Abstract
Ghrelin is a gut hormone that signals to the hypothalamus to stimulate growth hormone release, increase food intake and promote fat deposition. The ghrelin receptor, aka Growth Hormone Secretagogue Receptor (GHS-R), is highly expressed in the brain, with highest expression in Agouti-Related Peptide (AgRP) neurons in the hypothalamus. Compelling evidence indicates that ghrelin serves as a survival hormone in maintaining blood glucose and body weight during nutritional deficiencies. Recent studies demonstrated that AgRP neurons are involved in metabolic and behavioral adaptation to energy deficit to improve survival. Here, we used a neuronal subtype-specific GHS-R knockout mouse (Agrp-Cre;Ghsrf/f) to investigate the role of GHS-R in hypothalamic AgRP neurons in metabolic and behavioral adaptation to hypocaloric restricted feeding. We subjected the mice to a restricted feeding regimen of 40% mild calorie restriction (CR), with 1/4 food allotment given in the beginning of light cycle and 3/4 given at the beginning of dark cycle, to mimic normal mouse intake pattern. The CR-fed Agrp-Cre;Ghsrf/f mice exhibited reductions in body weight, fat mass and blood glucose. Metabolic profiling of these CR-fed Agrp-Cre;Ghsrf/f mice showed a trend toward reduced basal metabolic rate, significantly reduced core body temperature, and decreased expression of thermogenic genes in brown adipose tissue (BAT); this suggests a metabolic reset to a lower threshold. Significantly increased physical activity, a trend toward increased food anticipatory behavior, and altered fuel preferences were also observed in these mice. In addition, these CR-fed Agrp-Cre;Ghsrf/f mice exhibited a decreased counter-regulatory response, showing impaired hepatic glucose production. Lastly, hypothalamic gene expression in Agrp-Cre;Ghsrf/f mice revealed increased AgRP expression and decreased expression of genes in β-oxidation pathways. In summary, our data suggest that GHS-R in AgRP neurons is a key component of the neuro-circuitry involved in metabolic adaptation to calorie restriction.
Keywords: ghrelin, AgRP neurons, calorie restriction, glucose homeostasis, hepatic glucose production
Introduction
Ghrelin is a 28-amino acid peptide hormone released primarily from the gut, originally characterized for its capacity to stimulate growth hormone secretion (1). Ghrelin signaling is primarily mediated through its receptor, the Growth Hormone Secretagogue Receptor (GHS-R), which is most highly expressed in the hypothalamus, specifically in the agouti-related protein (AgRP) neurons (2, 3). The AgRP/neuropeptide Y (NPY) neurons are recognized as the “hunger neurons” that are activated during energy deficit to promote food-seeking behavior and locomotor activity (4, 5). We and others have shown that GHS-R in AgRP neurons contributes to the orexigenic effects of exogenous ghrelin (6–8). Furthermore, using electrophysiological recording, we have shown that ghrelin-induced activation of NPY neurons is abolished in GHS-R null mice (9). Interestingly, ghrelin has been demonstrated to exhibit daily rhythms of secretion that are synchronized by meal timing in both humans and rodents, showing pre-prandial increases in circulating acyl-ghrelin levels in anticipation of scheduled meals (10–12). Furthermore, transcriptomic studies have identified GHS-R as one of the top G-protein coupled receptors to be significantly upregulated by food deprivation compared to fed state in isolated AgRP neurons (13). However, in contrast to the pharmacological effects of ghrelin, a recent study using an adult-ablation of ghrelin mouse model showed that re-introducing pre-prandial concentrations of endogenous ghrelin is insufficient to stimulate food intake (14); this raises questions about the physiological role of endogenous ghrelin-GHS-R signaling in AgRP neurons under negative energy balance.
Emerging evidence suggests that ghrelin acts as an endogenous survival hormone, increasing levels in circulation as part of the adaptive response to nutritional and other stressors (15). A 50% caloric restriction (CR) produced hypoglycemia in Ghrelin−/− and Ghsr−/− mice in the first 16–20 days; this gradually normalized to levels comparable to CR littermate wild-type mice during a 40 day CR period (16), while a year-long chronic 40% CR produced hypoglycemia in Ghrelin−/− mice but not in Ghsr−/− mice (17). Mice with acyl ghrelin deficiency due to GOAT knockout showed life-threatening hypoglycemia in response to one-week-long severe CR (60%) (18), while re-expression of Ghsr in AgRP neurons in Ghsr-null mice rescued hypoglycemia induced by 60% CR (6). Hence, GHS-R in AgRP neurons plays a role in the counter-regulatory response under severe energy deficit; however, whether it regulates metabolic adaptations to mild energy deficit remains undetermined.
Energy homeostasis is achieved by balancing energy intake with energy expenditure, adapting feeding-related behaviors and metabolism to nutrient supply) (19). Since nutrient availability is often intermittent, organisms develop anticipatory responses to food presentation, especially when access to food is restricted. In rodent models, these adaptive responses include reduction in core body temperature to reduce metabolic rate and preserve energy, increased home cage activity and running wheel behaviors, and secretion of endocrine hormones related to metabolic control (20). Here, we investigated the role of GHS-R in AgRP neurons in regulating adaptive responses to energy deficiency, using AgRP neuron-specific GHS-R knockout mice (Agrp-Cre;Ghsrf/f) under a mild CR scheduled feeding paradigm. Our study revealed an important role of GHS-R in AgRP neurons in managing body weight, glucose control, metabolism and glycemic counter-regulatory response under negative energy balance.
Materials and Methods
Animals and procedures
All experimental procedures were approved and conducted in compliance with the Institutional Animal Care and Use Committee at Baylor College of Medicine. Mice were kept in a pathogen-free facility at Baylor College of Medicine under controlled temperature (75±1°F) and 12 hours (h) light/dark cycles (6 A.M. to 6 P.M.), with free access to water and food (chow diet; Harlan-Teklad 2920X). Mice were group-housed with 4–5 mice per cage to prevent isolation stress, unless otherwise stated. Ghsrf/f mice were bred with AgRP-Cre mice to generate Agrp-Cre;Ghsrf/f mice and littermate control Ghsrf/f (WT) mice, as we previously described (7). Whole-body composition (fat and lean mass) of mice was monitored using Echo MRI-100 whole-body composition analyzer (Echo Medical Systems, Houston, TX, USA).
