Abstract
Breast cancer is one of the most common malignancies in women. Despite advances in treatment of endocrine-dependent tumors, the complete molecular basis of transformation is still unknown. What is clear is that a variety of genetic lesions and epigenetic modifications are present in the neoplasm. Disregulation of several signaling pathways is known to be associated with breast cancer development, among them is the wingless and integration site growth factor (Wnt) pathway. While genetic mutations of certain components of this pathway, such as APC, are significant contributing factors for colorectal cancers, they are typically not the predominate mechanism associated with breast cancer. Instead, it appears that DNA hypermethylation leads to aberrant regulation of the Wnt pathway in breast cancer, and as such, this review focuses on the epigenetic regulation of Wnt pathway components in breast cancer.
Keywords: breast cancer, DNA methylation, epigenetics, Wnt signaling, β-catenin, cancer stem cell
Introduction
Breast cancer is the most common cancer among women and is second only to lung cancer in mortality. According to the World Health Organization, approximately 1.2 million people worldwide were diagnosed with breast cancer in 2007. The American Cancer Society estimates for women that 213,000 new cases and 41,000 deaths will be attributed to invasive breast cancer in the United States this year. The chance of developing invasive breast cancer during a woman’s lifetime is approximately 1 in 8. Although much less common, approximately 1,700 cases of male breast cancer occur each year.
A combination of genetic and epigenetic changes, which lead to disregulation of genes and pathways controlling cell growth, contribute to breast cancer development. The two most common epigenetic alterations involving DNA methylation and histone modification result in changes in gene expression patterns without mutations in the gene. Though reversible, these modifications are maintained after DNA synthesis, resulting in heritable structural changes (reviewed by Widschwendter and Jones1). At the molecular level, DNA methylation is the enzymatic addition of methyl groups to the 5-position of cytosines that are most often located within CpG islands found in the promoter region of genes. Promoter hypermethylation effectively represses the transcription and subsequent gene expression, and occurs in many genes involved in breast cancer.1 Methylation is mediated by three known DNA cytosine methyltransferases, DNMT1, 3α and 3β.1 DNMT1 is the most abundant and is responsible for maintenance of established methylation patterns from the parent strand to the daughter strand in DNA replication. DNMT 3α and 3β are involved in de novo methylation and are up regulated in aging cells.1,2 DNMT1 and DNMT3β show increased expression in breast cancer.3,4
The histone modifications of acetylation, methylation, phosphorylation, polyADP-ribosylation and ubiquitination direct chromatin into transcription repressive or permissive configurations.2 For example, histone acetylation [through histone acetyl transferase (HAT)] allows protein access to DNA and favors transcription, whereas deacetylation by histone deacetylase (HDAC) enzymes, compacts the DNA into a transcription-repressive chromatin complex.
This review focuses on the Wnt signaling pathway and it’s epigenetic regulation in breast cancer. Unlike colorectal cancers,5 evidence for genetic mutations of Wnt pathway components such as adenomatous polyposis coli (APC) being an early and necessary step in breast malignancies is rare.6–9 However, various lines of evidence suggest that this pathway is disregulated in breast cancer,10–12 presumably through epigenetic mechanisms.
