Abstract
Background: Public health systems today face the dual challenges of controlling infections and curbing the increase in antimicrobial resistance manifested in drug-resistant microorganisms in hospitals and elsewhere. In the last ten years, research has been conducted to develop new materials with antimicrobial properties to be used in medical devices, increasingly found to harbour critical nosocomial infections. Methods: Two next-generation composites using the antimicrobial qualities of silver were tested against Escherichia coli, Staphylococcus aureus and Candida albicans with the purpose of evaluating their antimicrobial and antifungal activity. These tests applied the standardized method according to ISO-2216: Plastics-Measurement of Antibacterial Activity on Plastics Surfaces. Testing was carried out using polyethylene (PE) enriched with AgNO3 as a positive control and PE as a negative control. Results: The antimicrobial activity of the composites proved to be between medium (bacteriostatic) and very good (bactericidal). In particular, PE2 showed the highest scores against all microorganisms, with values ranging from good to very good. Instead, PE1 had lower scores, with a value of medium for Escherichia coli and slight for Candida albicans. Statistical analysis carried out with the t-test for unpaired data showed a statistically significant difference between the positive control and the other polymers (p< .0001). Conclusions: Based on our findings, we conclude that the test, conducted to ISO-2216 standards, could be extended to include fungal strains and that the new composites could be used to produce antimicrobial surfaces for medical devices, for example, intubation tubes, urinary catheters, vascular prostheses, and mechanical heart valves. This would reduce the risk of microbial contamination and biofilm formation, ensuring better health outcomes for patients treated with these devices. Further testing should be done to evaluate potential future applications of these composites and the possibility of adding fungal strains to the IS0-2216 standard. (www.actabiomedica.it)
Keywords: antimicrobial resistance, silver composites, nosocomial infections, antimicrobial activity
Introduction
Even today, infectious diseases, especially in hospitals, continue to present a major public health problem with socio-economic and cultural consequences (1-5). Even though the standards of health care and medical technology have risen significantly, the World Health Organization (WHO) declared that infections occur in 15% of patients under medical care in hospital, particularly in Intensive Care Units (ICUs) (2,6).
In 2015, the European Center for Disease Control reported that 8.3% of hospitalized patients in intensive care for more than two days acquired at least one healthcare associated infection (HAI). Specifically, 6% of patients were affected by pneumonia, 4% by bloodstream infections (BSI), and finally 2% by urinary tract infections (UTIs) (7). Intubation is the cause of infection in 97% of patients, catheters have been associated with 43% of BSIs, and urinary catheters with 97% of UTIs (7). Regarding the most common bacteria, Pseudomonas aeruginosa was identified in pneumonia episodes, Staphylococcus spp in bloodstream infections, and Escherichia coli in urinary catheter infections. In fact, the surfaces of medical devices that remain in place for days provide a substrate for biofilm production and the growth of these microorganisms (4-7). In the last decade, Candidemia has also been defined as a severe and often life-threatening infection. Candida strains can cause invasive candidiasis (IC) in tertiary care hospitalized patients and also in catheter-associated urinary tract infections (CAUTI) (11,12).
Hospital-acquired infections can be caused by contamination due to incorrect conduct on the part of healthcare workers, for example, failure to wash their hands properly, to store food at the correct temperature, or prepare it in properly hygienic conditions (11-14). This superficial attitude can be attributed to the high levels of stress to which these professional categories are subjected and the speed required for the performance of their duties (15-24).
Another factor in the spread of infections is the public’s limited awareness and understanding of the proper use of medicines. Better education about this would improve the effectiveness of treatment and reduce costs for patients and society, thus avoiding risks for health and the waste of resources (25, 26). One of the first steps to take in encouraging the correct use of medicines is to improve the public’s health literacy, defined by the WHO as “the cognitive and social skills which determine the motivation and ability of individuals to gain access to, understand and use information in ways which promote and maintain good health” (27,28). One area in particular to be improved is the understanding of the medical terminology commonly used in manuals or package inserts.
Furthermore, in the last decade the over-prescribing of antibiotics or their misuse by patients has increased the risk of hospital infections caused by drug-resistant microorganisms (29). The WHO has recorded an increase in antimicrobial resistance (AMR) among clinical bacteria and has defined this phenomenon one of the most critical threats for human health, in particular in vulnerable patients (29).