Caloric restriction (CR)
For all calorie restriction experiments, mice were individually housed in feeder cages for the entire study, using a computer-controlled gating system which eliminated human intervention and error (Center Feeder Cages with food access control, Columbus Instruments, Columbus, OH, USA).
Cohorts 1 and 2 at 40% CR.
8-month-old, weight-matched male mice were transferred into separate feeder cages. Each experimental mouse was allowed daily ad libitum food intake which was assessed for a week; the mice were then allowed 60% of their daily intake to achieve an individualized 40% CR. To mimic normal food intake pattern, 3/4 of the daily 60% food allowance was given at 6PM (the beginning of dark cycle) and 1/4 of the 60% food allowance was given at 6 A.M. (the beginning of light cycle). Blood glucose was sampled weekly between 5–6 P.M., before feeders opened. A drop of tail blood was used to measure glucose using OneTouch Ultra blood glucose meter (LifeScan, New Brunswick, NJ, USA), and 50 μL of blood was collected into EDTA-coated capillary tubes for insulin analysis. Plasma insulin was measured using Mouse Insulin ELISA kit (Cat: 10–1247-10, Mercodia, Uppsala, Sweden) according to manufacturer’s instructions. Plasma glucagon was measured by the Vanderbilt University Medical Center Hormone Assay and Analytical Services Core. In parallel, a group of mice were maintained with ad libitum food access and served as the control for the CR regimen mice.
At end of study for cohort 1, mice were fasted after their morning meal until the next morning and then sacrificed (total of approximately 24 h fasting); tissues were collected, snap frozen and stored at −80°C until further analysis. For cohort 2, mice were given their morning meal, then sacrificed 1 h before the beginning of dark phase (total of approximately 10 h fasting); tissues were collected snap frozen and stored at −80°C until further analysis.
Indirect calorimetry
All metabolic parameter data were obtained using an Oxymax (Columbus Instruments, Columbus, OH, USA) open-circuit indirect calorimetry system, as we previously described (21). This system is equipped with 16 chambers, so to compare diet (ad libitum and CR) and genotype effects, ad libitum-fed control Ghsrf/f and AgRP-Cre;Ghsrf/f mice (n = 3 for each group), and CR-fed Ghsrf/f and AgRP-Cre;Ghsrf/f mice (n = 5 for each group) were subjected to indirect calorimetry measurements in the same session. Energy expenditure was normalized to both lean mass and body weight. Resting metabolic rate (determined at 24°C ambient temperature, or RMR24), was calculated from the average of the 3 lowest points of energy expenditure values between 10 A.M. and 2 P.M. (mid-phase of light cycle) on the fasting day, when mice have been fasted from 6 A.M., as we previously described (21). This approximates the definition for RMR (that animals are at rest and post-absorptive), but did not meet the criteria for being at thermoneutrality (22). Hence, we use the term RMR24 to indicate that the RMR in this study is determined at 24°C. Locomotor activity was measured on x- and z-axes using infrared beams to count the number of beam breaks during the recording period.
Glucose tolerance test (GTT), insulin tolerance test (ITT) and pyruvate tolerance test (PTT)
For GTT, overnight-fasted mice were i.p. injected with 2.0 g/kg D-glucose (Sigma, St. Louis, MO, USA). For PTT, mice were fasted overnight, and i.p. injected with 2 g/kg sodium pyruvate. Blood glucose was measured at different time points (0, 15, 30, 60, 120 min) using the OneTouch glucose meter. For ITT, mice received their morning meal at 6 A.M., were then fasted for 3 h starting at 9 A.M., and i.p. injected with 0.5 U/kg Humulin (Eli Lilly Company, Indianapolis, IN, USA). Blood glucose was measured at time points 0, 30, 45 and 60 min.
RNA Extraction and qRT-PCR
Total RNA was isolated using RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. Reverse transcription was performed with Superscript III First Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Quantitative real-time PCR reaction was performed in triplicate with SsoAdvanced SYBR Green Mastermix (Bio-Rad) on a Biorad CFX384 model (Bio-Rad Lab., Hercules, CA, USA). Relative gene expression levels were normalized using 18S as the housekeeping gene. The stability of the housekeeping gene is shown in Supplemental Table 1. Ghsr-1α primers were as described below: forward primer 5׳-GGACCAGAACCACAAACAGACA-3׳, reverse primer 5׳-CAGCAGAGGATGAAAGCAAACA-3׳ (23). This primer set flanks the intron, which allows us to distinguish its expression distinct from GHS-R1β. The rest of the primer information is available upon request. The raw Cq values of the housekeeping gene 18s are listed in the supplement table as a measure of RNA quality and stability.
Statistical analysis
Data are represented as mean ± SEM. Graph-Pad Prism version 6.0 software (La Jolla, CA, USA) was used in statistical analysis, and P < 0.05 was considered statistically significant. Normality and equal variance tests were conducted using D’Agostino-Pearson normality test. In the case of small sample size, data were first analyzed with non-parametric Mann-Whitney tests. Two-way ANOVA with repeated measures (with Time and Genotype as independent factors) was used to analyze body weight, fat mass and percentage, blood glucose, GTT, ITT and PTT data. Two-way ANOVA, with Diet (ad libitum vs. CR) and Genotype as independent factors, was used to analyze other data, unless otherwise stated. Šidák’s multiple comparisons test was used for post hoc analysis. Statistical significance of gene expression data was determined using the Holm-Šidák method, with alpha = 0.05, to correct for multiple t-tests.