Wnt Signaling Pathway
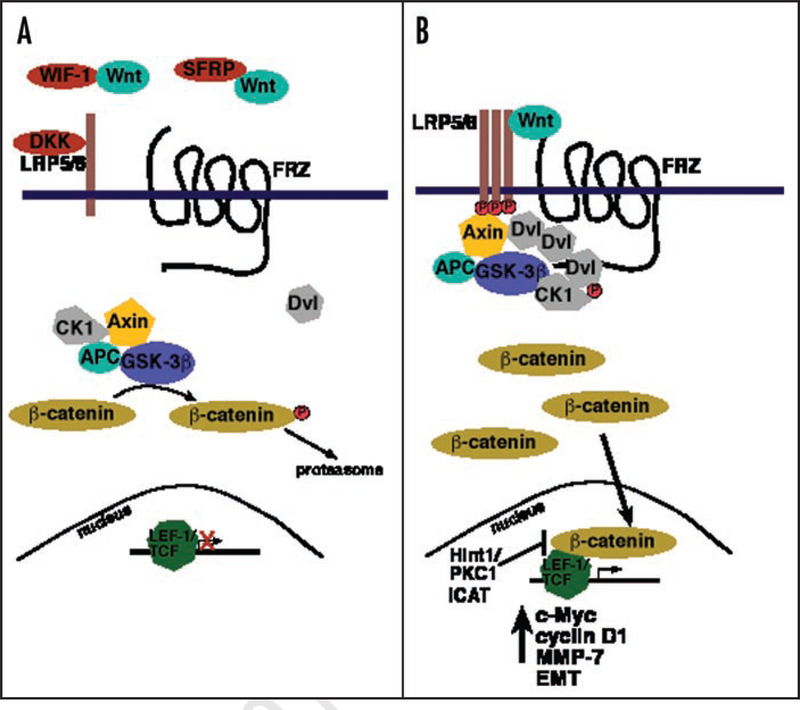
The Wnt signaling pathway is important in cell differentiation and proliferation, cell movement and polarity, and for maintenance of self-renewal in hematopoietic stem cells (reviewed in ref. 13), and defects in this pathway are implicated in the pathogenesis of several tumor types, including breast cancer (reviewed in refs. 14 and 15). Thus, evidence is mounting that the Wnt pathway is a significant contributor to tumorigenesis. Wnt signals are transduced through two different pathways, the canonical and the non-canonical,13 largely due to the fact that Wnt is a family of closely related, secreted glycoproteins with at least 19 members that can bind to different cell surface receptors or different combinations of receptors that ultimately determine which specific pathway is activated.16,17 This review will focus primarily on the canonical pathway in which secreted Wnt signals are transduced through the Frizzled (FRZ) family of transmembrane receptors and the low-density lipoprotein receptor-related protein (LRP5/6) correceptor to ultimately stimulate β-catenin, the critical transcription regulator of growth-promoting genes (Fig. 1). There are numerous positive and negative regulators of this pathway, and loss of negative regulation, which activates constitutive signaling and growth, is an important contributor to tumor development.12,18–20
Figure 1.
Simplified cartoon of the Wnt signaling pathway. (A) In the absence of Wnt or if Wnt cannot bind Frizzled (FRZ) receptor due to sequestration by Wnt inhibitory factor (WIF-1) or frizzled-related protein (SFRP), β-catenin is phosphorylated and degraded, and control over differentiation and proliferated is maintained by the cell. (B) Wnt binding to FRZ and low-density lipoprotein receptor-related protein (LRP) clusters assembled on a Dishevelled (Dvl) scaffold allows β-catenin to accumulate in the cytoplasm and then translocate to the nucleus where, in complex with transcription factors TCF/LEF-1, various genes for proliferation and epithelial-to-mesenchymal transition (EMT) are activated.
Wnt binding to Frizzled is followed by Dishevelled (Dvl) aggregates assembling a scaffold-like structure at the plasma membrane.21 This triggers formation of clusters of LRP5/6 that are then phosphorylated by casein kinase I (CKIγ). Subsequently Axin, in complex with glycogen synthase kinase 3β (GSK-3β) and the associated APC proteins, binds to complete the LRP signalosome.21 Thus the activity of GSK-3β is blocked and β-catenin accumulates in the cytoplasm.22 β-catenin is then able to translocate into the nucleus where it associates with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors that subsequently activate expression of a number of genes (in part by displacing Groucho and HDAC) including those involved in epithelial-to-mesenchymal transition and cell proliferation such as c-myc, c-jun, cyclin D1 and CD44 (Fig. 1). In the absence of Wnt binding, the protein ‘destruction complex’ of GSK-3β along with CKIα, APC and Axin, phosphorylates β-catenin, targeting it for proteolytic degradation (Fig. 1). However, Lee et al.,23 recently demonstrated that Dvl overexpression is itself enough to promote transcriptional activation of TCF/LEF-responsive genes, suggesting that if the concentration of Dvl is high enough to support self-association near the plasma membrane, LRP5/6 can assemble and form the LRP signalosome even in the absence of a Wnt ligand.