At the same time, the European Center for Disease Control highlighted the very rapid increase of infections caused by bacteria like carbapenemase-producing Enterobacteriaceae (CPE) in patients hospitalized in Italian and in European institutions from 2014 to 2017 (30-32). Therefore, public health services are facing a double challenge: controlling infections and limiting AMR diffusion (33, 34).
As part of the efforts to tackle these problems, researchers have been developing polymers with antimicrobial activity, and in the last decade, a new group of materials including antimicrobial peptides (AMPs), cationic synthetic polymers, and nanoparticles has been created (7, 35). In particular, antimicrobial-biocompatible polymers have been developed for medical devices in order to prevent the formation of biofilms and the insurgence of nosocomial infections, and as such provide an innovative method to combat these problems that is both gentle and safe. Two types of antimicrobial polymers can be used for the production of medical devices: polymers that already exhibit antimicrobial activity and polymers that are modified to confer antimicrobial properties (10).
In particular, among the wide range of existing antimicrobial plastics, metal-polymer-nano composites and in particular silver-polymers are the subject of increasing interest (36,37). Silver compounds are known to show strong antibacterial activity towards a broad spectrum of bacteria, and the interest in silver derivatives and their potential application as antimicrobial agents has led researchers to investigate different classes of ligands in order to obtain novel silver(I) complexes. The aim of this study was to evaluate possible antibacterial and antifungal activity of new silver-containing polymers (I) synthesized by the Chemistry Department of the University of Camerino (UNICAM), specifically as a potential antibacterial and antifungal agent to embed in plastics. The results obtained against Escherichia coli and Staphylococcus aureus were compared with the results obtained against Candida albicans.
Methods
1. Novel composites containing silver (I) acyl pyrazolonate additives
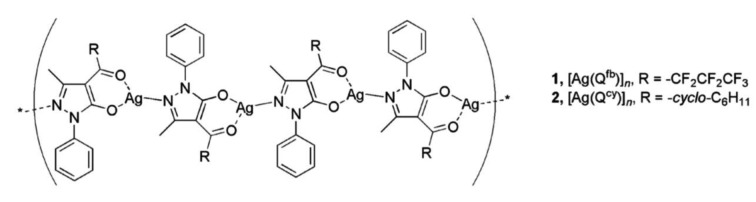
The polyethylene composite materials named PE1 and PE2 were prepared, by the Chemistry Department of UNICAM. PE1 was obtained by mixing, at 150 °C, the complex [Ag(Qfb)]n with polyethylene in a 1:1000 weight ratio, the final product was shaped into 50x50 mm square (10 mm thick). PE2 was similarly obtained by using the complex [Ag(Qcy)]n which differs only for the R substituent (Figure 1). The detailed preparation procedure and the complete characterization have been previously reported (39, 40).
Figure 1.
The antimicrobial action of the composite materials PE1 and PE2 has been tested against two microbial strains (E. coli, S. aureus) and one fungal strain (C. albicans).
Molecular structures of silver(I) acylpyrazolonato additives 1 and 2, respectively used in the composite polyethylene materials PE1 and PE2.