Results
Deletion of GHS-R in AgRP neurons exaggerates the reductions of body fat and blood glucose in response to mild CR
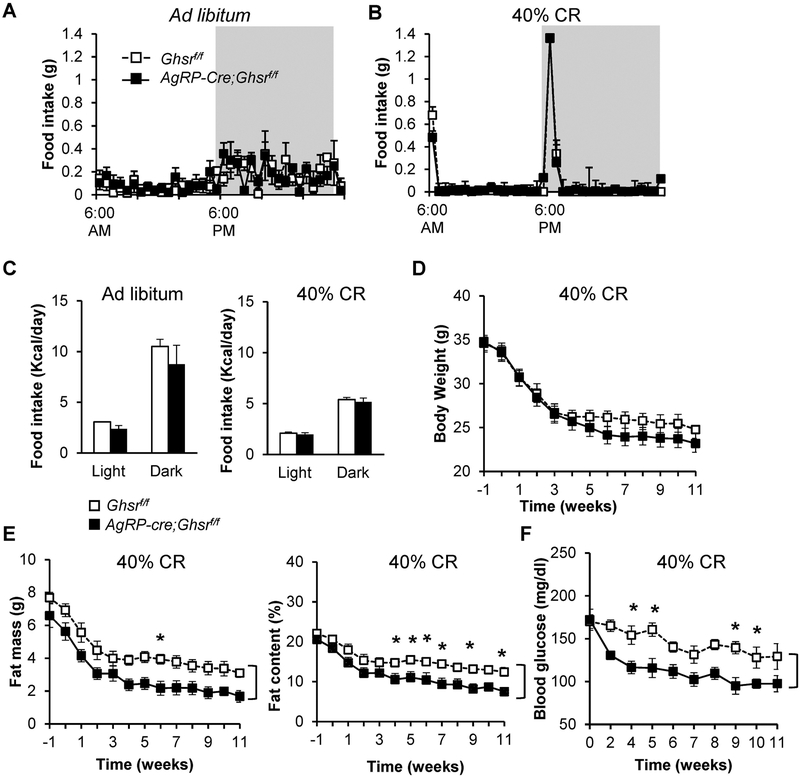
We have previously shown that under CR condition (50% CR), global ablation of GHS-R leads to greater reductions in glucose levels compared to their WT littermates, reaching nadir after 16 days (16). Recent literature suggested that AgRP neurons are required for the adaptation to CR and scheduled restricted feeding, as well as for integration of metabolic cues (24). To determine the role of GHS-R in AgRP neurons in the adaptation to scheduled food restriction and mild energy deficiency, we subjected 8-month-old male AgRP-Cre;Ghsrf/f and control Ghsrf/f mice to a restricted feeding regimen with 40% CR. For the first week, the daily ad libitum food intake of each experimental mouse was assessed; the mice were then allowed 60% of their daily intake to achieve 40% CR. To mimic normal food intake patterns, 3/4 of the daily 60% food allowance was given at 6 P.M. and 1/4 of the 60% daily allowance was given at 6 A.M. (Figs. 1A & 1B). Consistent with our previous report, AgRP-Cre;Ghsrf/f and Ghsrf/f mice showed no significant difference in daily total food intake under ad libitum or CR feeding regimens (Fig. 1C) (7). Under 40% CR, AgRP-Cre;Ghsrf/f mice showed a trend of decreased body weight compared to littermate Ghsrf/f control mice [Time: F(12, 108) = 84.43, p < 0.0001; Genotype: F(1, 9) = 3.25, p = 0.105; Time × Genotype: F(12, 108) = 2.12, p = 0.02] (Fig. 1D). The decrease in body weight in Ghsrf/f control mice reached nadir at approximately 4 weeks after initiation of CR, while AgRP-Cre;Ghsrf/f mice continued to lose weight and reached nadir at approximately 6 weeks after initiation of CR. Body composition analysis showed that AgRP-Cre;Ghsrf/f mice lost considerably more fat than Ghsrf/f mice [for comparison of fat mass, Time: F(12, 108) = 42.65, p < 0.0001; Genotype: F(1, 9) = 12.28, p = 0.0067; Time × Genotype: F(12, 108) = 0.24, p = 0.9958. For comparison of fat percentage, Time: F(12, 108) = 23.37, p < 0.0001; Genotype: F(1, 9) = 17.31, p = 0.0024; Time × Genotype: F(12, 108) = 0.36, p = 0.9732] (Fig. 1E). Despite the exaggerated loss of fat in calorie-restricted AgRP-Cre;Ghsrf/f mice, there was no significant difference in lean mass between the genotypes (Supplemental Fig. 1). To monitor glucose levels during CR, blood glucose was sampled weekly between 5–6 P.M., before feeders opened and mice acquired the larger meal (3/4 of daily allowance). Ghsrf/f control mice showed a gradual decrease in glucose levels after initiation of CR (Fig. 1F). Remarkably, glucose levels in AgRP-Cre;Ghsrf/f mice were significantly reduced compared to Ghsrf/f mice [Time: F(9, 81) = 13.56, p < 0.0001; Genotype: F(1, 9) = 18.44, p = 0.0020; Time × Genotype: F(9, 81) = 2.24, p = 0.0272] (Fig. 1F). The fastest decline in blood glucose levels occurred in the first 2 weeks of CR, after which they were maintained at relatively constant levels from the 4th week of CR onwards. Taken together, these data suggest that under energy deficiency, GHS-R deletion in AgRP neurons resets fat and glucose metabolism to a lower threshold.
Figure 1. Under mild calorie restriction, deletion of GHS-R in AgRP neurons decreases adiposity and blood glucose.
(A, B) Daily feeding schedules of ad libitum and 40% CR feeding regimen. (C) Daily food intakes in ad libitum and 40% CR feeding regimen. (D-F) Metabolic characterization of mice under CR. (D) Body weights of Ghsrf/f and AgRP-Cre;Ghsrf/f mice. (E) Fat mass and fat percentages of Ghsrf/f and AgRP-Cre;Ghsrf/f mice. (F) Blood glucose levels, measured 1 hour before onset of dark phase. Cohort 1, n = 5–6, by two-way ANOVA with repeated measures (Time and Genotype), with Šidák’s post hoc analysis. “]” indicates significant genotype effect; “*” indicates p < 0.05 in post hoc analysis, Ghsrf/f vs. AgRP-Cre;Ghsrf/f. Similar data were found in an independent cohort (Cohort 2, n = 8).