As part of the normal regulation of the Wnt pathway to maintain controlled growth, several secreted Wnt antagonists prevent Wnt from binding FRZ [such as Wnt inhibitory factor-1 (WIF1) and secreted frizzled-related proteins (SFRPs)], or to LRP5/6 [DICKKOPF proteins (DKKs)]. These inhibitors prevent the downstream signal transduction and thus help to prevent TCF/LEF-responsive gene expression (reviewed in refs. 24 and 25). The observation that β-catenin expression and autocrine Wnt signaling in breast cancer cells are responsive to exogenous DKK1 and SFRP1 supports the notion that loss of Wnt antagonist expression is an important mechanism in tumorigenesis.18
Regulation of Wnt Pathway Components through Methylation
Aberrant DNA methylation is common in human neoplasia, and hypermethylation of gene-promoter regions is the predominant mechanism leading to loss of gene expression.1,26 Hypermethylation of numerous genes has been documented in various tumor types. Recently, promoter methylation of genes involved in the Wnt-β-catenin pathway was observed in breast cancer (Table 1).12,27–30 This is not surprising since it is known that β-catenin is localized in the nucleus in breast cancer,11,31,32 and activating genetic mutations in Wnt pathway members are rare.
Table 1.
Known and potential methylation-regulated Wnt pathway genes in breast cancer
Wnt inhibitory factor-1 (WIF1, Fig. 1) is a conserved Wnt-binding protein that is downregulated in many cancer types, including prostate, breast, lung, melanoma, colorectal and bladder, and thus is a potential tumor suppressor.30 Ai et al., examined DNA methylation of the WIF1 promoter in both human breast cancer cell lines and in human breast tumors.27 In tumor tissue, methylation-specific PCR (MSP, reviewed in ref. 33) revealed that 16 of 24 tumors (67%) displayed WIF1 promoter hypermethylation. These results agree with those of Wissmann et al., who determined that WIF1 expression was downregulated in 63% (17/27) of invasive ductal carcinomas and in 50% (4/8) of non-ductal invasive breast carcinomas.30 Interestingly, gene knockout studies of DNMT1 and DNMT3β result in significant re-expression of the WIF1 whereas single disruption of either of these genes alone resulted in only minor re-expression of WIF1.27 The re-expression was correlated with a 90% decrease in WIF1 methylation. These results indicate that DNMT1 and DNMT3β work in a cooperative fashion to methylate the WIF1 promoter.27 In summary, these findings suggest that epigenetic silencing of the WIF1 gene is a common event and contributing factor in breast tumorigenesis.
Several frizzled-related proteins (SFRP1–5) antagonize Wnt signaling, and SFRP1 gene is silenced in a number of different cancers, including breast cancer.34 One study12 evaluated 43 tumor and matching normal DNA samples for loss of heterozygosity at the SFRP1 locus and found 11 tumors had allelic loss in chromosome 8p, a site frequently altered in several tumors types. No genetic changes were detected on the remaining gene copy, suggesting SFRP1 may be silenced through methylation. SFRP1 promoter methylation was detected in eight breast cancer cell lines via MSP. More importantly, 79 of 130 (61%) primary breast cancers evaluated also displayed SFRP1 promoter methylation. Kaplan-Meier analysis showed SFRP1 gene hypermethylation was associated with a shorter overall survival of patients with invasive breast cancer, suggesting SFRP1 methylation status may be of predictive value. Another study found similar results despite using fewer samples.35
Recently, Suzuki et al., examined the methylation status of SFRPs 1, 2 and 5 in multiple breast cancer cell lines and patient samples.28 Gene expression analysis using RT-PCR and MSP confirmed that SFPR1 is downregulated and was methylated in 64% of breast cancer cell lines, and they reported for the first time that SFRP2 is methylated in 100% of the cells lines while SFRP5 is methylated in 90% of the breast cancer cell lines examined. As expected, gene expression was restored after treatment with DNMT inhibitors. Analysis of 78 breast tumor samples revealed methylation frequencies of 40, 77 and 71% for SFRP1, 2 and 5, respectively.
