Abstract
Objective: Honey and its polyphenolic compounds are of main natural antioxidants that have been used in traditional medicine. The aim of this review was to identify the protective effects of honey and chrysin (a polyphenol available in honey) against the chemical and natural toxic agents. Method: The scientific databases such as MEDLINE, PubMed, Scopus, Web of Science and Google Scholar were searched to identify studies on the antidotal effects of honey and chrysin against toxic agents. Results: This study found that honey had protective activity against toxic agents-induced organ damages by modulating oxidative stress, inflammation, and apoptosis pathways. However, clinical trial studies are needed to confirm the efficacy of honey and chrysin as antidote agents in human intoxication. Conclusion: Honey and chrysin may be effective against toxic agents. (www.actabiomedica.it)
Keywords: honey, chrysin, natural toxic agent, chemical toxic agent
1. Introduction
Nowadays, antioxidants are used for reducing risk of various diseases such as cancer, cardiovascular, neurodegenerative, renal failure, gastrointestinal, and respiratory diseases (1-3). Honey is one of the main natural antioxidants which is used as a nutritional product and an alternative treatment in traditional medicine (4). Honey is a natural liquid consisting of at least 181 ingredients including proteins, amino acids, minerals, vitamins, and organic acids (5). Honey also contains polyphenols, flavonoids, glycosides, quinone, alkaloids, cardiac glycosides, and volatile substances (6). The honey composition is related to the plant source, seasonal and environmental properties as well as honeybee species. Honey is also comprised of different impurity (7). Honey contains less fructose and glucose when compared to sugar, but contains more calories. Honey and sugar are both carbohydrates, consisting of the two types of sugar: glucose and fructose. Refined fructose, which is found in sweeteners, is metabolized by the liver and has been associated with: obesity. Although, Sugar is sugar, however, honey is (mostly) sugar. On the other hand, sugar is higher on the glycemic index (GI) than honey, meaning it raises blood sugar levels more quickly. This is due to its higher fructose content, and the absence of trace minerals. The difference between the digestion of honey compared to the digestion of sugar lies in the composition of enzymes in each of these products. Sucrose (table sugar) passes through the stomach without any digestion happening because of its disaccharide (a sugar composed of two monosaccharides) composition. This means, honey also has trace elements in it. These will depend on region, so depending on the source of your honey, it could have varying small amounts of minerals like zinc and selenium, as well as some vitamins. Where honey shines is in its content of bioactive plant compounds and antioxidants. Various constituents of honey are found to have antioxidant activities. Flavonoids and polyphenols such as chrysin in honey indicated the strong antioxidants properties (7). The pharmacological properties of honey are associated with the presence of polyphenols in honey. Although, honey may be low in vitamins and minerals but is high in some plant compoundsincluding chrysin (7). According to the recent scientific literature, honey and chrysin may be effective against a wide range of diseases from a wound to cancer (9-13). Various studies have also reported protective effects of honey and chrysin against natural and chemical toxic agents in different tissues (14). Thus, the present study was designed to review the protective effects of honey and chrysin against tissue injuries induced by natural and chemical agents.
2. Methods
We searched the literature for basic and clinical studies concerned with the antidote and protective effects of honey and chrysin against toxic agents. The electronic databases including PubMed-Medline, Embase, Google Scholar, and Scopus were searched from 1990 to 2018. Keywords were Honey, chrysin, natural toxic agent, toxic chemical agent, and protective effects combined in different ways to retrieve related articles. After reading the abstracts, the full texts of 58 articles were critically scrutinized.
3. Neuroprotection
3.1 Honey and neurotoxic agents
Lead
Lead (Pb) is one of the major toxic heavy metals causing severe tissue injury in both human and animal. Oxidative stress has been found as an essential mechanism involved in lead-induced neurodegenerative diseases such as memory impairment and locomotor disability. Lead exposure reduces locomotor and exploratory activities and also increases anxiety and memory impairment via disrupting the oxidant-antioxidant system in animal models. The study has indicated that administration of honey (1 and 1.5 ml/kg, PO) inhibited lead induced-neurotoxicity as seen through improvement of memory and locomotor functions in animals. The study suggested that honey protected brain against lead via enhancing antioxidant activities as evidenced by increasing superoxide dismutase (SOD), glutathione-S-transferase (GST), and glutathione peroxidase (GPx) activities and glutathione (GSH) level as well as decreasing brain level of lipid peroxidation (15).
Aluminum
Aluminum (Al) is one of the most abundant heavy metals in the Earth’s crust. It exists in the water, air, natural and commercial food, and medicinal agents such as drugs. Aluminum has been recognized as a neurotoxic agent inducing neurodegenerative diseases. Aluminum induces oxidative stress and increases amyloid beta level in the brain of animal models. Sub-chronic exposure to aluminum elevates lipid peroxidation and decreases GSH levels as well as catalase (CAT), SOD, and GST activities in brain. Aluminum also increases serum levels of tumor indices including arginase and a-l-fucosidase by modifying glutamate GABA system. It was reported that co-administration of AlCl3 with honey syrup (500 mg/kg, PO) increased antioxidant enzymes activities and decreased lipid peroxidation level in mouse brain. Honey administration also reduced serum tumor indices levels by modulating expression of BCL-W gene which is an anticancer gene in brain cells (16).
Lipopolysaccharide
Lipopolysaccharide (LPS) causes neurodegenerative diseases by inducing inflammatory mediators in the brain of experimental models (17, 18). It is suggested that inhibition of microglia-mediated neuroinflammation is an effective strategy for treatment of neurodegenerative diseases. In this regards, Candiracci et al., 2012 (19) indicated that honey extract (0.5 and 1 μg/ml) decreased secretion of pro-inflammatory mediators such as interleukin (IL-1β) and tumor necrotic factor (TNF-α) induced by LPS in N13 microglia. Honey decreased the inducible nitric oxide synthetize (iNOS) expressions and production of reactive oxygen species (ROS) (20). The study indicated honey may act as a potent inhibitor of microglial activation and prevent the neurodegenerative diseases (19) (Fig. 1).
Figure 1.
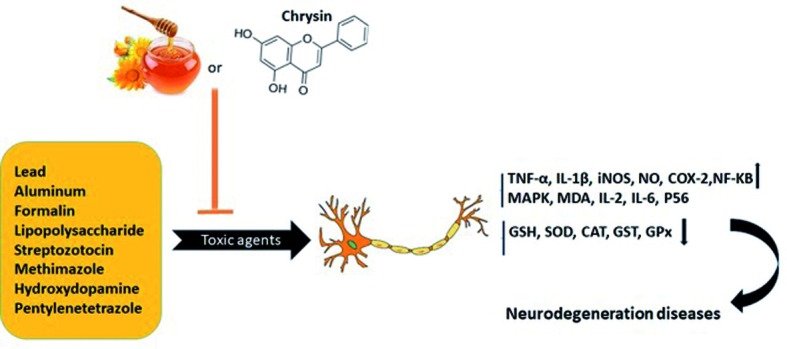
The protective effect of honey and chrysin against neurotoxic agents
3.2 Chrysin and Neurotoxic agents
Formalin
Formalin is used for inducing neuropathic pain in the animal models. Chrysin (50, 100 and 150 mg/kg, IP, 60 min before formalin injection) was effective against formalin-induced pain in rats by modulating corticosterone and noradrenaline levels in serum (21). Chrysin (5 and 10 mg/kg, IP) have indicated analgesic and anti-inflammatory effects via modulating COX-2 activity (22).
Lipopolysaccharide
Chrysin (1, 5 and 10 μM) prevented LPS-stimulated microglia cells by decreasing NO, TNF-α, IL-1β productions and expressions of iNOS and COX-2. The findings indicated chrysin ameliorated neuro-inflammation through modulating c-Jun N-terminal kinase and Nuclear factor-κB (NF-κB) signaling molecules (23). Chrysin inhibited LPS-induced vascular cell adhesion molecule-1 (VCAM-1) expression in cerebral vascular endothelial (bEnd.3) mouse cells by preventing NF-κB translocation and p38 mitogen-activated protein kinase (MAPK) signaling. It seems, the neuroprotective effects of chrysin is related to its anti-inflammatory activities (24).
Streptozotocin
Streptozotocin (STZ, 2-deoxy-2-(3-(methyl-3-nitrosoureido)-D-glucopyranose) is produced by Streptomycetes achromogenes inducing diabetes mellitus in the animal models. Brain is susceptible to hyperglycemia induced by STZ. Oxidative stress has a vital role in developing neurovascular complications in diabetes. Natural antioxidants such as chrysin can penetrate to blood-brain barrier that may be effective against diabetic neuropathy. In this context, it was reported chrysin (30 and 100 mg/kg for 26 days) combated against STZ-induced diabetes-associated cognitive decline in rats by modulating oxidative stress indices (MDA, CAT, SOD, and GSH), NF-κB, TNF-α, IL-6, IL-1β, as well as caspase-3 in cerebral cortex and hippocampus (25). Chrysin (20, 40, 80 mg/kg/day) prevented STZ-induced brain toxicity by modulating oxidative stress and decreasing MDA levels as well as an increase in total protein, SOD, CAT, and GST in rats’ brain (26).