2. ISO-2216: Plastics-Measurement of Antibacterial Activity on Plastics Surfaces
The antimicrobial activity by contact exerted by each of the PE composites was measured according to ISO standard-2216 (ISO-22196: Plastics – Measurement of Antibacterial Activity on Plastics Surfaces) (38). Square pieces of the composites measuring 50x50 mm (10 mm in thickness) were tested against two bacteria strains and one fungal strain in triplicate: (Gram-positive) Staphylococcus aureus ATCC 25923, (Gram-negative) Escherichia coli ATCC 25922 and the fungal strain Candida albicans ATCC 24433. Unloaded PE samples were used as negative control; PE loaded with AgNO3 was used as positive control. The appropriate culture medium was inoculated with the test microbes and cultivated for 24 h at 35±1°C under aerobic conditions to achieve the concentration of 107 CFU/mL. Bacterial suspensions (0.4 mL) were inoculated onto the test surface (in triplicate) and the inoculums were covered with a piece of polyethylene film (40x40mm), gently pressed down to spread the inoculum to the edges. The Petri dishes containing the inoculated test specimens were incubated at (35±1)°C with a relative humidity of no less than 90% for 24±1h. After the incubation time, the inoculum was processed by adding 10 mL SCDLP broth (Soybean casein digest broth with lecithin and polyoxyethylene sorbitan monooleate). From the SCDLP broth, tenfold serial dilutions were made in phosphate-buffered physiological saline (PBS-saline) and aliquots of 1 mL for each dilution were placed in Petri dishes, and 15 mL of plate count agar (PCA) was added to disperse the bacteria. The inverted Petri dishes were incubated at (35±1)°C for 48 h. After incubation, the number of colonies in the Petri dishes was counted. The number of bacteria surviving on the specimens tested was compared to the number of colonies present on the negative controls. Antimicrobial performance (R) was determined according to the Japanese industrial standards method (JIS L-1902: 2002, Testing Method for Antibacterial Activity of Textiles) (41) and based on the following classification: no antimicrobial activity = ≤0.5 log microbial growth reduction (<68.4% reduction); slight antimicrobial activity = 0.5-1log microbial growth reduction (<68.4% to<90% reduction); medium antimicrobial activity = >1 to ≤ 2 log microbial growth reduction (90% to <99% reduction); good antimicrobial activity = 2 to <3 log microbial growth reduction (99% to <99.9% reduction); very good antimicrobial activity = >3log microbial growth reduction (>99.9% reduction). In addition, the % of reduction was interpreted for bacteria strains in terms of the bactericidal activity (>99.9% of inoculum reduction) or bacteriostatic activity (90 to 99.9% of inoculum reduction) of each composite.
3. Statistical analysis
To verify whether there may be a difference between the resilience and coping values before and after rescue, the unpaired t-test was used and data was processed with the XLstat software (XLSTAT. Statistical software and data analysis add-on for Excel. Addinsoft (2017)) (42).
Results
To verify the antimicrobial activity of the loaded composites by contact, the results of the experiments were elaborated according to the JIS L-1902:2002: Testing Method for Antibacterial Activity of Textiles (41), applying the standardized method (ISO-2216) (38). After an incubation of 24h, the data obtained relating to microbial charge found on different types of composites was transformed into logarithmic units to calculate antimicrobial performance as Log reduction (R value). The evaluation of bactericidal, bacteriostatic and antifungal activity provided us with much more detailed information about the behavior of the substances tested against two microbial and a fungal species.
In general, an inhibition of microbial growth on the contact surface between the PE composite squares and the inoculums of the microorganisms was revealed.
Our data show that within 24h of exposure, both PE1 and PE2 exhibited antimicrobial activity against both bacteria, ranging between a medium and very good level, in line with the behavior shown by the positive control (PEAgNO3). Tested against C. albicans, PE1, unlike PE2, showed only slight activity, measuring between 68% and 90%. The positive control (PEAgNO3) achieved a medium-good antibacterial performance against all bacteria strains.
In detail, tested against E. coli, composites PE1 and PE2 show bacteriostatic activity (<99.9%), with PE2 performing better than PE1 (Figure 2). In fact, PE1 achieved an R value from 90% to <99%, corresponding to good antimicrobial performance. The R value of PE2, instead, was within 99% to 99.9%. In addition, compared with the positive control (PEAgNO3), PE1 achieved only 1.59 log microbial growth reduction, whereas PE1 showed a value of 2.81 against the 2.21 reached by PEAgNO3 (Figure 2).
Figure 2.
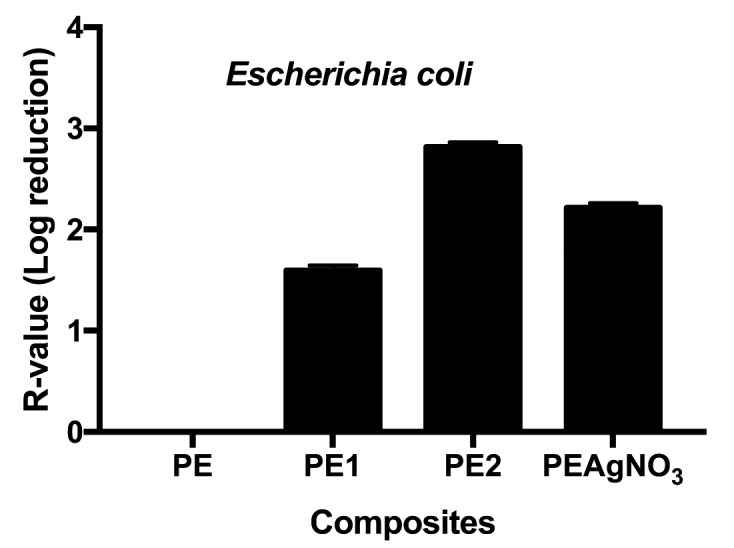
R-values obtained for Escherichia coli; unpaired t test of PE1 vs PAgNO3 was t=131.5 with a ***p value< .0001, and of PE2 t=127.3 with a ***p value <.0001
Tested against S. aureus, composites PE1 and PE2 achieved very good antimicrobial performance, corresponding to bactericidal activity largely exceeding a log value of 3. Instead, PEAgNO3 showed only a medium antimicrobial performance, with a range of antimicrobial performance from 90% to <99% (Figure 3).