These data demonstrate that loss of various antagonists of the Wnt ligand through promoter methylation leads to overactivation of the Wnt signaling pathway, promoting tumorigenesis in human mammary tissues.12 Additional evidence implicating the Wnt pathway comes from the observation that β-catenin (and other downstream targets such as cyclin D1 and LEF) are upregulated in over 40% of breast cancers.10,11 Other Wnt pathway components are also modified through DNA methylation. For example, several studies showed that the APC promoter is methylated in ~35–50% of breast cancer tumors and cell lines.7,9,29,36 and that APC expression is correlated with methylation status. Though APC mutations occur in less than 20% of primary tumor isolates,6,8 LOH is detected in ~25% of breast tumors.9,37,38 In addition, the APC promoter is also regulated by methylation in prostate cancer.39 Loss of APC favors β-catenin accumulation and TCF/LEF-induced transcription. Wnt-independent accumulation of β-catenin might also be promoted by methylation of CDHI, E-cadherin. Membrane-anchored E-cadherin binds β-catenin in the cytoplasm, and thus its downregulated expression permits cytoplasmic β-catenin accumulation.40 However, data from Sarrio et al., unexpectedly found no increase in β-catenin in lobular breast carcinomas containing methylation-silenced CDHI.9 Thus epigenetic silencing of E-cadherin in breast cancer may lead exclusively to other consequences such as epithelialto-mesenchymal transition and increased invasiveness.41
Other Wnt pathway negative regulators are also candidates for epigenetic gene silencing in cancer. The expression of DKK1, which blocks Wnt signals by targeting LRP5/6 for degradation, is silenced by methylation in colon cancer42 and is repressed in some breast cancer cell lines.28,43 One study found that c-Myc expression results in silencing of DKK1, presumably through transcriptional repression mediated by promoter binding by Myc as determined by ChIP assays.43 However, the authors have not ruled out the contribution of methylation-induced silencing of DKK1,43 and recently DKK1 was found to be methylated in 27% and 19% of breast cancer cell lines and patient samples, respectively.28 DKK3, which blocks Wnt7a but not LRP5/6, is downregulated through promoter methylation in acute lymphoblastic leukemia, multiple myeloma and lung cancer.44–46 Hint1/PKC1 (histidine triad nucleotide-binding protein 1/protein kinase C inhibitor) represses TCF-β-catenin transcription activity and, unless silenced, should block Wnt signal propagation.47 While the Hint1 promoter is methylated in colon and non-small cell lung cancers,48,49 no studies containing rigorous analysis of methylation-induced silencing of these genes in breast cancer have been published.
However, silencing some genes that negatively regulate growth-promoting pathways may be lethal. For example ICAT, or inhibitor of β-catenin and TCF, blocks the interaction of β-catenin and TCF and thus stops the Wnt signal transduction.50 Loss of ICAT expression leads to developmental defects of the kidney,51 and to date there are no published studies reporting epigenetic regulation of ICAT in cancer.
Cancer Stem Cells and Wnt
Recent evidence suggests that cancer stem cells (CSCs) mediate solid tumor initiation and progression (reviewed in ref. 52). The CSC constitutes only a minor subpopulation of cells within the tumor, but can self-renew and also give rise to differentiated tumor cells. In contrast the bulk of the tumor is composed of highly differentiated cells with limited proliferative potential. Wnt, together with Notch and Hedgehog pathways, are involved in stem cell self-renewal in hematopoietic, leukemia and some solid tumors (reviewed in ref. 53 and 54). The Wnt pathway is also upregulated in prostate tumor-initiating cells.55 While it is known that Wnt signaling is important for normal stem cell self-renewal and mammary gland development,56 it remains to be determined if and how Wnt signaling and its epigenetic regulation in the CSC contribute to breast carcinogenesis.