Methimazole
Methimazole (MTZ) is useful for treatment of overactive thyroid patients. In animal models, MTZ is used for inducing hypothyroidism. Hypothyroidism usually causes depression. Chrysin (20 mg/kg, IP) represented protective effects against MTZ-induced depressive-like behavior in female mice. Chrysin modulated serotonin (5HT) in the prefrontal cortex, hippocampus as well as dopamine in hippocampus of theanimals (27).
Hydroxydopamine
6-hydroxydopamine (6-OHDA) is a neurotoxic catecholaminergic agent which is used for inducing Parkinson’s disease in animal models. 6-OHDA affects inflammatory responses and neurotrophic factors in Parkinson’s disease. Chrysin (10 mg/kg, PO) showed a protective effects against 6-OHDA-induced Parkinson’s disease in rats. Chrysin decreased TNF-α, IL-1β, IL-2, IL-6, and NFKB levels and increased IL-10 level, total antioxidant capacity in the striatum, as well as brain-derived neurotrophic factor, nerve growth factor, and glial cell line-derived neurotrophic factor levels. Chrysin also increased dopamine, 3,4-dihydroxyphenylacetic acid, tyrosine hydroxylase, and homovanylic acid levels. Chrysin improved Parkinson’s disease through modulating inflammatory cytokines and neurotrophic factors in the brain of rats with Parkinson’s diseases (28).
Ammonium chloride
Hyperammonemia changes ammonia metabolism and increases inflammatory cytokines production in brain. Chrysin (100mg/kg, PO) showed protective effects on ammonium chloride (NH4Cl)-induced neuroinflammation in rats. Chrysin up-regulated glutamine synthetase (GS) activity and glial fibrillar acidic protein (GFAP) expression as well as down-regulated TNF-α, IL-1β, IL-6, p65 NF-κB, iNOS, and COX-2 expression in hyperammonemic rats brain (29).
Pentylenetetrazole
Pentylenetetrazole (PTZ) is a selective blocker of chloride channel coupled to ɣ-aminobutyric acid type A (GABAA) receptor complex. PTZ has been used as a circulatory and respiratory stimulant drug and in convulsive therapy. However, the side effects such as uncontrolled seizure limited its use. Recently, PTZ is applied for inducting seizure in animal models. Chrysin (2.5, 5, and 10 mg/kg) representes anticonvulsant properties against PTZ-induced convulsions in rats (30) (Fig. 1).
4. Respiratory protection
4.1 Honey and respiratory toxic agents
Ovalbumin
Ovalbumin (OVA) is the main protein of egg white applying for induction of allergic reaction such as airway hyper-responsiveness in experimental models. It was reported that the aerosolized honey at a dose of (25% (v/v) and 50% (v/v) inhibited of ovalbumin (OVA)-induced asthma on airway tissues in a rabbit model. The findings indicated aerosolized honey ameliorated OVA-induced structural changes in epithelium, mucosa, and submucosal regions of airway. Honey decreased the number of airway inflammatory cells in bronchoalveolar lavage fluid and also inhibited the goblet cell hyperplasia (31). Shamshuddin et al. 2016 (32) indicated honey at a dose of 10% (v/v), 40% (v/v) and 80% (v/v) inhibited OVA-induced allergic asthma in mice model by decreasing inflammatory cell infiltration and beta-hexosaminidase level in bronchoalveolar lavage fluid. All histopathological evaluations confirmed the preventive effects of honey against OVA-induced allergic asthma. It evidenced through improving epithelium thickness, number of mast cell and mucus expression in the treated animals (32) (Fig. 2).
Figure 2.
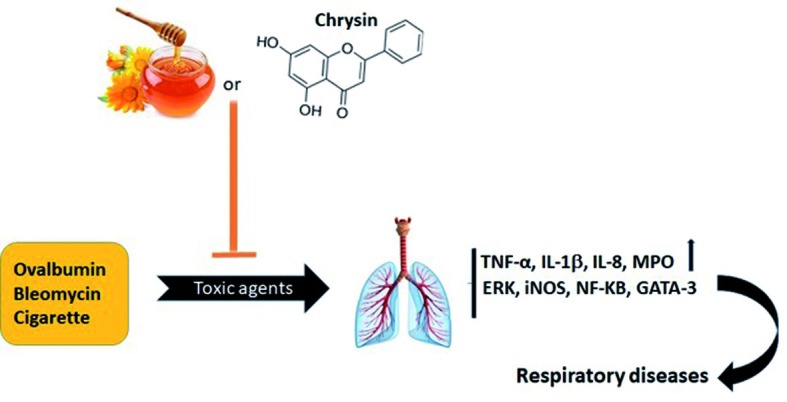
The protective effects of honey and chrysin against respiratory agents
4.2 Chrysin and respiratory toxic agents
Ovalbumin
OVA induces lung inflammation and airway hyperresponsiveness via shifting immune response toward a T-helper type 2 (Th2) profile in mice airway. Chrysin (50 mg/kg, PO) had protective effects against ovalbumin-induced inflammation by regulating transcription factors T-bet and GATA-3 in mice (33). Chrysin (3, 10, and 30 mg/kg, PO) also ameliorated histopathological lung injury in the rats exposed to ovalbumin. It was suggested that anti-asthmatic effects of chrysin might be associated with its protective effects on Th1/Th2, iNOS and NF-κB expressions (34).
Bleomycin
Bleomycin is an anti-cancer drug inducing pulmonary inflammation and fibrosis. Bleomycin induces lung injury through producing inflammatory cytokines such as IL-18 and IL-1β in and oxidative stress (35). Chrysin (50 mg/kg, PO) indicated protective effects against bleomycin-induced lung inflammation and fibrosis in rats by modulating oxidant-antioxidant system (36).
Cigarette smoke
Cigarette smoke induces airway inflammation through increasing inflammatory cytokine in serum levels such as TNF-α, IL-1β, IL-8, and MPO. Chrysin pretreatment (10 and 20 mg/kg, IP) inhibited cigarette smoke-induced airway inflammation via modulating ERK and p38 phosphorylation (37) (Fig. 2).
5. Cardiovascular protection
5.1 Honey and cardiovascular toxic agents
Cadmium
Cadmium (Cd) is one of the major heavy metals that is associated with various diseases by inducing cellular toxicity (12). Cd produces cardiovascular abnormalities via modifying oxidative and inflammatory responses. Cd elevated mean corpuscular volume and reduced white and red blood cells counts, lymphocytes, platelets, hemoglobin, and hematocrit. However, it was reported honey (50% concentration, PO) increased hemoglobin and white blood cell count in rats exposed CdCl2 (38). Abdelaziz et al. 2013 (39), also reported orally administration of honey (9 mg/kg, 1 h before a single dose of CdCl2 injection) decreased in hematotoxic effects of Cd by ameliorating the changed blood parameters (39).
Lead
Some studies have indicated the hematotoxic effects of lead in animal models. It was indicated that honey (1 mg/kg, o) improved lead-induced anemia in rabbits. The study suggested the protective effects of honey against lead-induced blood toxic effects (40).
Copper sulfate
Copper sulfate (CuSo4), an inorganic compound, is used as a pesticide and herbicide that its toxicity is related to copper content. Copper is an “essential mineral” that is very toxic at high concentrations. Copper causes oxidative stress by inducting Fenton-type redox reactions. However, copper toxicity usually occurs following disruption of absorption, distribution, and excretion of this metal. Copper toxicity might be associated with cardiovascular diseases. In vitro study conducted by Makedou et al., 2012 (41) have indicated that Greek pine tree honey (100, 200 and 400 μg/ml diluted serum, and 10, 20 and 40 μg/ml diluted LDL, respectively) prevented cardiotoxicity of copper sulfate by decreasing susceptibility of human LDL-c to oxidation. The study indicated Greek honey could delay LDL oxidation induced by copper sulfate, and may be useful for treating cardiovascular diseases (41).
Cigarette
Cigarette smoking plays a principal role in initiating and progression of cardiovascular diseases by inducing inflammation. Honey consumption (20 g/day, PO) ameliorated inflammatory indices including high sensitive C-reactive protein (h-CRP), IL-6, and TNF-α among chronic smokers from Quit Smoking Clinic and Health Campus, Universiti Sains Malaysia. The study suggested that honey inhibited the development of cardiovascular problems by ameliorating inflammatory response (42).
Isoproterenol
Isoproterenol is one of the non-selective β agonist drugs that are effective for treatment of bradycardia, heart block, and asthma. However, several studies have indicated the cardiotoxicity effects of isoproterenol in the animal models (43-45). Isoproterenol increased the cardiac enzymes activity including lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), aspartate transaminase (AST), alanine transaminase (ALT), as well as the cardiac troponin I (cTnI) triglycerides (TG), total cholesterol (36), low-density lipoprotein-cholesterol (LDL-C), and lipid peroxidation levels. Isoproterenol also decreased the activities of antioxidants enzymes and GSH levels in heart as well as high-density lipoprotein-cholesterol (HDL-C) serum levels. Pretreatment of Tualang honey (3 g/kg, PO) and Sundarban honey (5 g/kg, PO) in the ischemic rats inhibited isoproterenol-induced myocardial infarction through modulating oxidative stress and dyslipidemia (46, 47).