GHS-R deficiency in AgRP neurons increases physical activity and enhances carbohydrate utilization under mild CR
To assess the metabolic adaptations to 40% CR with scheduled feeding, we performed indirect calorimetry in mice that have been calorie-restricted for 12 weeks (n = 5 for each genotype, together with a small group of ad libitum-fed control mice (n = 3 for each genotype). Due to the small sample size in ad libitum groups, normality tests could not be performed; however, non-parametric tests showed no significant differences in physical activities, RER, energy expenditure and RMR24 between ad libitum-fed Ghsrf/f and AgRP-Cre;Ghsrf/f mice, consistent with our previous report (7). We placed the mice in automated metabolic cages; after 3 days of baseline recording, the mice were subjected to a 24-h fast followed by 24-h ad libitum refeeding (Supplemental Figs. 2A & 2B). In the CR groups, the mice received their morning meal before a 24-h fast. Since we found no significant differences between genotypes under fasting or refeeding conditions in physical activity and respiratory quotients, only baseline data are shown here, the rest can be found in Supplemental Figure 2. CR with scheduled feeding altered the pattern of physical activity, synchronizing peak physical activities to feeding times at 6 A.M. and 6 P.M., respectively (Fig. 2A). Despite the change in activity pattern, the total physical activity levels (expressed as number of beam break counts per hour) were comparable between Ghsrf/f mice under both ad libitum feeding and CR. Interestingly, total physical activity was significantly increased in calorie-restricted AgRP-Cre;Ghsrf/f mice compared to Ghsrf/f mice [Diet: F(1, 12) = 6.42, p = 0.0278; Genotype: F(1, 12) = 2.09, p = 0.1758; Diet × Genotype: F(1, 12) = 3.78, p = 0.0778] (Fig. 2B).
Figure 2. GHS-R deletion in AgRP neurons increases food anticipatory behavior and metabolic flexibility under calorie restriction.
Indirect calorimetry analysis of mice under ad libitum feeding and after 12 weeks of 40% CR regimen. After a 7-day acclimatization period to metabolic cages, metabolic parameters were recorded for 3 days baseline, 24 hours fasting, and 24 hours refeeding. (A) Physical activity (beam breaks counts) for the 24 hours baseline recording before fasting. (B) Total physical activity (beam breaks per hour). (C) Physical activity in the 4 hours before onset of dark phase (expressed as beam breaks per hour); this is considered as food anticipatory activity (FAA). (D) Respiratory exchange ratio (RER, = VCO2/VO2 Ratio). (E) Average RER. For 40% CR group, mice from Cohort 1 were measured, n = 3–5 for each genotype. Two-way ANOVA (Diet and Genotype), with Šidák’s post hoc analysis. * p < 0.05.
AgRP neurons have been shown to modulate appetitive behaviors as well as consummatory behaviors (25, 26). In addition, previous studies have reported a reduction in food anticipatory activity (FAA) in global GHS-R knockout mice (27–29). FAA is characterized by an increase in locomotor activity in anticipation of mealtime during calorie restriction or restricted feeding. Here we calculated physical activity in the 4 hours prior to onset of dark phase (27, 29) and found that, indeed, scheduled CR feeding significantly increased FAA in both genotypes [Diet: F(1, 12) = 34.09, p < 0.0001; Genotype: F(1, 12) = 1.763, p = 0.2090; Diet × Genotype: F(1, 12) = 0.95, p = 0.349] (Fig. 2C). Interestingly, deletion of GHS-R in AgRP neurons led to a trend of increased FAA under CR.
Consistent with previous report (30), CR elevated metabolic flexibility, showing a greater range of respiratory quotients (RER) during peak and trough periods when compared to ad libitum-fed mice (Fig. 2D). This suggests more efficient switching between fat and carbohydrate metabolism in the CR mice, depending on fuel availability. Calorie-restricted AgRP-Cre;Ghsrf/f mice exhibited a significant increase in average RER compared to Ghsrf/f mice, suggesting greater fuel preference for carbohydrate utilization [Diet: F(1, 12) = 5.21, p = 0.0416; Genotype: F(1, 12) = 2.78, p = 0.1215; Diet × Genotype: F(1, 12) = 8.30, p = 0.0138] (Fig. 2E). Reinforcing this notion, there was a slower switch to fat utilization (RER plots of AgRP-Cre;Ghsrf/f mice shift to right in figure) in the dark phase in calorie-restricted AgRP-Cre;Ghsrf/f mice when compared to Ghsrf/f mice (Fig. 2D).
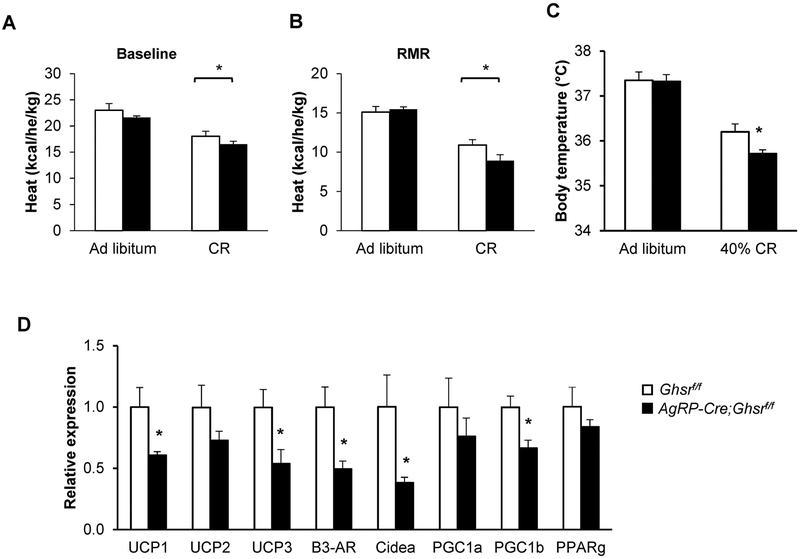
Under mild CR, GHS-R deficiency in AgRP neurons decreases resting metabolic rate, core body temperature and brown adipose tissue thermogenesis
As expected, metabolic assessment showed that overall energy expenditure (heat) was reduced in control Ghsrf/f mice after 12-week CR when compared to mice on ad libitum feeding [Diet: F(1, 12) = 30.90, p = 0.0002; Genotype: F(1, 12) = 2.67, p = 0.1307; Diet × Genotype: F(1, 12) = 0.005, p = 0.9443] (Fig. 3A). Interestingly, despite the increase in physical activity in calorie-restricted AgRP-Cre;Ghsrf/f mice, their overall energy expenditure was unchanged compared to control Ghsrf/f mice (Fig. 3A). The resting metabolic rate, determined at an ambient temperature of 24°C (RMR24), was generally reduced in CR mice compared to ad libitum-fed mice; interestingly, the calorie-restricted AgRP-Cre;Ghsrf/f mice showed a trend toward decreased RMR24 as compared to Ghsrf/f mice [Diet: F(1, 12) = 56.92, p < 0.0001; Genotype: F(1, 12) = 1.33, p = 0.2736; Diet × Genotype: F(1, 12) = 2.76, p = 0.1250] (Fig. 3B). In addition, we measured core body temperature 1 h before the onset of the dark phase; we found that core body temperature was reduced in CR mice compared to those with ad libitum feeding, and calorie-restricted AgRP-Cre;Ghsrf/f mice exhibited significantly decreased core body temperature compared to Ghsrf/f mice [Diet: F(1, 16) = 86.49, p < 0.0001; Genotype: F(1, 16) = 3.42, p = 0.083; Diet × Genotype: F(1, 16) = 2.83, p = 0.1117] (Fig. 3C).