In summary, acquisition of breast and numerous other cancers are modulated in part through epigenetic silencing of genes that negatively regulate cell proliferation pathways. Thus, the processes of gene hypermethylation and histone hypoacetylation are therapeutically attractive targets because these non-genetic alterations are reversible.2 However, the inherent cytotoxicity of DNA methyltransferase inhibitors such as decitabine [5-aza-2’-deoxycytidine (5-aza-dC)] needs to be reduced if they are to have a prominent role in cancer therapy.57 Perhaps second-generation inhibitors, those that single-out and act directly and specifically on DNMTs and/or HDAC enzymes, or even drugs targeting cancer stem cells may prove to be successful in managing these diseases. In addition, Schlange et al.,58 demonstrated that interference with autocrine Wnt signaling decreases survival and proliferation of breast cancer cells. These data support the idea that targeted inhibition of Wnt pathways components may be an effective means to control breast cancer.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations:
- DNMT
DNA methyltransferase
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
- FRZ
Frizzled
- LRP
low density lipoprotein receptor-related protein
- CK1
casein kinase 1
- GSK-3β
glycogen synthase kinase 3β
- APC
adenomatous polyposis coli
- Dvl
Disheveled
- TCF/LEF
T-cell factor/lymphoid enhancer factor
- WIF1
Wnt inhibitory factor 1
- SFRPs
secreted frizzled-related proteins
- DKKs
DICKKOPF proteins
- MSP
methylation-specific PCR
- Hint1/PKC1
histidine triad-nucleotide binding protein/protein kinase C inhibitor
- ICAT
inhibitor of β-catenin
- CSC
cancer stem cell
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
References
- 1.Widschwendter M and Jones PA. DNA methylation and breast carcinogenesis. Oncogene 2002; 21:5462–82. [DOI] [PubMed] [Google Scholar]
- 2.Lu Q, Qiu X, Hu N, Wen H, Su Y and Richardson B. Epigenetics, disease, and therapeutic interventions. Ageing Research Reviews 2006; 5:449–67. [DOI] [PubMed] [Google Scholar]
- 3.Agoston AT, Argani P, Yegnasubramanian S, De Marzo AM, Ansari Lari MA, Hicks JL, Davidson NE and Nelson WG. Increased protein stability causes DNA methyltransferase 1 dysregulation in breast cancer. J Biol Chem 2005; 280:18302–10. [DOI] [PubMed] [Google Scholar]
- 4.Girault I, Tozlu S, Lidereau R and Bieche I. Expression analysis of DNA methyltransferases 1, 3A, and 3B in sporadic breast carcinomas. Clin Cancer Res 2003; 9:4415–22. [PubMed] [Google Scholar]
- 5.Jaiswal AS, Balusu R and Narayan S. Involvement of adenomatous polyposis coli in colorectal tumorigenesis. Front Biosci 2005; 10:1118–34. [DOI] [PubMed] [Google Scholar]
- 6.Furuuchi K, Tada M, Yamada H, Kataoka A, Furuuchi N, Hamada J, Takahashi M, Todo S and Moriuchi T. Somatic mutations of the APC gene in primary breast cancers. Am J Pathol 2000; 156:1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Z, Tamura G, Tsuchiya T, Sakata K, Kashiwaba M, Osakabe M and Motoyama T. Adenomatous polyposis coli (APC) gene promoter hypermethylation in primary breast cancers. Br J Cancer 2001; 85:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashiwaba M, Tamura G and Ishida M. Aberrations of the APC gene in primary breast carcinoma. J Cancer Res Clin Oncol 1994; 120:727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarrio D, Moreno Bueno G, Hardisson D, Sanchez Estevez C, Guo M, Herman JG, Gamallo C, Esteller M and Palacios J. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer 2003; 106:208–15. [DOI] [PubMed] [Google Scholar]
- 10.Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP and Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA 2006; 103:3799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG and Hung MC. Betacatenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 2000; 97:4262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veeck J, Niederacher D, An H, Klopocki E, Wiesmann F, Betz B, Galm O, Camara O, Durst M, Kristiansen G, Huszka C, Knuchel R and Dahl E. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene 2006; 25:3479–88. [DOI] [PubMed] [Google Scholar]
- 13.Mohinta S, Wu H, Chaurasia P and Watabe K. Wnt pathway and breast cancer. Front Biosci 2007; 12:4020–33. [DOI] [PubMed] [Google Scholar]