Ethanol
Clinical observation in a patient presenting with a history of alcohol abuse and experimental studies indicated the association between ethanol consumption and cardiovascular diseases. The protective effect of Anzer honey against ethanol- increased vascular permeability in rats has been studied. The results indicated that Anzer honey (0.275 g/kg, PO) with 25.44 mg/g ascorbic acid inhibited vascular permeability after ethanol exposure. It was suggested that the vascular protective effects of Anzer honey against ethanol might be related to the antioxidant content including a high amount of the ascorbic acid (48).
Lipopolysaccharide
LPS induces innate immune responses leading to the release of cellular NO and other inflammatory mediators as well as macrophage migration which causes sepsis (12). High doses of LPS is associated with organ failure and induces a systemic inflammatory response and septic shock. The effect of honey against a lethal dose of LPS-induced organ failure in rabbit has been studied. Honey (1 mL of 500 mg/kg in saline, IV) protected cardiac and lipid profiles against a lethal dose of LPS as evidenced by ameliorating red blood cell (RBC), white blood cell (WBC) and thrombocyte counts, blood pH, neutrophil infiltration and MPO activity. The study suggested that honey may be protected organs failure during inflammatory disorders (49).
High-Fat Diet
A high-fat diet is one of the leading risk factors for obesity and cardiovascular diseases distributing lipid profile. Several studies indicated that honey consumption is useful for controlling lipid hemostasis. Gelam honey decreased the percentage of adiposity index including body mass index (BMI) and the TC, TG, leptin, and resistin plasma levels in rats fed high-fat diet. The study suggested honey consumption decreased the risk of obesity and cardiovascular diseases by modulating lipid metabolism (50) (Fig. 3).
Figure 3.
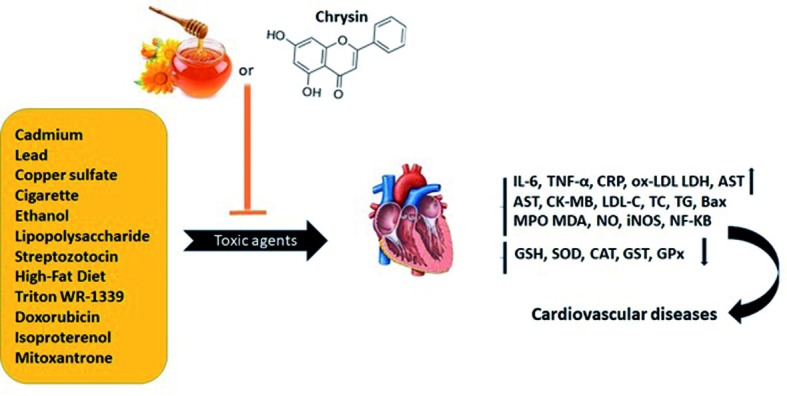
The protective effects of honey and chrysin against cardiotoxic agents
5.2 Chrysin and cardiovascular toxic agents
Triton WR-1339
Triton WR-1339 is used for inducting hypercholesterolemia in the animal models. Triton WR-1339 causes hypercholesterolemia by inhibiting lipoprotein lipase activity. Hypercholesterolemia is one the main risk factors for progressing coronary artery disease such as atherosclerosis. Chrysin (200 mg/kg, PO) improved Triton WR-1339-induced hypercholesterolemia by increasing the enzymatic and nonenzymatic antioxidants in rats (51).
Doxorubicin
Doxorubicin (DOX) is one of the anticancer chemotherapeutic drugs with severe side effects including cardiotoxicity (52). Chrysin (25 and 50 mg/kg, PO) indicated protective effects against DOX-induced acute cardiotoxicity via modulating oxidative stress indices (GSH, CAT, SOD and MDA), inflammatory markers (NF-κB, iNOS, COX-2, TNF-α and NO) as well as apoptotic components (Bax, Bcl-2, cytochrome c, caspase-3) (53).
Streptozotocin
Chrysin (20, 40 and 80 mg/kg, IP) inhibited hyperlipidemia induced by STZ trough modulating oxidative stress in rats liver (26).
Mitoxantrone
Methotrexate (MTX) belongs to the chemotherapeutic drugs; however, its toxic effects including cardiotoxicity limited its clinical uses. MTX causes cardiomyopathy with a reduction in left ventricular ejection fraction and heart failure (54). Chrysin inhibited MTX-induced cardiotoxicity by modulating apoptosis indices such as an increase in the Bcl-2 and also a reduction in Bax and caspase-3 expressions (55) (Fig. 3).
6. Hepatoprotective effects
6.1 Honey and hepatotoxic agents
Lead
Lead is recognized as a potent hepatotoxic agent. Several underlying mechanisms, including oxidative stress, inflammation, and apoptosis are involved in hepatotoxicity induced by lead. Lead disturbs liver metabolism, decreases liver antioxidant content and increases serum levels of liver enzymes. Carob honey (1 g/kg, PO) inhibited lead-induced hepatotoxicity in rabbits as noted by decreasing the liver enzymes serum levels (40).
Aluminum
Aluminum exposure disturbs mitochondrial energy metabolism by inducing mitochondrial oxidative stress, which leads to liver dysfunction. Honey (500 mg/kg, PO) inhibited hepatotoxicity induced by aluminum chloride (AlCl3) in mice as evidenced through decreasing liver enzymes, total bilirubin, and lipid peroxidation activities. The study suggested that honey decreased the hepatotoxic effects of AlCl3 by modulating oxidative stress (56).
Carbon tetrachloride
Carbon tetrachloride (CCl4) is a hepatotoxic agent that causes liver injury by induction of ROS generation, inflammation, apoptosis and cell death. CCl4 increases the liver enzymes serum levels including ALT, AST, ALP, and GGT. Slow or rapid intravenous injection of honey (40 and 80 mg/kg, PO) was effective against CCl4-induced liver injury in sheep (57). It was also indicated that total food restriction with 50% honey decreased the ALT and AST serum levels in rats exposed to CCl4 (38). Sider honey (5 g/kg, PO) ameliorated CCl4-induced hepatotoxicity in rats as noted by decreasing in the ALT, AST, ALP, and bilirubin serum levels as well as the MDA content. Honey increased liver weight, GSH content while CCl4 decreased these parameters. Honey improved pathological damages such as cell necrosis, vacuolar degeneration, mononuclear cellular infiltration pyknotic nuclei, and apoptotic bodies in the liver of animal exposed to CCl4 (58). The protective effects of Saudi Sider honey (SSH) against CCl4 induced oxidative stress and liver injury have been investigated in the rat. Administration of SSH (0.5 and 1.0 g/kg/day, PO) prior exposure to CCl4 decreased the enzyme markers serum levels including ALT, AST, ALP, GGT and also bilirubin. SSH also increased total protein content and reduced malondialdehyde (MDA) levels in the rats liver exposed to CCl4. The histopathological study of liver confirmed the hepatoprotective effects of honey which is seen by a reduction in liver lesions (59). Cheng et al., 2015 (60) have investigated the impact of buckwheat honey on CCl4-induced liver injury in mice. Administration of buckwheat (0.22 g/10 g, PO) decreased serum lipoprotein oxidation and elevated serum oxygen radical absorbance capacity. Additionally, buckwheat honey ameliorated the ALT and AST activities that are induced by CCl4. The hepatic levels of MDA were reduced and the antioxidant enzymes (SOD and GST) activities elevated following honey administration. The findings indicated that the hepatoprotective effects of buckwheat honey might be related to its antioxidant activity (60). Sadek et al., 2016 (61) also reported that honey inhibited CCl4-induced liver injury via increasing phosphorylase activity in liver and decreasing carbohydrate intolerance and insulin resistance index (HOMA-IR). Moreover, the modulatory effects of honey on cytokine genes such as TNF-α and transducing growth factor beta (TGF-β) have been involved in the hepatoprotective effects of honey (61).
Acetaminophen
Acetaminophen (paracetamol or N-acetyl-p-aminophenol; APAP) belongs to analgesic drugs that its overdose is accompanied with hepatotoxicity. The hepatotoxicity of acetaminophen is initially associated with over-production of N-acetyl-p-benzoquinone imine (NAPQI) by cytochrome p450. NAPQI is eliminated by binding to GSH following exposure to therapeutic doses of acetaminophen. Overdosing of APAP decreases the cellular GSH content, permitting NAPQI to attach to the cellular proteins and cause lipid peroxidation (LPO), which can lead to hepatotoxicity. Acetaminophen increases the ALT, AST, ALP, GGT, total bilirubin and MDA serum levels as well as proinflammatory cytokines such as TNF-α and IL-1β levels in liver tissue. Acetaminophen overdose causes liver failure in experimental animals and humans. Pretreatment with honey inhibited acetaminophen-induced hepatotoxicity by modulating oxidative stress and inflammatory responses in rats liver. Honey (5, 10 and 20 g/kg, PO) inhibited liver injury as evidenced by a decrease in the AST and ALT activities serum as well as the Il-1β levels in the animals exposed to acetaminophen. Honey increased the GSH content and GPx activity as well as decreased MDA levels in liver. The histopathological study indicated that honey improved the liver lesions induced by acetaminophen (62). Afroz et al., 2014 (63) has shown that Sundarban honey from Bangladesh (5 g/kg, PO) inhibited acetaminophen-induced liver injury by modulating oxidative stress. A hepatic histopathological evaluation conducted by Galal et al., 2012 (62) and Gupta et al., 2016 (64) indicated the protective effects of honey against acetaminophen, noted by decreasing in congestion, necrosis, hemorrhage, distorted hepatic architecture and nuclear inclusion in the rat and mice models.