Figure 3.
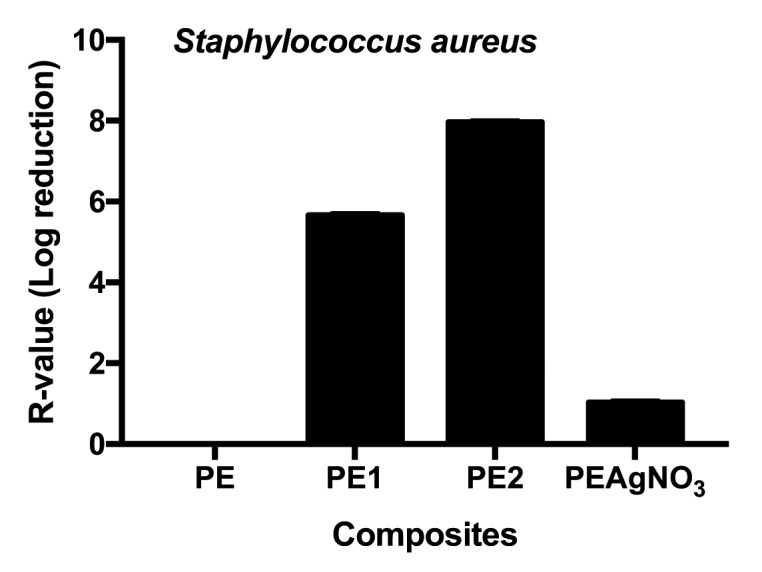
R-values obtained for Staphylococcus aureus; Unpaired t test of PE1 vs PAgNO3 was t=983.6 with a ***p value< .0001, and of PE2 t=1041 with a ***p value <.0001
Tested against Candida albicans, only PE2 achieved good antimicrobial performance. PE1 showed only slight antimicrobial activity. The observation that PE2 and PEAgNO3 had similar activity suggests that the ISO standard test can be used to test new polymers against fungi (Figure 4).
Figure 4.
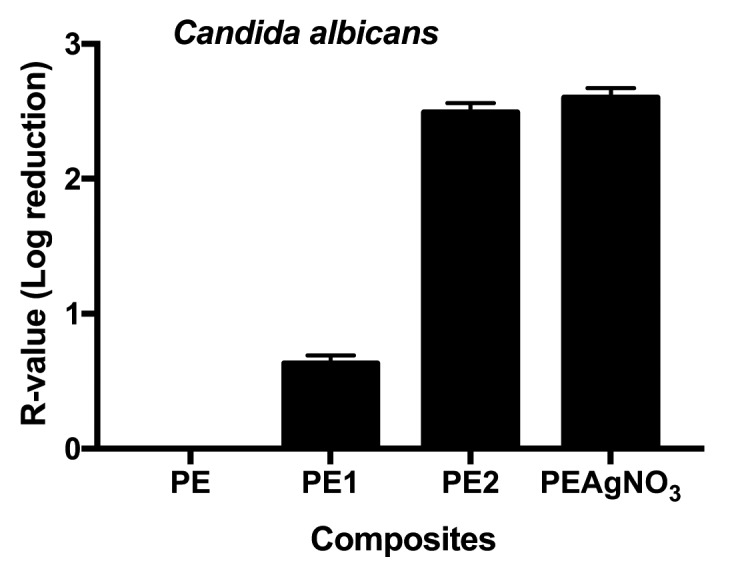
R-values obtained testing PE, PE1, PE2 and PAgNO3 against Candida albicans; unpaired t test of PE1 vs PAgNO3 was t=575.29 with a ***p value<.0001, and of PE2 t=26.99 with a ***p value <.0001
Discussion
All experiments performed on the composites against two bacterial and one fungal strain in accordance with the ISO 22196:2007 (38) confirmed the antimicrobial and antifungal activity of all polymers tested. In general, the results showed that the composites embedded in PE maintain the broad-spectrum activity of silver, targeting both gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria and the Candida albicans fungus.