Figure 3. AgRP-Cre;Ghsrf/f mice on 40% CR show lower basal metabolic rate, core body temperature and expression of thermogenic genes in brown adipose tissue.
(A-B) Indirect calorimetry analysis of mice under ad libitum feeding and 40% CR regimen. (A) Energy expenditure adjusted by body weight; (B) Resting metabolic rate (RMR), measured during light cycle, between 10 A.M. to 2 P.M., and normalized by body weight. n = 3–5. (C) Core body temperature, measured 1 hour before onset of dark phase. Cohort 1, n = 6. Data in A-C analyzed with two-way ANOVA (Diet and Genotype), followed by Šidák’s post hoc analysis. * p < 0.05. (D) Expression of brown adipose tissue (BAT) thermogenic function-related genes. Cohort 1, n =5. * p < 0.05, Ghsrf/f vs. AgRP-Cre;Ghsrf/f, data analyzed with Holm-Šidák method with correction for multiple t-tests.
Since the main thermogenic tissue in rodents is BAT (31), we assessed expression levels of genes involved in thermogenic function and BAT activation (32). Consistently, we found markedly reduced expression of the following in the BAT of calorie-restricted AgRP-Cre;Ghsrf/f mice: β3-adrenergic receptor (β3-AR), thermogenic regulatory genes uncoupling protein-1 and −3 (Ucp1, Ucp3), peroxisome proliferator-activated receptor γ coactivator 1β (PGC1β), and cell death-inducing DNA fragmentation factor alpha-like effector A (CIDEA) (Fig. 3D). However, expression of PPARγ, PGC1α and Ucp2 were not significantly changed. Taken together, these data suggested that GHS-R deficiency in AgRP neurons leads to metabolic adaptation to CR including increased physical activity, fuel preference towards carbohydrate metabolism, and energy conservation via reductions of RMR, core body temperature and BAT thermogenesis.
GHS-R deficiency in AgRP neurons diminishes counter-regulatory response under mild CR
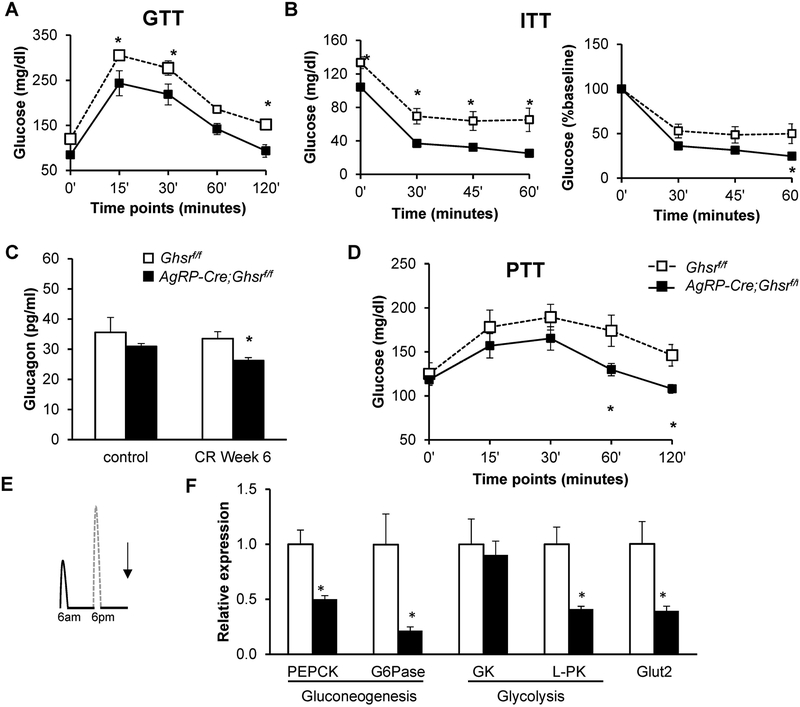
Besides energy metabolism, the hypothalamus plays important roles in glycemic regulation. We performed glucose tolerance tests (GTT) and insulin tolerance tests (ITT) to assess glycemic control and insulin sensitivity. We previously reported that in ad libitum-fed mice, deletion of GHS-R in AgRP neurons did not alter insulin sensitivity as measured by GTT and ITT (7). Here, under 40% CR, we found that glucose excursion was significantly reduced in AgRP-Cre;Ghsrf/f mice during GTT [Time: F(4, 56) = 89.83, p < 0.0001; Genotype: F(1, 14) = 10.02, p = 0.0069; Time × Genotype: F(4, 56) = 0.496, p = 0.7385] and ITT [Time: F(3, 36) = 88.54, p < 0.0001; Genotype: F(1, 12) = 14.54, p = 0.0025; Time × Genotype: F(3, 36) = 0.353, p = 0.7874] (Figs. 4A & B). During ITT, blood glucose levels dropped to less than 40 mg/dl in AgRP-Cre;Ghsrf/f mice and these mice appeared shaky; therefore, the experiment was terminated at 60 min and mice were administered glucose solution to restore glucose levels. A previous study has shown that global GHS-R deletion is associated with lower plasma glucagon (33). In agreement, after 6 weeks of 40% CR, plasma glucagon levels were significantly reduced in AgRP-Cre;Ghsrf/f mice [Diet: F(1, 32) = 0.1153, p = 0.7376; Genotype: F(1, 21) = 5.766, p = 0.0257; Diet × Genotype: F(1, 21) = 0.8502, p = 0.3670] (Fig. 4C), while insulin levels were similar between AgRP-Cre;Ghsrf/f and Ghsrf/f mice (data not shown). When subjected to a bolus of pyruvate, CR AgRP-Cre;Ghsrf/f mice showed an attenuated glucose excursion compared to Ghsrf/f mice, suggesting reduced hepatic glucose production [Time: F(4, 56) = 8.25, p < 0.0001; Genotype: F(1, 13) = 3.306, p = 0.0921; Time × Genotype: F(4, 52) = 3.56, p = 0.0122] (Fig. 4D).