Bromobenzene
Bromobenzene is an industrial chemical that used for producing pesticides and dyes. This agent causes hepatotoxicity by converting into bromobenzene 3, 4-oxide, which produces oxidative stress and liver injury. Apis cerana honey (5, 10 and 20 g/kg, twice a day, PO) had protective effects against bromobenzene-induced hepatotoxicity in mice. Honey decreased MDA level and transforming growth factor β1 (TGF-β) expression and increased the SOD and GPx activities in the liver of mice exposed to bromobenzene (65).
Metanil-yellow
Metanil yellow is one of the mono azo dyes with high water solubility. The use of metanil yellow as a colorant agent is banded, However, in developing countries, it is still used as a colorant in several food industries such as ice-creams, sweet meat, beverages, and soft drinks. Metanil yellow causes hepatotoxicity in animal models as evidenced by an increase in the enzymes levels liver including ALT, AST, ALP and gamma-glutamyl transpeptidase (GGT). It also induces the NF-κB signaling pathways and increases the inflammatory mediators expression such as TNF- α and IL-1 β in liver. Honey (2.5 mg/kg, PO) suppressed metanil yellow-induced the NF-κB expression and TNF- α and IL-1 β levels in rats liver. The study suggested that honey has hepatoprotective activity against metanil-yellow-induced liver injuries due to its anti-inflammatory properties (66) (Table 1).
Table 1.
Hepatoprotective effects of honey and chrysin against chemical or natural toxic agents
| Results | Constituents | In vitro/In vivo | Toxic agents | References |
| Decreased the serum levels of liver enzymes | Honey | Rabbit | Pb | [40] |
| Decreased the serum levels of liver enzymes, total bilirubin, and lipid peroxidation | Honey | Mice | Al | [56] |
| Decreased the serum levels of liver enzymes | Honey | Sheep | CC14 | [57] |
| Decreased the serum levels of ALT and AST. | Honey | Rat | [38] | |
| Decreased the elevated serum levels ALT, AST, ALP, and bilirubin Decreased the liver levels of MDA Increased liver weight, GSH content AND total protein t Improved cell necrosis, vacuolar degeneration, mononuclear cellular infiltration pyknotic nuclei and apoptotic bodies in the liver Increased the phosphorylase activity Decreased the carbohydrate intolerance and insulin resistance index (HOMA-IR) Modulated the expression of TNF-α and TGF-β. |
[58, 59, 61] | |||
| Decreased serum lipoprotein oxidation, MDA content Increased the activities of ALT , AST SOD and GST Decreased inflammatory cascade via Blockage of TNF-α-converting enzyme activity and TNF-α production | Honey Chrysin |
Mice | CCL4 | [60, 75] |
| Decreased the activities of serum AST, ALT, Il-1β and MDA content; Increased the GSH content and GPx activity; Improved the liver lesions Inhibited apoptosis and autophagy via decreasing the activity of caspase-3 and LC3B level. Decreased congestion, necrosis, hemorrhage, distorted hepatic architecture and nuclear inclusion |
Honey Chrysin |
Rat | Acetaminophen | [62, 63, 64] [68] |
| Decreased MDA level and expression of TGF-β Increased the activities of SOD and GPx | Honey | Rat | Bromobenzene | [65] |
| Decraesed NF-κ B expression, the liver levels of TNF-α and IL-1 β. | Honey | Rat | Metanil-yellow | [66] |
| Modulated the (ERK2)/Nrf2/ARE signaling pathways in hepatocytes. | Chrysin | Rat | tBHP | [70] |
| Decreased lipid peroxidation, XO activity; Increased GSH content, CAT, GR, SOD, GPx, GST, G6PDD and QR enzyme activities; Ameliorated expression of COX-2, iNOS, levels of NF-κB, and TNF-α. |
Chrysin | Rat | Cisplatin | [72] |
| Modulated the activities of ADH, XO and CYP 2E1 a Decreased the levels of TBARS, lipid hydroperoxides, and conjugated dienes Increased the activity of SOD, CAT, GPx, GR, GST, levels of GSH, vitamin C, and vitamin E. |
Chrysin | Rat | Ethanol | [73, 74] |
| Ameliorated hepatic enzyme activities, lipid peroxidation, activities of SOD, CAT, GPx, GR, GST and the levels of GSH, vitamin C, and vitamin E. | Chrysin | Rat | GalN | [76] |
| Modulated the oxidative stress. | Chrysin | Rat | STZ | [92] |
| Increasing hepatic glutathione GSH, GPx, GR, SOD, and CAT Decreased lipid peroxidation. | Chrysin | Rat | MTX | [67] |
Pb: Lead; SOD: superoxide dismutase; GST: glutathione-S-transferase; GSH-Px: glutathione peroxidase; GSH: glutathione; Al Aluminum; CAT: Catalase; IL-1β: interleukin-1β; TNF-α: tumor necrotic factor NF-κB: Nuclear factor-κB; STZ: Streptozotocin MTX: Methotrexate; CCl4: Carbon tetrachloride; tBHP: Tert-butyl hydroperoxide; GalN: D-galactosamine
6.2 Chrysin and hepatotoxic agents
Methotrexate
MTX causes hepatotoxicity via inducing oxidative stress. Chrysin (40 and 80 mg/kg, PO) showed a protective effect against MTX-induced hepatotoxicity via increasing hepatic glutathione GSH, GPx, GR, SOD, and CAT levels and decreasing lipid peroxidation in rats (67).
Acetaminophen
Chrysin (25 and 50 mg/kg, IP) ameliorated hepatotoxicity induced by acetaminophen in rats. Chrysin decreased lipid peroxidation which led to an elevation in the antioxidant enzymes activities. Chrysin modulated inflammatory responses via elevating the TNF-α and IL-1β levels. Chrysin inhibited apoptosis and autophagy through decreasing caspase-3 activity and LC3B level. Chrysin indicated a protective effect on acetaminophen-induced hepatotoxicity by reducing oxidative stress, inflammation, apoptotic and autophagic pathways (68).
Tert-butyl hydroperoxide
Tert-butyl hydroperoxide (tBHP) causes hepatotoxicity via increasing generation of cellular ROS (69). Chrysin (5, 10 and 25 μM) improved the hepatotoxicity of tBHP by modulating ERK2/Nrf2/ARE signaling pathways in rat primary hepatocytes (70).
Cisplatin
Cisplatin causes hepatotoxicity through inducing oxidative stress and inflammation (71). Chrysin (25 and 50 mg/kg, PO) can improve cisplatin-caused hepatotoxicity via inhibiting oxidative stress and inflammatory responses. Chrysin decreased lipid peroxidation, xanthine oxidase (XO) activities and increased GSH, CAT, GR, SOD, GPx, GSTlevels as well as glucose-6 phosphate dehydrogenase (G6PDD) and quinone reductase (QR) enzyme activities. Chrysin ameliorated the cyclooxygenase-2 (COX-2), iNOS expressions and NF-κB, and TNF-α levels in the liver of cisplatin-treated rats (72).
Ethanol
Excessive ethanol consumption damages to the various organs including the liver. Ethanol induces hepatotoxicity in three stages including fatty liver, alcoholic hepatitis, and cirrhosis-fibrous. Chrysin (20 and 40 mg/kg, IV) indicated the hepatoprotective effects during ethanol consumption via modulating ADH, XO, CYP 2E1 and CAT activities in rats (73). Chrysin (20 mg/kg) ameliorated ethanol-induced hepatotoxicity through decreasing the thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides levels, and conjugated dienes. Chrysin increased the SOD, CAT, GPx, GR, GST activities, and even the GSH, vitamin C, and vitamin E levels in rats liver (74).
Carbon tetrachloride
Chrysin (50mg/kg, PO) ameliorated CCl4-induced acute liver by blocking TNF-α-converting enzyme activity and TNF-α production that leads to suppressing the inflammatory cascade (75).
D-galactosamine
D-galactosamine (GalN) is a hepatotoxic agent increasing serum hepatic enzyme activities and lipid peroxidation level in the liver. Chrysin (20, 50 and 100 mg/kg, PO) ameliorated the hepatic enzyme activities, lipid peroxidation, the antioxidant enzymes (SOD, CAT, GPx, GR, GST) activities as well as GSH, vitamin C and vitamin E levels in liver (76).
Streptozotocin
STZ causes liver toxicity by inducing oxidative stress synthesized. Chrysin (20, 40 and 80, IP) showed the hepatoprotective effects against STZ via modulating oxidative stress (26) (Table 1).
7. Nephroprotective effects
7.1 Honey and nephrotoxic agents
Acetaminophen
The overdose of acetaminophen induces kidney toxicity by disturbing oxidase isoenzymes function in kidney. Acetaminophen increases creatinine, urea, BUN levels, inflammatory mediators, and oxidative stress indices in kidney tissues. Sundarban honey (5 g/kg, PO) from Bangladesh indicated nephroprotective effects on acetaminophen via decreasing BUN, creatinine, and MDA serum levels in rats. Histopathological evaluation in kidneys also confirmed protective effects of honey against acetaminophen. The protective effect of honey may be caused by binding to acetaminophen metabolites and reducing of their affnity to cellular GSH. Thus, honey increased the GSH level and excretion of acetaminophen metabolites (63).