The positive results of the contact test, [standardized by the ISO 22916:2007 (38)], against two bacteria strains and also against a fungal strain (Candida albicans) suggest that these composites can be used against fungal strains as well. In depth, analysis of the results indicates some differences between the antimicrobial activities displayed by the various PE composites. The most important result was recorded for PE1, which showed very good antimicrobial performance measured as bactericidal activity against Escherichia coli and Staphylococcus aureus, and the highest performance (good) against Candida albicans. Even though PE1 showed only medium and slight performance against Candida albicans and Escherichia coli respectively, it performed very well against Staphylococcus aureus.
The higher performance of PE1 may indicate that the mechanism of interaction between this composite and the bacteria cell is stronger than that of the PE2 composite. The silver in the structure of polymer 1 probably exhibits stronger antimicrobial activity. In fact, the hypothesis that silver may act against microorganisms (43) is based on the observation that the oxidized form of silver (Ag+) can bind strongly to thiols, phosphates, and other electron donating functional groups, in an interaction that disrupts the microbial cell membrane, causing leakage of cellular contents and finally cell death. A consequence of this interaction is the disruption of the cell membrane, followed by the leakage of cellular contents and finally microbial cell death (44).
Our results confirmed the possible application of the novel polymers to control nosocomial infections and AMR. In fact, new polymers characterized by antimicrobial activity are being investigated for use as materials for medical devices (45). In particular, silver has been widely used as an alternative antimicrobial agent in medical devices such as vascular prostheses, urinary catheters, and mechanical heart valves, and the results obtained in the present research confirm this application (46,47).
In addition, this feature is useful in that the various PEs can be used in place of plastic composites of AgNPs, in order to avoid the negative side effects of the latter, such as silver release and harmful environmental impact. “Contact action by polymer/polymer composites,” achieved by embedding insoluble silver (I) coordination polymers in a polymeric matrix offers a new concept in the field of plastics endowed with permanent antimicrobial activity. Thus our composite plastics may prove useful in a number of different situations in which the accumulation of unwanted material is often overlooked.
Conclusions
In the last decade, there has been increased research to develop new antimicrobial polymeric materials free of negative effects on human health. Study of the mechanisms of interaction between such polymers and microorganisms should move forward with the use of standardized protocols. For this reason, we tested the antimicrobial and antifungal activity of two silver (I) coordination polymers, applying the standard methodology reported in ISO-22196: Plastics – Measurement of Antibacterial Activity on Plastics Surfaces. For future clinical applications, it will be important to test the composites against clinically isolated microbes, especially multidrug-resistant strains, and evaluate the in vitro and in vivo biocompatibility of composites.