Figure 4. GHS-R deletion in AgRP neurons increases insulin sensitivity and decreases hepatic glucose production under calorie restriction.
(A) Glucose levels during GTT after 18 hours overnight fast; (B) Glucose levels during ITT after 3 hours morning fast with glucose levels (left) and percentage of glucose change from baseline (right); (C) Plasma glucagon levels; (D) Glucose levels during PTT after 18 hours overnight fast that had been on 11 weeks of CR. Mice from Cohort 2 were used in the in vivo experiments for GTT, ITT and PTT, n = 8 for each group. (E) Diagram depicting the time at which liver tissues were collected for gene expression analysis, Cohort 1; (F) Expression of genes involved in hepatic glucose production. Tissues from Cohort 1, collected as indicated in E, were analyzed, n =5 for each group. Data in A, B, and D were analyzed with two-way ANOVA with repeated measures (Time and Genotype), followed by Šidák’s post hoc analysis. Data in C were analyzed with two-way ANOVA (Diet and Genotype), followed by Šidák’s post hoc analysis. Data in F were analyzed with Holm-Šidák method with correction for multiple t-tests. * p < 0.05, Ghsrf/f vs. AgRP-Cre;Ghsrf/f.
To test whether hepatic glucose production contributes to the reduced glucose excursion during pyruvate tolerance test, mRNA levels of several gluconeogenic and glycolysis genes in livers were measured. Liver tissues were collected in the morning when mice had been fasted for almost 24 hours since their morning meal from the previous day (Fig. 4E). The genes analyzed included phosphoenolpyruvate carboxykinase (Pepck), glucose-6 phosphatase (G6pc), glycerol kinase (Gk2), and pyruvate kinase (Pkm), as well as glucose transporter 2 (Glut2) genes. Consistent with the pyruvate tolerance test results, we detected significantly reduced expression of genes involved in gluconeogenesis (Pepck and G6pc), glycolysis (Gk2 and Pkm), as well as glucose transport (Glut2) in the livers of AgRP-Cre;Ghsrf/f mice (Fig. 4F).
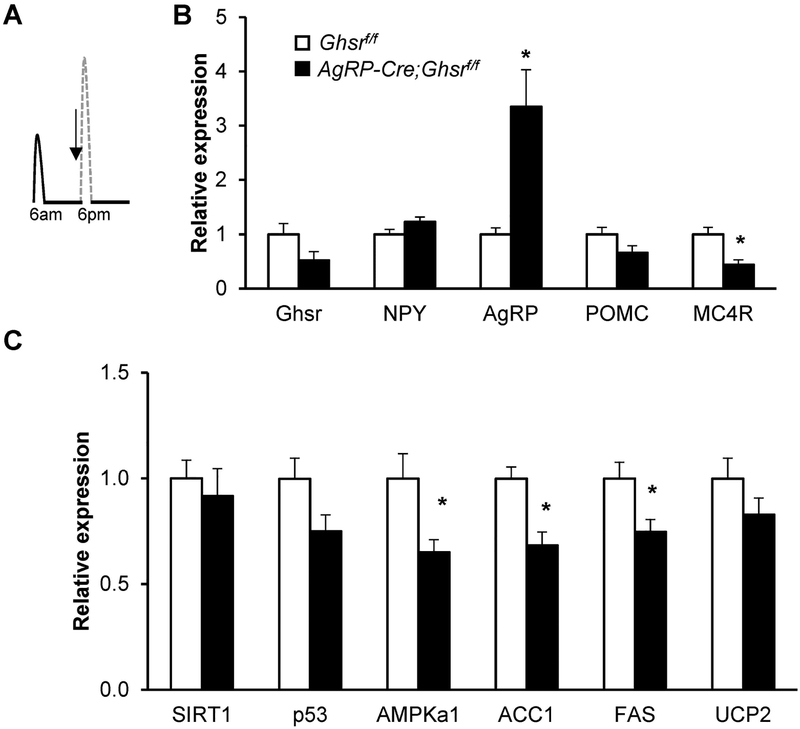
GHS-R deficiency in AgRP neurons attenuates hypothalamic Sirt1-p53 pathway under CR
As AgRP-Cre;Ghsrf/f mice on CR exhibited greater food anticipatory behavior and enhanced carbohydrate metabolism at the onset of the dark phase when mice were allowed access to a larger meal, we further explored the dynamic changes during this transition. We collected hypothalamus from the CR mice at the beginning of the dark phase (Fig. 5A). Expression of the orexigenic neuropeptide Agrp was significantly increased in AgRP-Cre;Ghsrf/f mice, while Npy expression was not significantly altered (Fig. 5B). Consistent with increased Agrp expression, there was a trend of decreased Pomc satiety signals; we also a detected significant reduction in its receptor Mc4r in the hypothalamus of AgRP-Cre;Ghsrf/f mice.