- 14.Aguilera O, Munoz A, Esteller M and Fraga MF. Epigenetic alterations of the Wnt/betacatenin pathway in human disease. Endocr Metab Immune Disord Drug Targets 2007; 7:13–21. [DOI] [PubMed] [Google Scholar]
- 15.Howe LR and Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther 2004; 3:36–41. [DOI] [PubMed] [Google Scholar]
- 16.Cadigan KM and Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 1997; 11:3286–305. [DOI] [PubMed] [Google Scholar]
- 17.Katoh M Regulation of WNT3 and WNT3A mRNAs in human cancer cell lines NT2, MCF-7 and MKN45. Int J Oncol 2002; 20:373–7. [PubMed] [Google Scholar]
- 18.Bafico A, Liu G, Goldin L, Harris V and Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell 2004; 6:497–506. [DOI] [PubMed] [Google Scholar]
- 19.Klopocki E, Kristiansen G, Wild PJ, Klaman I, Castanos Velez E, Singer G, Stohr R, Simon R, Sauter G, Leibiger H, Essers L, Weber B, Hermann K, Rosenthal A, Hartmann A and Dahl E. Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol 2004; 25:641–9. [PubMed] [Google Scholar]
- 20.Ugolini F, Adelaide J, Charafe Jauffret E, Nguyen C, Jacquemier J, Jordan B, Birnbaum D and Pebusque MJ. Differential expression assay of chromosome arm 8p genes identifies Frizzled-related (FRP1/FRZB) and Fibroblast Growth Factor Receptor 1 (FGFR1) as candidate breast cancer genes. Oncogene 1999; 18:1903–10. [DOI] [PubMed] [Google Scholar]
- 21.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M and Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007; 316:1619–22. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MD and Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 2006; 281:22429–33. [DOI] [PubMed] [Google Scholar]
- 23.Lee YN, Gao Y and Wang HY. Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, −2 and −3. Cell Signal 2008; 20:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano Y and Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003; 116:2627–34. [DOI] [PubMed] [Google Scholar]
- 25.Function Niehrs C. and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006; 25:7469–81. [DOI] [PubMed] [Google Scholar]
- 26.Robertson KD. DNA methylation and human disease. Nat Rev Genet 2005; 6:597–610. [DOI] [PubMed] [Google Scholar]
- 27.Ai L, Tao Q, Zhong S, Fields CR, Kim WJ, Lee MW, Cui Y, Brown KD and Robertson KD. Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis 2006; 27:1341–8. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Toyota M, Caraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, Mori M, Hirata K, Imai K, Shinomura Y, Baylin SB and Tokino T. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer 2008; 98:11447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virmani AK, Rathi A, Sathyanarayana UG, Padar A, Huang CX, Cunnigham HT, Farinas AJ, Milchgrub S, Euhus DM, Gilcrease M, Herman J, Minna JD and Gazdar AF. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter 1A in breast and lung carcinomas. Clin Cancer Res 2001; 7:1998–2004. [PubMed] [Google Scholar]
- 30.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, Knuechel R, Rosenthal A and Pilarsky C. WIF1, a component of the Wnt pathway, is downregulated in prostate, breast, lung, and bladder cancer. J Pathol 2003; 201:204–12. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki S, Ikeda S, Ishizaki Y, Kurihara T, Tokumoto N, Iseki M, Arihiro K, Kataoka T, Okajima M and Asahara T. Alterations and correlations of the components in the Wnt signaling pathway and its target genes in breast cancer. Oncol Rep 2005; 14:1437–43. [DOI] [PubMed] [Google Scholar]
- 32.Ryo A, Nakamura M, Wulf G, Liou YC and Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol 2001; 3:793–801. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal A, Murphy RF and Agrawal DK. DNA methylation in breast and colorectal cancers. Mod Pathol 2007; 20:711–21. [DOI] [PubMed] [Google Scholar]
- 34.Shulewitz M, Soloviev I, Wu T, Koeppen H, Polakis P and Sakanaka C. Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene 2006; 25:4361–9. [DOI] [PubMed] [Google Scholar]
- 35.Lo PK, Mehrotra J, D’Costa A, Fackler MJ, Garrett Mayer E, Argani P and Sukumar S. Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol Ther 2006; 5:281–6. [DOI] [PubMed] [Google Scholar]