Acknowledgments
Funding sources: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author’s contributions:
SS designed the study and interpreted the results. PF contributed to the manuscript writing. GI contributed to the manuscript revising. IL conducted the microbiological tests. MF coordinated the synthesis of polymers and helped to critically review the manuscript. DNC designed the study, interpreted the results, and contributed to critical revision of the manuscript.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Ng VW, Chan JM, Sardon H, Ono RJ, García JM, Yang YY, Hedrick JL. Antimicrobial hydrogels: a new weapon in the arsenal against multidrug-resistant infections. Adv Drug Deliv Rev. 2014 Nov 30;78:46–62. doi: 10.1016/j.addr.2014.10.028. doi: 10.1016/j.addr.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Khan HA, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478–82. [Google Scholar]
- 3.Siracusa M, Grappasonni I, Petrelli F. The pharmaceutical care and the rejected constitutional reform: what might have been and what is. Acta Biomed. 2017 Oct 23;88(3):352–359. doi: 10.23750/abm.v%vi%i.6376. doi: 10.23750/abm.v88i3.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Signorelli C, Odone A, Gozzini A, Petrelli F, Tirani M, Zangrandi A, Zoni R, Florindo N. The missed Constitutional Reform and its possible impact on the sustainability of the Italian National Health Service. Acta Biomed. 2017 Apr 28;88(1):91–94. doi: 10.23750/abm.v88i1.6408. doi: 10.23750/abm.v88i1.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrelli F, Contratti CM, Tanzi E, Grappasonni I. Vaccine hesitancy, a public health problem. Ann Ig. 2018 Mar-Apr;30(2):86–103. doi: 10.7416/ai.2018.2200. doi: 10.7416/ai.2018.2200. [DOI] [PubMed] [Google Scholar]
- 6.MacVane SH. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J Intensive Care Med. 2017 Jan;32(1):25–37. doi: 10.1177/0885066615619895. Epub 2016 Jan 15. Review. [DOI] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Annual Epidemiological Report for 2015. Stockholm: ECDC; 2017. Healthcare-associated infections acquired in intensive care units. In: ECDC. [Google Scholar]
- 8.Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009 Dec;73(4):378–85. doi: 10.1016/j.jhin.2009.03.030. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014 Oct;27(4):665–90. doi: 10.1128/CMR.00020-14. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polívková M, Hubáček T, Staszek M, Švorčík V, Siegel J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int J Mol Sci. 2017 Feb 15;18(2) doi: 10.3390/ijms18020419. pii: E419. doi: 10.3390/ijms18020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocmanová I, Lysková P, Chrenkova V, Olišarová P, Dobiáš R, Janouškovcová H, Soukupová H, Mallátová N, Svobodová L, Hamal P, Skružná M, Bartoníková N. Nosocomial candidemia in the Czech Republic in 2012-2015: results of a microbiological multicentre study. Epidemiol Mikrobiol Imunol. 2018 Spring;67(1):3–10. [PubMed] [Google Scholar]
- 12.Peng D, Li X, Liu P, Luo M, Chen S, Su K, Zhang Z, He Q, Qiu J, Li Y. Epidemiology of pathogens and antimicrobial resistanceof catheter-associated urinary tract infections in intensivecare units: A systematic review and meta-analysis. Am J Infect Control. 2018 Dec;46(12):e81–e90. doi: 10.1016/j.ajic.2018.07.012. doi: 10.1016/j.ajic.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Grappasonni I, Petrelli F, Scuri S, Mahdi SS, Sibilio F, Amenta F. Knowledge and Attitudes on Food Hygiene among Food Services Staff on Board Ships. Ann Ig. 2018 Mar-Apr;30(2):162–172. doi: 10.7416/ai.2018.2207. doi: 10.7416/ai.2018.2207. [DOI] [PubMed] [Google Scholar]
- 14.Siracusa M, Petrelli F. Trade of food supplement: food or drug supplement. Recenti Prog Med. 2016 Sep;107(9):465–471. doi: 10.1701/2354.25224. doi: 10.1701/2354.25224. [DOI] [PubMed] [Google Scholar]
- 15.Grappasonni I, Marconi D, Mazzucchi F, Petrelli F, Scuri S, Amenta F. Survey on food hygiene knowledge on board ships. Int Marit Health. 2013;64(3):160–7. [PubMed] [Google Scholar]
