Abstract
Hemobilia is an unusal cause of upper gastrointestinal bleeding and may be the result of the formation of an hepatic vessel pseudoaneurysm. This is a rare occurence after laparoscopic or open cholecistectomy. The most importants factor for pathogenesis are direct or indirect iatrogenic injuries during intervention and hepatic trauma. Clinical presentation may also be late and includes more frequently upper gastrointestinal bleeding due to pseudoaneurysm rupture, abdominal pain and jaundice secondary to bile duct compression. Therapies includes trans arterial embolization of feeding artery and percutaneous ingjection of embolic devices into the aneurysm. Surgery must be reserved for cathether based therapy failure. We report a case of a 66 year old man, presenting a month after cholecystectomy, complaining abdominal pain in the upper right quadrant and hematemesis. An EGDS exam showed hemobilia and computed tomography (CT) revealed a cistic artery pseudoaneurysm (PSA) wich have been successfully treated with hyperselective arterial embolization. Although this is a rare complication the surgeon must be aware of related symptoms and signs in order to sospect pseudoaneurysm as prompt recognition and treatment are essential. Untreated haemobilia may determine an immediate threat to life leading to acute haemodynamic instability We describe both diagnostic features and therapeutic strategies in comparison to the most recent literature. (www.actabiomedica.it)
Keywords: hemobilia, pseudoaneurysm, cholecystectomy, arterial embolization
Introduction
Post-operative Hemobilia due to pseudoaneurysm is a rare but well-known occurrence representing only 6% of all causes of upper gastrointestinal bleeding. Artery pseudoaneurysm is a continuous inflammatory process that leads to erosion in the elastic and muscular components of the arterial wall, ultimately resulting in pseudoaneurysm formation. The most importants factor for pathogenesis are direct or indirect iatrogenic injuries during intervention and hepatic trauma.
Clinical presentation may also be late and includes more frequently upper gastrointestinal bleeding due to pseudoaneurysm rupture, abdominal pain and jaundice secondary to bile duct compression (3-12). Therapies includes trans arterial embolization of feeding artery and percutaneous ingjection of embolic devices into the aneurysm. Surgery must be reserved for cathether based therapy failure (13-14). We report a case of a 66 year old man, presenting a month after cholecystectomy, complaining abdominal pain in the upper right quadrant and hematemesis. An EGDS exam showed hemobilia and computed tomography (CT) revealed a cistic artery pseudoaneurysm (PSA) wich have been successfully treated with hyperselective arterial embolization. Although this is a rare complication the surgeon must be aware of related symptoms and signs in order to sospect pseudoaneurysm. We describe both diagnostic features and therapeutic strategies in comparison to the most recent literature.
Case report
I.C., 66 years old, chronic heavy smoker, with a history of hypertension, recently subjected to pace maker implantation for atrial fibrillation, presented to our clinic for symptomatic cholelitiasis. US performed in previous months demonstrated multiple subcentimeter gallstones without any signs of acute cholecystitis. The patient had been subjected to Endoscopic retrograde cholangiopancreatography (ERCP) for choledocholithiasis. According to the program we performed a video laparoscopic cholecystectomy. During the surgical procedure an anomalous biliary duct for VI-VII hepatic segment, wich flows directly into the common hepatic duct over cystic duct is accidentally damaged. We therefore decided to convert the procedure from laparoscopic to open cholecystectomy. We also placed a Kehr type drain into the common hepatic duct from wich came some gallstone. Intraoperative cholangiography didn’t show sign of bile spillage. Post-operative course was completely normal and the patient has been discharged 4 days later. Two weeks after we performed another cholangiography wich showed no signs of leakage.
About one month after the patient presented again to our clinic with a history of hematemesis, melena and upper and right abdominal pain from 2 days. The abdomen resulted distended and the patiens was pale. When the patient arrived at hospital, his blood pressure was approximately 80/60 mm Hg and his pulse rate was 110 beats per minute.
We decided to perform an endoscopic exam wich showed clots and blood in descending duodenum, and in particular showed a clot exiting through the major duodenal papilla (Fig. 1-2).
Figure 1.

Bleeding from duodenal tract
Figure 2.

Bleeding from Vater’s Papilla
The patient has been hospitalized for hemobilia. Laboratory investigations revealed a significant fall in hemoglobin values (7.0 mg/dl). Other significant laboratory studies revealed the following values: white blood cell count, 18.3 × 103/uL; total bilirubin, 2,4 mg/dl.
A CT-SCAN analysis showed an oval blushing of contrast (10-11 mm)in arterial phase, next to a clip on hepatic pedicle as ay be seen in pseudoaneurysm. In delayed phase along the common biliary duct the CT-SCAN showed hyperdense material probably due to hematic losses. Hemobilia and dilatation of biliary ducts are also highlighted.