Cisplatin
Cisplatin causes severe nephrotoxicity in humans via inducing inflammation and oxidative stress (77). Cisplatin-induced inflammation and oxidative stress in kidney through activation of NF-κB pathways. Oxidants including hydrogen peroxide superoxide and hydroxyl radicals activate the NF-κB pathways. The nephrotoxicity of cisplatin is determined by an increase in urea, creatinine and uric acid levels in serum. Cisplatin causes significant tubulointerstitial injuries, increases α-smooth muscle actin (α-SMA), fibrogenic factors, TGF-β1, and reduces the cell proliferation index, bromodeoxyuridine (Brdu). Pretreatment with honey (500 mg/kg, PO) decreased cisplatin-induced tubular epithelial cell necrosis, infiltration of the immune component into kidney and also inflammatory cytokine and adhesion molecule expression (TNFα, IL-6, ICAM-1, MCP-1) in kidney of cisplatin-treated animals. The findings indicated that cisplatin-enhanced the expression of NF-κB that has been decreased with honey. The study has suggested that honey inhibited cisplatin-induced nephrotoxicity via suppressing the NF-κB pathway (78). Orally honey administration (100 mg/kg) reversed the increased serum levels of urea, creatinine, and uric acid, and also ameliorated the histopathologic alteration. Honey showed a decrease in the α-SMA and TGF-β1 expressions and an increase in the expression of Brdu (79).
The consumption of honey (3 days before the onset of cisplatin administration) decreased cisplatin-induced nephrotoxicity in cancer patients as noted by creatinine and urea serum levels (80).
Carbon tetrachloride
Carbon tetrachloride (CCl4) is recognized as a potent nephrotoxic agent inducing acute and chronic renal damage. CCl4 increases creatinine, ALP, BUN, Uric acid and NO serum levels as an index of kidney damage. Oxidative stress has a main role in nephrotoxicity induced by CCl4.
Sider honey (5 g/kg, PO) ameliorated CCl4-induced nephrotoxicity in the rats as noted by a decrease in ALP, urea, uric acid, creatinine and NO serum levels and also in the MDA content in kidney. Honey increased kidney weight, GSH content while CCl4 decreased these parameters. Honey also improved the pathological damages such as cell necrosis, intertubular infiltration and cloudy swelling in kidney of rats exposed to CCl4 (58).
The protective effects of Saudi Sider honey (SSH) against CCl4 induced oxidative stress, and kidney injury have been investigated in rats. Administration of SSH (0.5 and 1.0 g/kg, PO) prior exposure to CCl4 decreased creatinine, urea, and uric acid serum levels. SSH increased total protein content and reduced MDA levels in kidney of rats exposed to CCl4. The histopathological study of kidney indicated that honey reversed glomeruli, interstitial tubules, and blood vessels to the normal condition (59) (Table 2).
Table 2.
Nephroprotective effects of honey and chrysin against chemical or natural toxic agents
| Results | Constituents | In vitro/In vivo | Toxic agents | References |
| Decreased the serum levels of BUN, Cr, MDA,TNF-α, IL-1β, and IL-33 Increased the serum levels of GSH, the activities of SOD, CAT, and GPx and excretion of acetaminophen metabolites. |
Honey Chrysin |
Rat | Acetaminophen | [63,81] |
| Increasing GSH level,; Decreased; Inhibited apoptotic tissue damage by increasing caspase-3 activity and autophagic tissue injury by elevating the expression of LC3B | ||||
| Decreased the expression of NFkB. | Honey | Mice | Cisplatin | [78] |
| Decreased the serum levels of urea, Cr and uric acid Inhibited oxidative stress | Honey Chrysin |
Rat | [79, 88] | |
| Ameliorated the histopathologic alteration Decreased the expression of α-SMA and TGF-β1 | Honey | Human | [80] | |
| Decreased the serum levels of ALP, urea, uric acid, Cr, NO Decreased the kidney levels of MDA Increased the kidney levels of GSH Improved cell necrosis, intertubular infiltration and cloudy swelling in the kidney |
Honey Chrysin |
Rat | CC14 | [59, 89] |
| Inhibited glomerular podocyte apoptosis via reduction of DNA fragmentation, decreased the Bax/Bcl-2 ratio, Apaf-1 activation and cytochrome c in renal podocytes; Ameliorated proteinuria and abnormal alterations in glomerular Protected podocyte injury via regulating ER stress. |
Chrysin | Mice | Glucose | [82] |
| Regulated the PPAR-γ and NF-κB signaling pathway | Chrysin | Rat | Adenine | [83] |
| Increased the serum levels of GSH, CAT, GPx, and SOD Decreased lipid peroxidation | Chrysin | Rat | TCDD | [85] |
| Increased antioxidant content in kidney tissue | Chrysin | Rat | DOX | [86] |
| Alleviated oxidative stress and apoptotic damage. | Chrysin | Rat | 5-FU | [87] |
| Decreased MDA, CYP 2E1, ADH, and XO Increased GSH, Gpx, CAT, and GR Modulated the ROS production | Chrysin | Rat | Ethanol | [73] |
SOD: superoxide dismutase; GST: glutathione-S-transferase; GSH-Px: glutathione peroxidase; GSH: glutathione; CAT: Catalase; IL-1β: interleukin-βp; TNF-α: tumor necrotic factor; NF-κB: Nuclear factor-κB; DOX: Doxorubicin; MTX: Methotrexate; CCl4: Carbon tetrachloride;TCDD: Tetrachlorodibenzo-p-dioxin; 5-FU: 5-Fluorouracil.
7.2 Chrysin and Nephrotoxic agents
Acetaminophen
Chrysin (25 and 50 mg/kg, PO) prevented nephrotoxicity in rats due to its antioxidant, anti-apoptotic and anti-inflammatory activities. Chrysin modulated acetaminophen-induced oxidative stress in the kidney by increasing the GSH level and activities of antioxidant enzymes (SOD, CAT, and GPx). Chrysin additionally decreased the levels of inflammatory markers including TNF-α, IL-1β, and IL-33. Furthermore, chrysin inhibited apoptotic tissue damage through increasing caspase-3 activity. Chrysin also ameliorated autophagic tissue injury by elevating the light chain 3B (LC3B) expression (81).
Glucose
Excess of glucose consumption causes diabetes and its complications including podocyte damage and dysfunction in human and animals. Glomerular epithelial podocytes play a leading role in modulating the glomerular filtration barrier function through their foot processes. Chrysin indicated the renoprotective effects against glucose in podocytes and mouse kidneys. Chrysin (1-20 μmol/L) inhibited glomerular podocyte apoptosis through reduction of DNA fragmentation. Chrysin decreased the Bax/Bcl-2 ratio, Apaf-1 activations and cytochrome c in renal podocytes exposed to high glucose. Chrysin (10 mg/kg, PO) ameliorated proteinuria and the abnormal alterations in glomerular ultrastructure. Chrysin showed the protective effects against glucose-induced podocyte injury by regulating ER stress (82).
Adenine
Adenine is an organic compound of purine family inducing nephrotoxicity in animal models (20). Adenine increases creatinine, urea, neutrophil gelatinase-associated lipocalin serum levels as well as N-Acetyl-β-d glucosaminidase activity, uremic toxin 3-indoxyl sulfate, inflammatory cytokines, and urinary albumin concentration. Adenine also increases the antioxidant content and decreases lipid peroxidation in kidney. Chrysin (10, 50 and 250 mg/kg, PO) indicated the protective effects against adenine-induced renal failure in rats via regulating peroxisome proliferator-activated receptor γ and the NF-κB signaling pathways (83).
Tetrachlorodibenzo-p-dioxin
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin that causes nephrotoxicity (84). Chrysin (2, 20 and 50 mg/kg, PO) prevented TCDD-induced nephrotoxicity through elevating the GSH, CAT, GPx, and SOD levels and reducing lipid peroxidation (85).
Doxorubicin
Doxorubicin is associated with nephrotoxicity. This drug causes nephrotoxicity via inducing oxidative stress. Chrysin (40 and 80 mg/kg, PO) treatment attenuated nephrotoxicity induced by doxorubicin through increasing antioxidant content in kidney tissues (86).
5-Fluorouracil
5-Fluorouracil (5-FU) is an antineoplastic drug that its clinical use is limited due to its toxic effects including renal toxicity. 5-Fluorouracil causes renal toxicity via inducing oxidative stress, activating p53, Bax, and caspase-3 and down-regulation of Bcl-2 expression. Chrysin (50 and 100 mg/kg, PO) showed the protective effects against 5-FU-induced renal toxicity via ameliorating oxidative stress and apoptotic damage in rat kidney (87).
Cisplatin
Renal toxicity is one of the main side effects of cisplatin that leads to limit its usage in treatment of various cancers. Cisplatin causes renal injury via inducing ROS generation. Chrysin ameliorated cisplatin-induced renal toxicity as noted by a decrease in serotonin and BUN, lipid peroxidation serum levels and XO activity which is accompanied by an increase in antioxidant enzyme (CAT, GPx, GR, and GST) and GSH levels. Chrysin showed the protective effects against cisplatin-induced renal injury by ameliorating oxidative stress (88).