- 16.Grappasonni I, Paci P, Mazzucchi F, De Longis S, Amenta F. Awareness of health risks at the workplace and of risks of contracting communicable diseases including those related to food hygiene, among seafarers. Int Marit Health. 2012;63(1):24–31. [PubMed] [Google Scholar]
- 17.Scuri S, Tesauro M, Petrelli F, Peroni A, Kracmarova L, Grappasonni I. Implications of modified food choices and food-related lifestyles following the economic crisis in the Marche Region of Italy. Ann Ig. 2018 Mar-Apr;30(2):173–179. doi: 10.7416/ai.2018.2208. doi: 10.7416/ai.2018.2208. [DOI] [PubMed] [Google Scholar]
- 18.Petrelli F, Scuri S, Tanzi E, Nguyen C, Grappasonni I. Public health and burnout: a survey on lifestyle changes among workers in the healthcare sector. Acta Biomed. 2018 Nov 28;90(1):24–30. doi: 10.23750/abm.v90i1.7626. doi: 10.23750/abm.v90i1.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grappasonni I, Petrelli F, Traini E, Grifantini G, Mari M, Signorelli C. Psychological symptoms and quality of life among the population of L’Aquila’s “new towns” after the 2009 earthquake. Epidem Biostat Pub Health. 2017;14(2):e11690–1-13. doi: 10.2427/11690. [Google Scholar]
- 20.Petrelli F, Grappasonni I, Evangelista D, Pompei P, Broglia G, Cioffi P, Kracmarova L, Scuri S. Mental and physical effects of energy drinks consumption in an Italian young people group: a pilot study. J Prev Med Hyg. 2018 Mar 30;59(1):E80–E87. doi: 10.15167/2421-4248/jpmh2018.59.1.900. doi: 10.15167/2421-4248/jpmh2018.59.1.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scuri S, Petrelli F, Tesauro M, Carrozzo F, Kracmarova L, Grappasonni I. Energy drink consumption: a survey in high school students and associated psychological effects. J Prev Med Hyg. 2018 Mar 30;59(1):E75–E79. doi: 10.15167/2421-4248/jpmh2018.59.1.898. doi: 10.15167/2421-4248/jpmh2018.59.1.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grappasonni I, Petrelli F, Amenta F. Deaths on board ships assisted by the Centro Internazionale Radio Medico in the last 25 years. Travel Med Infect Dis. 2012 Jul;10(4):186–91. doi: 10.1016/j.tmaid.2012.06.006. doi: 10.1016/j.tmaid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Kračmarová L, Klusoňová H, Petrelli F, Grappasonni I. Tobacco, alcohol and illegal substances: experiences and attitudes among Italian university students. Rev Assoc Med Bras (1992) 2011 Sep-Oct;57(5):523–8. doi: 10.1590/s0104-42302011000500009. [DOI] [PubMed] [Google Scholar]
- 24.Spacilova L, Klusonova H, Petrelli F, Signorelli C, Visnovsky P, Grappasonni I. Substance use and knowledge among Italian high school students. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009 Jun;153(2):163–8. doi: 10.5507/bp.2009.028. [DOI] [PubMed] [Google Scholar]
- 25.Petrelli F, Grappasonni I, Peroni A, Kracmarova L, Scuri S. Survey about the potential effects of economic downturn on alcohol consumption, smoking and quality of life in a sample of Central Italy population. Acta Biomed. 2018 Mar 27;89(1):93–98. doi: 10.23750/abm.v89i1.7059. doi: 10.23750/abm.v89i1.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrelli F, Scuri S, Tanzi E, Nguyễn TTC, Grappasonni I. Lifestyles and discomfort in a sample of young Romanian students. J Prev Med Hyg. 2018 Sep 28;59(3):E230–E235. doi: 10.15167/2421-4248/jpmh2018.59.3.985. doi: 10.15167/2421-4248/jpmh2018.59.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grappasonni I, Scuri S, Tanzi E, Kracmarova L, Petrelli F. The economic crisis and lifestyle changes: a survey on frequency of use of medications and of preventive and specialistic medical care, in the Marche Region (Italy) Acta Biomed. 2018 Mar 27;89(1):87–92. doi: 10.23750/abm.v89i1.7068. doi: 10.23750/abm.v89i1.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen CTT, Scuri S, Nguyen BT, Petrelli F, Grappasonni I. Levels of understanding of the rules of correct medical usage among vietnamese pharmacy students: a cross-sectional study. J Prev Med Hyg. 2018 Dec 15;59(4):E261–E266. doi: 10.15167/2421-4248/jpmh2018.59.4.925. doi: 10.15167/2421-4248/jpmh2018.59.4.925. eCollection 2018 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grappasonni I, Petrelli F, Klusoňová H, Kračmarová L. Level of understanding of medical terms among italian students. Ceska Slov Farm. 2016 Winter;65(6):216–220. [PubMed] [Google Scholar]