We decided to manage the PSA with a celiac trunk selective arterial embolization via right femoral catheterization. Angiography showed the PSA located near the the surgical clip, close to hepatic artery bifurcation (Fig. 3). Through superselective cathterization we placed multiple spiral coils (MRI compatible) inducing PSA occlusion (Fig. 4).
Figure 3.

Cystic artery pseudoaneurism
Figure 4.
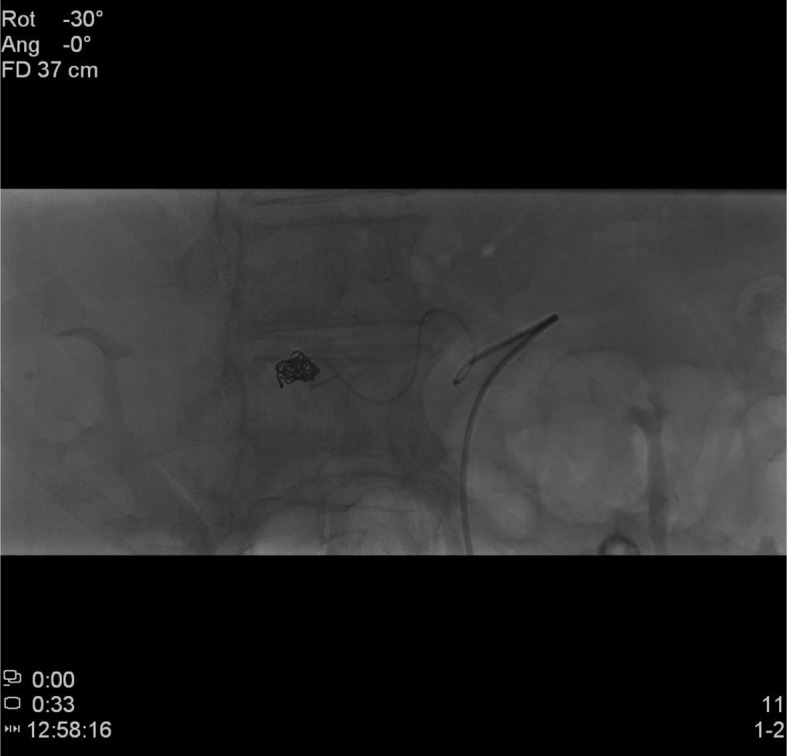
Embolization of pseudoaneurysm
In post procedural course we infuse two concentred herytrocytes bags with hemoglobin value raise to 9.0 mg/dl the day after and to 10 mg/dl three days after. The patient has been discharged three days after hospitalization in good conditions.
The patient presented to our clinic a week after, totally asymptomatic, showing a normal hemoglobin value (11.4 mg/dl). An imaging guided follow up has been performed a month after using color doppler ultra sound that has not highlighted abdominal collection nor bile ducts dilatations.
Discussion
Hemobilia was first reported by F. Glisson in 1654 and the defined by Sandblomm in 1948 as an hemorrage into the bilary tract from a passageway between blood vessels and bile ducts (7). The most frequent causes of this pathological condition are liver traumas and pseudoaneurysm of epathic arteries. Less frequently inflammatory conditions or neoplasms are involved in the ethiology. Iatrogenic causes are nowadays the most important factors (two-thirds of cases are iatrogenic), procedures as hepatobiliary surgery, laparoscopic cholecistectomy, biliary drainage and liver biopsy may be complicated by the formation of pseudo aneurysm. Vascular damages and thermal injuries during laparoscopic Calot’s triangle dissection are predisposing factors. However most cases described have a history of difficult or prolonged intervention (1, 5). Most likely, precipitating factors in the exposed case, include initial clip encroachment of the vessels, thermal or mechanical injury, and inflammation due to surgery.
The incidence of pseudoaneurysm formation is hard to determine, as asymptomatic aneurysm could not be easily determined or may thrombose spontaneously, while the risk of rupture is related to sizes (5, 9).
The time interval between procedures or surgery and onset of clinical symptoms is variable. Most of patients present a month after surgery but 5 years delayed presentation has been described.
Most of patients present with the classical symptoms: jaundice, biliary colic or upper abdominal pain, and gastrointestinal bleeding, but less then 40% patients present the complete triad first described by Quincke in 1871 (8, 9). In our case upper gastrointestinal bleeding from resulted from the cystic artery pseudoaneurysm’s communication with the cystic duct.