Ethanol
Chronic ethanol consumption disturbs renal functions through inducing oxidative stress. Chrysin (20 and 40 mg/kg, IP) represented the protective effects against ethanol-induced renal toxicity. Chrysin administration decreased MDA, CYP 2E1, ADH, and XO levels in kidney. Chrysin also increased GSH, Gpx, CAT, and GR levels in kidney of ethanol-treated rats. Chrysin reduced renal toxicity by modulating ROS production (73).
Carbon tetrachloride
CCl4 disturbs renal function through increasing the iNOS expression and MDA level as well as decreasing GSH and CAT, SOD, and Gpx levels in kidney. Chrysin (200mg/kg, IP) inhibited CCL4-induced renal toxicity by inhibiting oxidative damage in rat kidney (89) (Table 2).
8. Reproductive system protection
8.1. Honey and reproductive toxic agents
Nicotine
Nicotine is one of the major components of tobacco and cigarette. Nicotine exposure disturbs the cellular oxidant-antioxidant balance and induces organ toxicities. The reproductive system is one of the targets for nicotine toxicity. Nicotine decreases percentage of sperm motility, viability and counts as well as follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone levels in serum. Honey administration (1g/kg, PO) improved sperm motility, viability, counts, morphology as well as FSH, LH, and testosterone levels in rats exposed to nicotine. Additionally, honey ameliorated histopathological changes including the degenerative seminiferous tubule architecture induced by nicotine (90).
Cigarette
Cigarette smoking causes sexual dysfunctions and decreases the fertility in males. Honey (1.2 g/kg, PO) markedly elevated the percentages of achieving intromission and ejaculation and raised mating and fertility markers in the male rats exposed to cigarette (91).
Conclusion
Recent years, natural products are considered as the new strategy in treatment of various diseases due to their main activities such as antioxidant. Chemical and natural toxic agents cause toxicity in human and animal specially following chronic exposure or high doses. These toxic agents lead to toxicity in the various organs such as nervous, respiratory, cardiovascular, gastrointestinal, renal and reproductive systems. The studies have indicated natural products may be effective against toxic agents in vitro and animal experiments. One of the natural products with more pharmacological protective effects is honey. Several studies have suggested that honey and its polyphenol such as chrysin decreased neurotoxicity, lung toxicity, cardiotoxicity, hepatotoxicity, nephrotoxicity, reproductive toxicity, genotoxicity and immunotoxicity via modulating oxidative stress, inflammation, and apoptosis in the various organs. The above findings confirmed in vitro and animal studies. Therefore, clinical trial studies should be done to confirm the efficacy and safety of honey and chrysin for treating intoxication in humans.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Sallak N, Sadighara P. Nutritional-qualitative factors changes during ripening of canned olives at different temperatures storage. Carpathian Journal of Food Science & Technology. 2015 Dec 1;7(4) [Google Scholar]
- 2.Dong F, Zhang J, Zhu S, Lan T, Yang J, Li L. Chrysin alleviates chronic hypoxia-induced pulmonary hypertension by reducing intracellular calcium concentration in pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2019;74(5):426–435. doi: 10.1097/FJC.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 3.Song HY, Sik Kim W, Kim JM, Bak DH, Moo Han J, Lim ST, Byun EB. A hydroxyethyl derivative of chrysin exhibits anti-inflammatory activity in dendritic cells and protective effects against dextran sodium salt-induced colitis in mice. Int Immunopharmacol. 2019;77:105958. doi: 10.1016/j.intimp.2019.105958. [DOI] [PubMed] [Google Scholar]
- 4.Braakhuis A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients. 2019;11(11):pii: E2705. doi: 10.3390/nu11112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Waili N, Salom K, Al-Ghamdi A, Ansari MJ. Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. The scientific world Journal. 2012;2012 doi: 10.1100/2012/930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grecka K, Kuś PM, Worobo RW, Szweda P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules. 2018 Jan 28;23(2):260. doi: 10.3390/molecules23020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam MR, Pervin T, Hossain H, Saha B, Hossain SJ. Physicochemical and Antioxidant Properties of Honeys from the Sundarbans Mangrove Forest of Bangladesh. Preventive nutrition and food science. 2017 Dec;22(4):335. doi: 10.3746/pnf.2017.22.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gašić UM, Milojković-Opsenica DM, Tešić ŽL. Polyphenols as Possible Markers of Botanical Origin of Honey. Journal of AOAC International. 2017 Jul 1;100(4) doi: 10.5740/jaoacint.17-0144. [DOI] [PubMed] [Google Scholar]
- 9.Winter GF. Medical-Grade Honey Dressing Use in Developing Countries. Advances in skin & wound care. 2017 Nov 1;30(11):1–3. doi: 10.1097/01.ASW.0000526158.36473.dd. [DOI] [PubMed] [Google Scholar]
- 10.Lavaf M, Simbar M, Mojab F, Majd HA, Samimi M. Comparison of honey and phenytoin (PHT) cream effects on intensity of pain and episiotomy wound healing in nulliparous women. Journal of Complementary and Integrative Medicine. 2017 Oct 5;15(1) doi: 10.1515/jcim-2016-0139. [DOI] [PubMed] [Google Scholar]
- 11.Song HY, Kim HM, Mushtaq S, Kim WS, Kim YJ, Lim ST, Byun EB. Gamma-Irradiated chrysin Improves anticancer activity in HT-29 colon cancer cells through mitochondria-related pathway. J Med Food. 2019;22(7):713–721. doi: 10.1089/jmf.2018.4320. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadian F, Pilehvar-Soltanahmadi Y, Alipour S, Dadashpour M, Zarghami N. Chrysin alters microRNAs expression levels in gastric cancer cells: Possible molecular mechanism. Drug Res (Stuttg) 2017;67(9):509–514. doi: 10.1055/s-0042-119647. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Q, Wen M, Xu C, Chen A, Qiu YB, Cao JG, Zhang JS. Song ZW8-bromo-7-methoxychrysin targets NF-κB and FoxM1 to inhibit lung cancer stem cells induced by proinflammatory factors. J Cancer. 2019;10(21):5244–5255. doi: 10.7150/jca.30143. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.van Meeuwen JA, Korthagen N, de Jong PC, Piersma AH, van den Berg M. (Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicol Appl Pharmacol. 2007;221(3):372–83. doi: 10.1016/j.taap.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Abdulmajeed WI, Sulieman HB, Zubayr MO, Imam A, Amin A, Biliaminu SA, et al. Honey prevents neurobehavioural deficit and oxidative stress induced by lead acetate exposure in male wistar rats-a preliminary study. Metabolic brain disease. 2016 Feb 1;31(1):37–44. doi: 10.1007/s11011-015-9733-6. [DOI] [PubMed] [Google Scholar]
- 16.Shati AA, Elsaid FG, Hafez EE. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience. 2011 Feb 17;175:66–74. doi: 10.1016/j.neuroscience.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Liu M, Bing G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s disease 2011. 2011 doi: 10.4061/2011/327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007 Apr 1;55(5):453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candiracci M, Piatti E, Dominguez-Barragán M, García-Antrás D, Morgado B, Ruano D, et al. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. Journal of agricultural and food chemistry. 2012 Dec 5;60(50):12304–11. doi: 10.1021/jf302468h. [DOI] [PubMed] [Google Scholar]
- 20.Ali BH, Inuwa I, Al Za’abi M, Al Bahlani S, Al Issaei H, Ramkumar A, et al. Renal and myocardial histopathology and morphometry in rats with adenine-induced chronic renal failure: influence of gum acacia. Cellular Physiology and Biochemistry. 2014;34(3):818–28. doi: 10.1159/000363045. [DOI] [PubMed] [Google Scholar]
- 21.Farkhondeh T, Samarghandian S, Azimin-Nezhad M, Samini F. Effect of chrysin on nociception in formalin test and serum levels of noradrenalin and corticosterone in rats. International journal of clinical and experimental medicine. 2015;8(2):2465. [PMC free article] [PubMed] [Google Scholar]
- 22.Rauf A, Khan R, Raza M, Khan H, Pervez S, De Feo V, et al. Suppression of inflammatory response by chrysin, a flavone isolated from Potentilla evestita Th. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia. 2015 Jun 1;103:129–35. doi: 10.1016/j.fitote.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Ha SK, Moon E, Kim SY. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neuroscience letters. 2010 Nov 26;485(3):143–7. doi: 10.1016/j.neulet.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 24.Lee BK, Lee WJ, Jung YS. Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-κB Signaling. International journal of molecular sciences. 2017 Jul 3;18(7):1424. doi: 10.3390/ijms18071424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Zang A, Zhang L, Zhang H, Zhao L, Qi Z, et al. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurological Sciences. 2014 Oct 1;35(10):1527–32. doi: 10.1007/s10072-014-1784-7. [DOI] [PubMed] [Google Scholar]