Figure 5. GHS-R deletion in AgRP neurons increases AgRP expression, but decreases genes involved in lipid metabolism under calorie restriction.
(A) Diagram depicting the time at which hypothalamic samples were collected for gene expression analysis; mice were on CR for 12 weeks. (B-C) Expression of genes involved in hypothalamus. Tissues from Cohort 2, collected as indicated in A, were analyzed, n =4 for each group. Data were analyzed with Holm-Šidák method with correction for multiple t-tests. * p < 0.05, Ghsrf/f vs. AgRP-Cre;Ghsrf/f.
It has been reported that the central sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin (34). Furthermore, the orexigenic effect of ghrelin involves the activation of hypothalamic AMP-activated protein kinase (AMPK) and inactivation of enzymatic steps in the de novo fatty acid biosynthetic pathway (35). Here we found that while the expression of Sirt1 and p53 showed no significant changes, expression of Ampk, acetyl-CoA carboxylase 1 (Acaca), and fatty acid synthase (Fasn) were significantly reduced in the hypothalamus of AgRP-Cre;Ghsrf/f mice, suggesting altered fatty acid metabolism (Fig. 5C).
Discussion
Calorie restriction is a well-established intervention that improves lifespan and “healthspan” in mammals, but compliance to long-term CR is extremely difficult to accomplish in humans (36). We previously showed that global deletion of GHS-R yielded CR-like metabolic characteristics of reduced body weight, lower fasting-glucose and -insulin, and improved insulin sensitivity during aging (21). We hypothesized that these metabolic benefits may be centrally driven, and that GHS-R in AgRP neurons may be part of the neurocircuitry contributing to the metabolic adaptations to CR. In addition, the observations that ghrelin resistance exists in diet-induced obese mice, that ghrelin sensitivity can be restored by weight loss, and that the actions of ghrelin are enhanced during CR, supports the notion that ghrelin is a peripheral signal of energy deficit (37). In the current study, we set out to determine the role of GHS-R in AgRP neurons in transducing the energy deficit signal and regulating metabolic alterations in adaptation to CR. We employed a mild CR with scheduled feeding regimen; this has been reported to produce a better-defined food intake and more stable mean body temperature compared to the restricted feeding paradigm that involves temporally restricting access to food for just several hours in the middle of the light cycle (38). Since age impacts on metabolism and our previous reports have demonstrated age-dependent changes in the ghrelin system when comparing young and old mice, we performed the study on 8-month old mice (21, 23, 39, 40). As 8-month old mice are considered mature adults and body weight and growth have stabilized, we chose this age range to conduct the CR study to conform to the idea of “undernutrition without malnutrition” in CR (36, 41). Consistent with this notion, the CR-induced weight loss in our study is gradual, occurring over 5 weeks, instead of experimental anorexia nervosa models in which drastic reductions happen within 2–3 days (28).
In the current study, we showed that energy expenditure, specifically resting metabolic rate (RMR24), was reduced under CR in littermate control mice compared to ad libitum controls, as expected. Interestingly, the reduction in RMR24 was further diminished in AgRP-Cre;Ghsrf/f mice under CR. Core body temperature was decreased by CR, and AgRP-Cre;Ghsrf/f mice showed an even greater attenuation. The reductions in RMR24 and core body temperature in AgRP-Cre;Ghsrf/f mice could be secondary to the greater loss of fat mass in these mice under CR. Similarly, the apparent increase in insulin sensitivity in AgRP-Cre;Ghsrf/f mice under CR could be due to the lower fat mass in these mice, as a previous study showed that fat mass is a major factor in determining glucose tolerance and insulin sensitivity (42). Whether the greater loss of fat mass in calorie restricted AgRP-Cre;Ghsrf/f mice is due to reduced lipid accumulation or increased fat catabolism remains to be determined. Interestingly, gene expression analysis in BAT showed significant reductions in uncoupling proteins 1 and 3 (Ucp1, Ucp3), β3-AR, PGC1β, as well as the BAT marker CIDEA (43), suggesting reduced sympathetic activation in BAT in calorie restricted AgRP-Cre;Ghsrf/f mice (44, 45). On the other hand, we previously demonstrated that deletion of GHS-R in AgRP neurons attenuates diet-induced obesity via increased thermogenesis, supporting a role for GHS-R in fat metabolism under positive energy balance (7). Taken together, our data showed that deletion of GHS-R in AgRP neurons elevates thermogenesis under positive energy balance and attenuates high fat diet-induced obesity, while conversely diminishes thermogenesis under negative energy balance. While this seems paradoxical, these data are consistent with the notion that ghrelin resistance (deletion of GHS-R) is a mechanism designed to protect a higher body weight set-point established during energy excess, while ghrelin signaling is required to protect energy reserves during negative energy balance (37).
AgRP neurons have important regulatory roles in lipid metabolism and hepatic glucose production (46, 47). The body has a highly fine-tuned integrated system for maintaining optimal levels of blood glucose (48). Previous studies have demonstrated an important role for ghrelin signaling in the counter-regulatory response to hypoglycemia (6, 18, 49). It has been suggested that ghrelin directly decreases insulin secretion and increases glucagon release in the pancreas, which in turn promotes hepatic glucose production and ultimately raises blood glucose levels (33, 50). Here we demonstrated that deletion of GHS-R in AgRP neurons in mice leads to a more severe reduction of glucose levels when exposed to CR, indicating central effects of ghrelin signaling on glucose homeostasis. The lower glucagon levels in AgRP-Cre;Ghsrf/f mice suggests an impaired counter-regulatory response to hypoglycemia. Consistently, AgRP-Cre;Ghsrf/f mice exhibited decreased hepatic glucose production, as evidenced by reduced pyruvate tolerance and decreased expression of genes involved in gluconeogenesis in the liver. These data are in line with a previous report showing that re-expression of GHS-R in AgRP neurons restores the blood glucose levels observed under severe CR (6). Our data complement this finding, demonstrating that under CR, suppression of GHS-R in AgRP neurons resets the metabolism to a lower threshold. Previously, we showed that GHS-R deletion in AgRP neurons abolished ghrelin-induced growth hormone (GH) secretion (7). Whether the lower metabolism and reduced counter-regulatory response in AgRP-Cre;Ghsrf/f mice is mediated through reduced GH secretion or reduced activation of the sympathoadrenal axis is unknown; further studies are required to determine the downstream effectors and pathways. Nevertheless, our data suggest that GHS-R in AgRP neurons is part of the central molecular circuitries that maintain normal metabolism and euglycemia under negative energy balance.