Diagnosis is usually made with gastrointestinal endoscopy wich can demonstrate blood flow from duodenal papilla, if this procedure fails to show bleeding sources, urgent CT scan should be considered. Endoscopic retrograde cholangiopancreatography (ERCP) eventually followed by stent placement or sphincterotomy, is highly effective in diagnosing this threatening complication, giving possibility to control possible cystic duct stump leaks and treating obstructive jaundice.
CT scan may show abdominal collection, biliary tree dilatation, gastrointestinal distension, pleural effusion and, overall, suspected vascular abnormality, while catheter arteriography is used for therapeutic procedure as pseudoaneurysm or feeding vessel embolization. This procedure, performed with coils, gel foam thrombin and other agents, has replaced the surgical management of pseudoaneurysm wich requires aneursym resection and ligation of cistic artery. While transarterial embolisation is the treatment of choice for haemostasis, with a 75% to 100% success rate, surgery remains however the next step after an embolization failure (13,14).
Conclusions
In conclusion, patients who underwent laparoscopic cholecistectomy should be observed in post-operative course as cystyc artery pseudoaneurysm occurs as a rare complication with clinical symptoms often delayed in time. Although this is a rare complication the surgeon must be aware of related symptoms and signs in order to suspect pseudoaneurysm as prompt recognition and treatment are essential.
Untreated haemobilia may determine an immediate threat to life leading to acute haemodynamic instability, necessitating detection, access, and control of the vascular anomaly.
The suspect of PSA should be posed in symptomatic patients showing hemobilia, using endoscopy and imaging guidance as CT angiography. Therapy should be at first endovascular as more invasive surgical treatments may be used only after embolization failure.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Kumar A, Sheikh A, Patyka L, et al. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin Imaging. 2014 Jul Aug;38(4):522–525. doi: 10.1016/j.clinimag.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson T, Travis S, Ettles D, et al. Hepatic artery angiography and hembolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999;22:20–4. doi: 10.1007/s002709900323. [DOI] [PubMed] [Google Scholar]
- 3.Green MHA, Duell RM, Jhonson CD, Jamieson NV. Haemobilia. Br J Surg. 2002;88(6):773–86. doi: 10.1046/j.1365-2168.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee SP, Tasman-Jones C, Wattie WJ. Traumatic hemobilia: a complication of percutaneous liver biopsy. Gastroenterology. 1977;72:941–4. [PubMed] [Google Scholar]
- 5.Curet P, Baumer R, Rocher A, Grellet J, et al. Hepatic hemobilia of traumatic or iatrogenic origin: recent advances in diagnosis and therapy, review of the literature1976 to 1981. World J Durg. 1984;8:2–8. doi: 10.1007/BF01658356. [DOI] [PubMed] [Google Scholar]
- 6.Madanur MA, Battula N, Sethi H, Deshpande R, et al. Pseudoaneurysm following laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2007;6:294–8. [PubMed] [Google Scholar]
- 7.Glisson F. 1st Edition. Amsterdam, Janssonium and Weyerstraten: 1654. Anatomia Hepatis; p. 2. [Google Scholar]
- 8.Rencuzogullari A, Okoh A, Akcam T, et al. Hemobilia as a result of right hepatic artery pseudoaneurysm rupture: an unusual complication of laparoscopic cholecystectomy. International Journal of Surgery Case report. 2014;5:142–144. doi: 10.1016/j.ijscr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrou A, Brennan N, Soonawalla Z, et al. Hemobilia due to cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy: case presentation and literature review. Int Surg. 2012;97:140–144. doi: 10.9738/CC52.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napolitano V, Cirocchi R, Spizzirri A, et al. A severe case of hemobilia and biliary fistula following an open urgent cholecystectomy. World Journal of Emergency Surgery. 2009;4:37. doi: 10.1186/1749-7922-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madanur MA, Battula N, Sethi H, et al. Pseudoaneurysm folowing laparoscopic cholecystectomy. Hepatobilary pancreat dis in. 2007;6(3):294–298. [PubMed] [Google Scholar]
- 12.Croce MA, Fabian TC, Spiers JP, et al. Traumatic hepatic artery pseudoaneurysm with hemobilia. Am J Surg. 1994;168(3):235–238. doi: 10.1016/s0002-9610(05)80193-x. [DOI] [PubMed] [Google Scholar]
- 13.Rauws EA, Gouma DJ. Endoscopic and surgical management of bile duct injuryafter laparoscopic cholecystectomy. Best Pract res clin gastroenterol. 2005;18(5):829–46. doi: 10.1016/j.bpg.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K. The endovascular management of visceral artery aneurysm and pseudoaneurysm. J Vasc Surg. 2007 Feb;45(2):276–83. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]


