler Thromb Vasc Biol. 2019:39:446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009:326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan D, Creemers EE, Kassiri Z. Matrix as an interstitial transport system. Circ Res. 2014:114:889–902. [DOI] [PubMed] [Google Scholar]
- 79.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res. 2016:110:298–308. [DOI] [PubMed] [Google Scholar]
- 80.Wight TN. A role for proteoglycans in vascular disease. Matrix Biol. 2018:71-72:396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen YH, Lu HS, LeMaire SA, Daugherty A. Unfolding the story of proteoglycan accumulation in thoracic aortic aneurysm and dissection. Arterioscler Thromb Vasc Biol. 2019:39:1899–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992:267:10003–10010. [PubMed] [Google Scholar]
- 83.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999:19:1004–1013. [DOI] [PubMed] [Google Scholar]
- 84.Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2008:294:H1197–1205. [DOI] [PubMed] [Google Scholar]
- 85.Roccabianca S, Bellini C, Humphrey JD. Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J R Soc Interface. 2014:11:20140397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002:90:481–487. [DOI] [PubMed] [Google Scholar]
- 87.Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006:98:370–377. [DOI] [PubMed] [Google Scholar]
- 88.Lemire JM, Merrilees MJ, Braun KR, Wight TN. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002:190:38–45. [DOI] [PubMed] [Google Scholar]
- 89.Halushka MK, Angelini A, Bartoloni G, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovasc Pathol. 2016:25:247–257. [DOI] [PubMed] [Google Scholar]
- 90.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977:39:13–20. [DOI] [PubMed] [Google Scholar]
- 91.Cikach FS, Koch CD, Mead TJ, Galatioto J, Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F, Roselli EE, Apte SS. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin X, Wanga S, Fellows A, et al. Glycoproteomic analysis of the aortic extracellular matrix in Marfan patients. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA118312175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Humphrey JD. Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-beta. J Vasc Res. 2013:50:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol. 2014:13:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997:272:556–562. [DOI] [PubMed] [Google Scholar]
- 96.Dupuis LE, Nelson EL, Hozik B, Porto SC, Rogers-DeCotes A, Fosang A, Kern CB. Adamts5(−/−) mice exhibit altered acan (aggrecan) proteolytic profiles that correlate with ascending aortic anomalies. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119313077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suna G, Wojakowski W, Lynch M, et al. Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation. 2018:137:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fava M, Barallobre-Barreiro J, Mayr U, Lu R, Didangelos A, Baig F, Lynch M, Catibog N, Joshi A, Barwari T, Yin X, Jahangiri M, Mayr M. Role of ADAMTS-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2018:38:1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ren P, Hughes M, Krishnamoorthy S, Zou S, Zhang L, Wu D, Zhang C, Curci JA, Coselli JS, Milewicz DM, LeMaire SA, Shen YH. Critical role of ADAMTS-4 in the development of sporadic aortic aneurysm and dissection in mice. Sci Rep. 2017:7:12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen YH, LeMaire SA. Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections. Curr Probl Surg. 2017:54:95–155. [DOI] [PMC free article] [PubMed] [Google Scholar]