- 26.Samarghandian S, Azimi-Nezhad M, Samini F, Farkhondeh T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Canadian journal of physiology and pharmacology. 2015 May 13;94(4):388–93. doi: 10.1139/cjpp-2014-0412. [DOI] [PubMed] [Google Scholar]
- 27.Bortolotto VC, Pinheiro FC, Araujo SM, Poetini MR, Bertolazi BS, de Paula MT, et al. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. European journal of pharmacology. 2018 Mar 5;822:78–84. doi: 10.1016/j.ejphar.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Goes AT, Jesse CR, Antunes MS, Ladd FV, Ladd AA, Luchese C, et al. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chemico-biological interactions. 2018 Jan 5;279:111–20. doi: 10.1016/j.cbi.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Mani R, Natesan V, Arumugam R. Neuroprotective effect of chrysin on hyperammonemia mediated neuroinflammatory responses and altered expression of astrocytic protein in the hippocampus. Biomedicine & Pharmacotherapy. 2017 Apr 1;88:762–9. doi: 10.1016/j.biopha.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Kumari A, Gulati A, Krishnamurthy S, Hemalatha S. Chrysin isolated from Pyrus pashia fruit ameliorates convulsions in experimental animals. Nutritional neuroscience. 2017 Dec;29:1–9. doi: 10.1080/1028415X.2017.1418786. [DOI] [PubMed] [Google Scholar]
- 31.Kamaruzaman NA, Sulaiman SA, Kaur G, Yahaya B. Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC complementary and alternative medicine. 2014 Dec;14(1):176. doi: 10.1186/1472-6882-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamshuddin NS, Zohdi RM. Gelam honey attenuates ovalbumin-induced airway inflammation in a mice model of allergic asthma. Journal of traditional and complementary medicine. 2016 Nov 4 doi: 10.1016/j.jtcme.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Q, Gu X, Cai J, Huang M, Su M. Chrysin attenuates allergic airway inflammation by modulating the transcription factors T-bet and GATA-3 in mice. Molecular medicine reports. 2012 Jul 1;6(1):100–4. doi: 10.3892/mmr.2012.893. [DOI] [PubMed] [Google Scholar]
- 34.Wadibhasme PG, Ghaisas MM, Thakurdesai PA. Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharmaceutical biology. 2011 May 1;49(5):508–15. doi: 10.3109/13880209.2010.521754. [DOI] [PubMed] [Google Scholar]
- 35.Nikbakht J, Hemmati AA, Arzi A, Mansouri MT, Rezaie A, Ghafourian M. Protective effect of gallic acid against bleomycin-induced pulmonary fibrosis in rats. Pharmacological Reports. 2015 Dec 1;67(6):1061–7. doi: 10.1016/j.pharep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Kilic T, Ciftci O, Cetin A, Kahraman H. Preventive effect of chrysin on bleomycin-induced lung fibrosis in rats. Inflammation. 2014 Dec 1;37(6):2116–24. doi: 10.1007/s10753-014-9946-6. [DOI] [PubMed] [Google Scholar]
- 37.Shen Y, Tian P, Li D, Wu Y, Wan C, Yang T, et al. Chrysin suppresses cigarette smoke-induced airway inflammation in mice. International journal of clinical and experimental medicine. 2015;8(2):2001. [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Waili NS, Saloom KY, Al-Waili TN, Al-Waili AN, Akmal M, Al-Waili FS, et al. Influence of various diet regimens on deterioration of hepatic function and hematological parameters following carbon tetrachloride: a potential protective role of natural honey. Natural product Research. 2006 Nov 1;20(13):1258–64. doi: 10.1080/14786410600906475. [DOI] [PubMed] [Google Scholar]
- 39.Abdelaziz I, Elhabiby MI, Ashour AA. Toxicity of cadmium and protective effect of bee honey, vitamins C and B complex. Human & experimental toxicology. 2013 Apr;32(4):362–70. doi: 10.1177/0960327111429136. [DOI] [PubMed] [Google Scholar]
- 40.Fihri AF, Al-Waili NS, El-Haskoury R, Bakour M, Amarti A, Ansari MJ, et al. Protective effect of morocco carob honey against lead-induced anemia and hepato-renal toxicity. Cellular Physiology and Biochemistry. 2016;39(1):115–22. doi: 10.1159/000445610. [DOI] [PubMed] [Google Scholar]
- 41.Makedou K, Iliadis S, Kara E, Gogou M, Feslikidis T, Papageorgiou G. Honey and its protective role against oxidation of human low density lipoproteins and total serum lipoproteins. Hippokratia. 2012 Jul;16(3):287. [PMC free article] [PubMed] [Google Scholar]
- 42.Ghazali WS, Romli AC, Mohamed M. Effects of honey supplementation on inflammatory markers among chronic smokers: a randomized controlled trial. BMC complementary and alternative medicine. 2017 Dec;17(1)):175. doi: 10.1186/s12906-017-1703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sniecinski RM, Wright S, Levy JH. Chapter 3 - Cardiovascular Pharmacology, Cardiothoracic Critical Care, Butterworth-Heinemann, Philadelphia. 2007:33–52. [Google Scholar]
- 44.Shukla AC. Steven JM. McGowan Jr FX. CHAPTER 16 - Cardiac Physiology and Pharmacology A2 - Coté, Charles J, in: J. Lerman, I.D. Todres (Eds.), A Practice of Anesthesia for Infants and Children (Fourth Edition), W.B. Saunders, Philadelphia. 2009:361–395. [Google Scholar]
- 45.Papadakos PJ, Koh Y. CHAPTER 38 - Respiratory Pharmacology and Aerosol Therapy, Mechanical Ventilation, W.B. Saunders, Philadelphia. 2008:428–442. [Google Scholar]
- 46.Afroz R, Tanvir EM, Karim N, Hossain M, Alam N, Gan SH, Khalil M. Sundarban honey confers protection against isoproterenol-induced myocardial infarction in Wistar rats. BioMed research International 2016. 2016 doi: 10.1155/2016/6437641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalil M, Tanvir EM, Afroz R, Sulaiman SA, Gan SH. Cardioprotective effects of tualang honey: amelioration of cholesterol and cardiac enzymes levels. BioMed research International 2015. 2015 doi: 10.1155/2015/286051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doğan A, Kolankaya D. Protective effect of Anzer honey against ethanol-induced increased vascular permeability in the rat stomach. Experimental and Toxicologic Pathology. 2005 Nov 15;57(2):173–8. doi: 10.1016/j.etp.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Kassim M, Mansor M, Al-Abd N, Yusoff KM. Gelam honey has a protective effect against lipopolysaccharide (LPS)-induced organ failure. International journal of molecular sciences. 2012 May 23;13(5):6370–81. doi: 10.3390/ijms13056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samat S, Kanyan Enchang F, Nor Hussein F, Ismail W, Iryani W. Four-Week Consumption of Malaysian Honey Reduces Excess Weight Gain and Improves Obesity-Related Parameters in High Fat Diet Induced Obese Rats. Evidence-Based Complementary and Alternative Medicine 2017. 2017 doi: 10.1155/2017/1342150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anandhi R, Annadurai T, Anitha TS, Muralidharan AR, Najmunnisha K, Nachiappan V, et al. Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in Triton WR-1339-induced hypercholesterolemic rats. Journal of physiology and biochemistry. 2013 Jun 1;69(2):313–23. doi: 10.1007/s13105-012-0215-6. [DOI] [PubMed] [Google Scholar]
- 52.Pecoraro M, Del Pizzo M, Marzocco S, Sorrentino R, Ciccarelli M, Iaccarino G, et al. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicology and applied pharmacology. 2016 Feb 15;293:44–52. doi: 10.1016/j.taap.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. European journal of pharmacology. 2014 Apr 5;728:107–18. doi: 10.1016/j.ejphar.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 54.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents. Drug safety. 2000 Apr 1;22(4):263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 55.Anghel N, Cotoraci C, Ivan A, Suciu M, Herman H, Balta C, et al. Chrysin attenuates cardiomyocyte apoptosis and loss of intermediate filaments in a mouse model of mitoxantrone cardiotoxicity. Histology and histopathology. 2015 Dec;30(12):1465–75. doi: 10.14670/HH-11-641. [DOI] [PubMed] [Google Scholar]
- 56.Shati AA, Alamri SA. Role of saffron (Crocus sativus L.) and honey syrup on aluminum-induced hepatotoxicity. Saudi medical Journal. 2010;31(10):1106–13. [PubMed] [Google Scholar]
- 57.Al-Waili NS. Intravenous and intrapulmonary administration of honey solution to healthy sheep: effects on blood sugar, renal and liver function tests, bone marrow function, lipid profile, and carbon tetrachloride-induced liver injury. Journal of medicinal food. 2003 Oct 1;6(3):231–47. doi: 10.1089/10966200360716652. [DOI] [PubMed] [Google Scholar]