- 36.Dulaimi E, Hillinck J, Ibanez de Caceres I, Al-Saleem T and Cairns P. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res 2004; 10:6189–93. [DOI] [PubMed] [Google Scholar]
- 37.Medeiros AC, Nagai MA, Neto MM and Brentani RR. Loss of heterozygosity affecting the APC and MCC genetic loci in patients with primary breast carcinomas. Cancer Epidemiol Biomarkers Prev 1994; 3:331–3. [PubMed] [Google Scholar]
- 38.Thompson AM, Morris RG, Wallace M, Wyllie AH, Steel CM and Carter DC. Allele loss from 5q21 (APC/MCC) and 18q21 (DCC) and DCC mRNA expression in breast cancer. Br J Cancer 1993; 68:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastian PJ, Ellinger J, Wellmann A, Wernert N, Heukamp LC, Muller SC and von Ruecker A. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin Cancer Res 2005; 11:4097–106. [DOI] [PubMed] [Google Scholar]
- 40.Yang SZ, Kohno N, Yokoyama A, Kondo K, Hamada H and Hiwada K. Decreased Ecadherin augments beta-catenin nuclear localization: studies in breast cancer cell lines. Int J Oncol 2001; 18:541–8. [PubMed] [Google Scholar]
- 41.Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ and Cleton Jansen AM. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer 2006; 94:661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, Garcia JM, Munoz A, Esteller M and Gonzalez Sancho JM. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 2006; 25:4116–21. [DOI] [PubMed] [Google Scholar]
- 43.Cowling VH, D’Cruz CM, Chodosh LA and Cole MD. c-Myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol Cell Biol 2007; 27:5135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chim CS, Pang R, Fung TK, Choi CL and Liang R. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 2007; 21:2527–36. [DOI] [PubMed] [Google Scholar]
- 45.Roman Gomez J, Cordeu L, Agirre X, Jimenez Velasco A, San Jose Eneriz E, Garate L, Calasanz MJ, Heiniger A, Torres A and Prosper F. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood 2007; 109:3462–9. [DOI] [PubMed] [Google Scholar]
- 46.Yue W, Sun Q, Dacic S, Landreneau RJ, Siegfried JM, Yu J and Zhang L. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis 2008; 29:84–92. [DOI] [PubMed] [Google Scholar]
- 47.Weiske J and Huber O. The histidine triad protein Hint1 interacts with Pontin and Reptin and inhibits TCF-beta-catenin-mediated transcription. J Cell Sci 2005; 118:3117–29. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Zhang Y, Li H, Xu Z, Santella RM and Weinstein IB. Hint1 inhibits growth and activator protein-1 activity in human colon cancer cells. Cancer Res 2007; 67:4700–8. [DOI] [PubMed] [Google Scholar]
- 49.Yuan BZ, Jefferson AM, Popescu NC and Reynolds SH. Aberrant gene expression in human non small cell lung carcinoma cells exposed to demethylating agent 5-aza-2’-deoxycytidine. Neoplasia 2004; 6:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H and Akiyama T. Inhibition of Wnt signaling by ICAT, a novel betacatenin-interacting protein. Genes Dev 2000; 14:1741–9. [PMC free article] [PubMed] [Google Scholar]
- 51.Hasegawa Y, Satoh K, Iizuka Kogo A, Shimomura A, Nomura R, Akiyama T and Senda T. Loss of ICAT gene function leads to arrest of ureteric bud branching and renal agenesis. Biochem Biophys Res Commun 2007; 362:988–94. [DOI] [PubMed] [Google Scholar]
- 52.Lobo NA, Shimono Y, Qian D and Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol 2007; 23:675–99. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, Dontu G and Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res 2005; 7:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reya T and Clevers H. Wnt signalling in stem cells and cancer. Nature 2005; 434:843–50. [DOI] [PubMed] [Google Scholar]
- 55.Patrawala L, Calhoun T, Schneider Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L and Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006; 25:1696–708. [DOI] [PubMed] [Google Scholar]
- 56.Liu BY, McDermott SP, Khwaja SS and Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci USA 2004; 101:4158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stresemann C, Brueckner B, Musch T, Stopper H and Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res 2006; 66:2794–800. [DOI] [PubMed] [Google Scholar]
- 58.Schlange T, Matsuda Y, Lienhard S, Huber A and Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res 2007; 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]