- 30.Van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017 May 19;8(4):460–469. doi: 10.1080/21505594.2016.1222343. doi: 10.1080/21505594.2016.1222343. Epub 2016 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Organization WH. Antimicrobial Resistance: Global Report on Surveillance. 2014 http://wwwwhoint/drugre sistance/documents/surveillancereport/en/ [Google Scholar]
- 32.European Centre for Disease Prevention and Control. Stockholm: ECDC;; 2017. Healthcare-associated infections acquired in intensive care units. In: ECDC. Annual epidemiological report for 2015. [Google Scholar]
- 33.Iacchini S, Sabbatucci M, Gagliotti C, Rossolini GM, Moro ML, Iannazzo S, D’Ancona F, Pezzotti P, Pantosti A. Bloodstream infections due to carbapenemase-producing Enterobacteriaceae in Italy: results from nationwide surveillance, 2014 to 2017. Euro Surveill. 2019 Jan;24(5) doi: 10.2807/1560-7917.ES.2019.24.5.1800159. doi: 10.2807/1560-7917.ES.2019.24.5.1800159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization (WHO) Global Action Plan on Antimicrobial Resistance. 2015 doi: 10.7196/samj.9644. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 . [DOI] [PubMed] [Google Scholar]
- 35.Paino-Alvarez M, Bonilla-Muñoz A, Garcia-Fernandez M. Antimicrobial Polymers in the Nano-World. Nanomaterials. 2017;7(2):1–48. doi: 10.3390/nano7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo MD, Won HS, Kim JH, Mishig-Ochir T, Lee BJ. Antimicrobial peptides for therapeutic applications: a review. Molecules. 2012 Oct 18;17(10):12276–86. doi: 10.3390/molecules171012276. doi: 10.3390/molecules171012276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Victor W.L. Ng, Julian M.W, Chan Haritz Sardon, Robert J, Ono Jeannette M, García Yi, Yan Yang, James L. Hedrick Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Advanced Drug Delivery Reviews. 2014;78:46–62. doi: 10.1016/j.addr.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 38.ISO-22196: 2007, Plastics – Measurement of Antibacterial Activity on Plastics Surfaces [Google Scholar]
- 39.Marchetti F, Palmucci J, Pettinari C, Pettinari R, Marangoni M, Ferraro S, Giovannetti R, Scuri S, Grappasonni I, Cocchioni M, Maldonado Hodar FJ, Gunnella R. Preparation of Polyethylene Composites Containing Silver(I) Acylpyrazolonato Additives and SAR Investigation of their Antibacterial Activity. ACS Appl Mater Interfaces. 2016 Nov 2;8(43):29676–29687. doi: 10.1021/acsami.6b09742. Epub 2016 Oct 20. [DOI] [PubMed] [Google Scholar]
- 40.Marchetti F, Palmucci J, Pettinari C, Pettinari R, Scuri S, Grappasonni I, Cocchioni M, Amati M, Lelj F, Crispini A. Linkage Isomerism in Silver Acylpyrazolonato Complexes and Correlation with Their Antibacterial Activity. Inorg Chem. 2016 Jun 6;55(11):5453–66. doi: 10.1021/acs.inorgchem.6b00495. doi: 10.1021/acs.inorgchem.6b00495. [DOI] [PubMed] [Google Scholar]
- 41.JIS L-1902: 2002, Testing Method for Antibacterial Activity of Textiles [Google Scholar]
- 42.XLSTAT. Statistical software & data analysis add-on for Excel. Addinsoft (2017) [Google Scholar]
- 43.Kumar R, Münstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005 May;26(14):2081–8. doi: 10.1016/j.biomaterials.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Su HL, Chou CC, Hung DJ, Lin SH, Pao IC, Lin JH, Huang FL, Dong RX, Lin JJ. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009 Oct;30(30):5979–87. doi: 10.1016/j.biomaterials.2009.07.030. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Schacht VJ, Neumann LV, Sandhi SK, Chen L, Henning T, Klar PJ, Theophel K, Schnell S, Bunge M. Effects of silver nanoparticles on microbial growth dynamics. J Appl Microbiol. 2013 Jan;114(1):25–35. doi: 10.1111/jam.12000. doi: 10.1111/jam.12000. [DOI] [PubMed] [Google Scholar]
- 46.Karchmer TB, Giannetta ET, Muto CA, Strain BA, Farr BM. A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Arch Intern Med. 2000 Nov 27;160(21):3294–8. doi: 10.1001/archinte.160.21.3294. [DOI] [PubMed] [Google Scholar]
- 47.Brutel de la Riviere A, Dossche KM, Birnbaum DE, Hacker R. First clinical experience with a mechanical valve with silver coating. J Heart Valve Dis. 2000 Jan;9(1):123–9. [PubMed] [Google Scholar]