- 58.El Denshary ES, Al-Gahazali MA, Mannaa FA, Salem HA, Hassan NS, Abdel-Wahhab MA. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. Experimental and Toxicologic Pathology. 2012 Nov 1;64(7-8):753–60. doi: 10.1016/j.etp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Al-Yahya M, Mothana R, Al-Said M, Al-Dosari M, Al-Musayeib N, Al-Sohaibani M, et al. Attenuation of CCl4-induced oxidative stress and hepatonephrotoxicity by Saudi Sidr honey in rats. Evidence-Based Complementary and Alternative Medicine 2013. 2013 doi: 10.1155/2013/569037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng N, Wu L, Zheng J, Cao W. Buckwheat honey attenuates carbon tetrachloride-induced liver and DNA damage in mice. Evidence-Based Complementary and Alternative Medicine 2015. 2015 doi: 10.1155/2015/987385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadek K, Beltagy D, Saleh E, Abouelkhair R. Camel milk and bee honey regulate profibrotic cytokine gene transcripts in liver cirrhosis induced by carbon tetrachloride. Canadian journal of physiology and pharmacology. 2016 May 30;94(11):1141–50. doi: 10.1139/cjpp-2015-0596. [DOI] [PubMed] [Google Scholar]
- 62.ZakiPHD HF. Potential protective effect of honey against paracetamol-induced hepatotoxicity. Archives Iranian Med. 2012 Nov;15:674–80. [PubMed] [Google Scholar]
- 63.Afroz R, Tanvir EM, Hossain M, Gan SH, Parvez M, Islam A, Khalil M. Protective effect of Sundarban honey against acetaminophen-induced acute hepatonephrotoxicity in rats. Evidence-Based Complementary and Alternative Medicine 2014. 2014 doi: 10.1155/2014/143782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta P, Tripathi A, Agrawal T, Narayan C, Singh BM, Kumar M, et al. Synergistic protective effect of picrorhiza with honey in acetaminophen induced hepatic injury. [PubMed] [Google Scholar]
- 65.Zhao H, Cheng N, He L, Peng G, Liu Q, Ma T, et al. Hepatoprotective Effects of the Honey of Apis cerana Fabricius on Bromobenzene-Induced Liver Damage in Mice. Journal of food science. 2018 Feb;83(2):509–16. doi: 10.1111/1750-3841.14021. [DOI] [PubMed] [Google Scholar]
- 66.Al-Malki AL, Sayed AA. Bees’ honey attenuation of metanil-yellow-induced hepatotoxicity in rats. Evidence-Based Complementary and Alternative Medicine 2013. 2013 doi: 10.1155/2013/614580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Ali N, Rashid S, Nafees S, Hasan SK, Sultana S. Beneficial effects of Chrysin against Methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Molecular and cellular biochemistry. 2014 Jan 1;385(1-2):215–23. doi: 10.1007/s11010-013-1830-4. [DOI] [PubMed] [Google Scholar]
- 68.Eldutar E, Kandemir FM, Kucukler S, Caglayan C. Restorative effects of Chrysin pretreatment on oxidant–antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: An experimental and biochemical study. Journal of biochemical and molecular toxicology. 2017 Nov;31(11):e21960. doi: 10.1002/jbt.21960. [DOI] [PubMed] [Google Scholar]
- 69.Kim EY, Hong KB, Suh HJ, Choi HS. Protective effects of germinated and fermented soybean extract against tert-butyl hydroperoxide-induced hepatotoxicity in HepG2 cells and in rats. Food & function. 2015;6(11):3512–21. doi: 10.1039/c5fo00785b. [DOI] [PubMed] [Google Scholar]
- 70.Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, et al. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Archives of toxicology. 2013 Jan 1;87(1):167–78. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- 71.Ateyya H, Yosef H, Nader MA. Ameliorative effect of trimetazidine on cisplatin-induced hepatotoxicity in rats. Canadian journal of physiology and pharmacology. 2015 Aug 19;94(2):225–30. doi: 10.1139/cjpp-2015-0304. [DOI] [PubMed] [Google Scholar]
- 72.Rehman MU, Ali N, Rashid S, Jain T, Nafees S, Tahir M, et al. Alleviation of hepatic injury by chrysin in cisplatin administered rats: probable role of oxidative and inflammatory markers. Pharmacological Reports. 2014 Dec 1;66(6):1050–9. doi: 10.1016/j.pharep.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Tahir M, Sultana S. Chrysin modulates ethanol metabolism in Wistar rats: a promising role against organ toxicities. Alcohol and alcoholism. 2011 Apr 29;46(4):383–92. doi: 10.1093/alcalc/agr038. [DOI] [PubMed] [Google Scholar]
- 74.Sathiavelu J, Senapathy GJ, Devaraj R, Namasivayam N. Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-induced toxicity in female albino rats. Journal of Pharmacy and Pharmacology. 2009 Jun;61(6):809–17. doi: 10.1211/jpp/61.06.0015. [DOI] [PubMed] [Google Scholar]
- 75.Hermenean A, Mariasiu T, Navarro-González I, Vegara-Meseguer J, Miuțescu E, Chakraborty S, et al. Hepatoprotective activity of chrysin is mediated through TNF-α in chemically-induced acute liver damage: An in vivo study and molecular modeling. Experimental and therapeutic medicine. 2017 May 1;13(5):1671–80. doi: 10.3892/etm.2017.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pushpavalli G, Kalaiarasi P, Veeramani C, Pugalendi KV. Effect of chrysin on hepatoprotective and antioxidant status in D-galactosamine-induced hepatitis in rats. European journal of pharmacology. 2010 Apr 10;631(1-3):36–41. doi: 10.1016/j.ejphar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 77.Khan MA, Gupta A, Kumar S, Ahmad S, Sastry JL. Hepatoprotective activity of a new polyherbal formulation against paracetamol and D-galactosamine induced hepatic toxicity. Journal of pharmacy & bioallied sciences. 2015 Oct;7(4):246. doi: 10.4103/0975-7406.168018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamad R, Jayakumar C, Ranganathan P, Mohamed R, El-Hamamy MM, Dessouki AA, et al. Honey feeding protects kidney against cisplatin nephrotoxicity through suppression of inflammation. Clinical and Experimental Pharmacology and Physiology. 2015 Aug;42(8):843–88. doi: 10.1111/1440-1681.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ibrahim A, Eldaim MA, Abdel-Daim MM. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology. 2016 Aug 1;68(4):1039–48. doi: 10.1007/s10616-015-9860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osama H, Abdullah A, Gamal B, Emad D, Sayed D, Hussein E, et al. Effect of Honey and Royal Jelly against Cisplatin-Induced Nephrotoxicity in Patients with Cancer. Journal of the American College of Nutrition. 2017 Jul 4;36(5):342–6. doi: 10.1080/07315724.2017.1292157. [DOI] [PubMed] [Google Scholar]
- 81.Kandemir FM, Kucukler S, Eldutar E, Caglayan C, Gülçin I. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: A Multi-biomarker approach. Scientia pharmaceutica. 2017 Jan 26;85(1):4. doi: 10.3390/scipharm85010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang MK, Park SH, Kim YH, Lee EJ, Antika LD, Kim DY, et al. Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Acta Pharmacologica Sinica. 2017 Aug;38(8):1129. doi: 10.1038/aps.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali BH, Adham SA, Al Za’abi M, Waly MI, Yasin J, Nemmar A, et al. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS One. 2015 Apr 24;10(4):0125285. doi: 10.1371/journal.pone.0125285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu CF, Wang YM, Peng SQ, Zou LB, Tan DH, Liu G, et al. Combined effects of repeated administration of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyls on kidneys of male rats. Archives of environmental contamination and toxicology. 2009 Nov 1;57(4):767–76. doi: 10.1007/s00244-009-9323-x. [DOI] [PubMed] [Google Scholar]
- 85.Ciftci O, Ozdemir I, Vardi N, Beytur A, Oguz F. Ameliorating effects of quercetin and chrysin on 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced nephrotoxicity in rats. Toxicology and industrial health. 2012 Nov;28(10):947–54. doi: 10.1177/0748233711430978. [DOI] [PubMed] [Google Scholar]
- 86.Rashid S, Ali N, Nafees S, Ahmad ST, Arjumand W, Hasan SK, et al. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicology mechanisms and methods. 2013 Jun 1;23(5):337–45. doi: 10.3109/15376516.2012.759306. [DOI] [PubMed] [Google Scholar]
- 87.Rashid S, Ali N, Nafees S, Hasan SK, Sultana S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food and Chemical Toxicology. 2014 Apr 1;66:185–93. doi: 10.1016/j.fct.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 88.Sultana S, Verma K, Khan R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. Journal of Pharmacy and Pharmacology. 2012 Jun;64(6):872–81. doi: 10.1111/j.2042-7158.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 89.Anand KV, Anandhi R, Pakkiyaraj M, Geraldine P. Protective effect of chrysin on carbon tetrachloride (CCl4)—induced tissue injury in male Wistar rats. Toxicology and industrial health. 2011 Nov;27(10):923–33. doi: 10.1177/0748233711399324. [DOI] [PubMed] [Google Scholar]
- 90.Kolawole TA, Oyeyemi WA, Adigwe C, Leko B, Udeh C, Dapper DV. Honey Attenuates the Detrimental Effects of Nicotine on Testicular Functions in Nicotine Treated Wistar Rats. Nigerian Journal of Physiological Sciences. 2015;30(1-2):10–6. [PubMed] [Google Scholar]
- 91.Mohamed M, Sulaiman SA, Sirajudeen KN. Protective effect of honey against cigarette smoke induced-impaired sexual behavior and fertility of male rats. Toxicology and industrial health. 2013 Apr;29(3):264–71. doi: 10.1177/0748233711432568. [DOI] [PubMed] [Google Scholar]
- 92.Samarghandian S, Azimi-Nezhad M, Samini F, Farkhondeh T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Canadian journal of physiology and pharmacology. 2015 May 13;94(4):388–93. doi: 10.1139/cjpp-2014-0412. [DOI] [PubMed] [Google Scholar]