Recent studies show that AgRP neurons control appetitive and consummatory behaviors including food anticipation, food foraging, food hoarding, and food intake (24–26). In the current study, we showed that total physical activity was significantly increased in AgRP-Cre;Ghsr f/f mice under CR compared to littermate control mice. In addition, CR with scheduled feeding significantly increased food anticipatory activity (FAA), consistent with a recent report showing significant increases in wheel-running activity occurring concomitantly with feeding in mice under 30% CR (51). We also found a trend for further increases in FAA in calorie-restricted AgRP-Cre;Ghsr f/f mice. In line with a trend for increased FAA, gene expression analysis showed that expression of the orexigenic peptide AgRP was significantly increased in the hypothalamus of AgRP-Cre;Ghsr f/f mice before the onset of scheduled meals in the beginning of dark phase. AgRP expression, measured at 1 h before food presentation, has been shown to correlate positively with FAA (52). However, this seemed counter-intuitive, since ghrelin signaling is a hunger signal that activates AgRP neurons to increase feeding (7, 9, 10). Indeed, previous studies have reported reduction in FAA in Ghsr KO mice, in restricted feeding paradigms where mice were given access to food at ZT4-ZT8 (27, 29). Hence, it is puzzling that loss of GHS-R specifically in AgRP neurons increases locomotor activity. Interestingly, using Fos as an index of neuronal activation, Blum et al. (27) showed that after 7 days of food restriction, Ghsr KO mice had significantly fewer Fos-immunoreactive cells in the dorsomedial, paraventricular and lateral hypothalamic nuclei in comparison with wild-type controls; on the other hand, Fos expression in the arcuate nucleus was lower in Ghsr KO mice but the differences did not achieve statistical significance. Thus, it is possible that GHS-R expressed in other hypothalamic nuclei compensated for the loss of GHS-R in AgRP neurons in our study. In line with this idea, re-expression of GHS-R in AgRP neurons in adult mice only partially restored food intake; this suggests that GHS-R in other hypothalamic nuclei or extra-hypothalamic sites may mediate the full extent of ghrelin’s effect on food intake (6). Future studies employing different experimental paradigms focusing on FAA, monitoring activity in the early phase of adaptive responses and acute pharmacological treatments, are required to define the role of GHS-R in the neurocircuitry governing food anticipatory behaviors.
Metabolic flexibility is the capacity to efficiently reprogram metabolism based on fuel sources and availability. On the other hand, metabolic inflexibility could exacerbate pre-existing elevated circulating glucose and reduced fatty acid oxidation, leading to obesity and type 2 diabetes (53, 54). Consistent with the metabolic benefits of CR, our data showed an increased range of peaks and troughs in RER in the wild-type control mice under CR when compared to those on ad libitum feeding, suggesting that CR increased metabolic flexibility. Interestingly, we found that AgRP-Cre;Ghsr f/f mice under CR exhibited increased fuel preference for carbohydrates, and a delayed switch to fatty acid metabolism after ingestion of scheduled meal in the dark phase. This contradicts the well-accepted effect of centrally-delivered ghrelin in increasing RER and adiposity (55, 56). On the other hand, the increase in RER in AgRP-cre;Ghsrf/f mice is in line with our previous report showing that deletion of GHS-R in all forebrain neurons (Syn1-cre;Ghsrf/f mice) increased RER, but decreased body weight and percentage of fat in chow-fed Syn1-cre;Ghsrf/f mice (57). The perceived contradiction in RER in our genetic model and pharmacological effects of ghrelin could be due to the congenital loss of GHS-R in AgRP neurons, such that the energy deficit signal is not correctly transduced and thus leading to uncoordinated metabolic responses to energy deficient state.
Recent studies showed that AgRP neurons are important in regulating metabolic flexibility and peripheral nutrient partitioning (58–60). It has been shown that ablation of AgRP neurons increased fatty acid utilization and attenuated body weight gain in mice fed a high-fat diet (59). Our calorie-restricted AgRP-Cre;Ghsr f/f mice exhibited a similar phenotype, showing a lower percentage of fat compared to littermate controls. Interestingly, we found that expression of AMPK, Acaca and Fasn were significantly reduced in the hypothalamus of AgRP-Cre;Ghsr f/f mice, suggesting decreased activation of the Sirtuin 1/p53 pathway and reduced lipid metabolism (34, 35). The role of AMPK in AgRP neurons in response to different glucose levels has been studied. Lockie et al. (61) showed that in euglycemic condition, knockdown of AMPK in AgRP neurons (via AAV delivery of dominant negative AMPK) did not affect ghrelin-induced feeding. However, under hyperglycemic condition, glucose suppressed ghrelin-induced feeding, and this suppression was abolished by AMPK knockdown in AgRP neurons. On the other hand, in hypoglycemic condition (glucoprivation by 2-deoxy-D-glucose, 2-DG), ghrelin-induced feeding was enhanced, and this enhancement was not affected by knockdown of AMPK. Importantly, 2-DG-induced food intake was not affected by AMPK knockdown. Together, these data suggest that: 1) AMPK in AgRP neurons is important for transducing glucose signals but not ghrelin signals in AgRP neurons in terms of effects on food intake, and 2) AMPK in AgRP neurons does not transduce the glucoprivation signals. In our study, we found that AMPK expression was reduced in the hypothalamus of AgRP-cre;Ghsrf/f mice under CR at 1 hour before onset of dark phase and presentation of food; this is concomitant with the lower glucose levels in AgRP-cre;Ghsrf/f mice under CR, as measured in the same time frame. We do not know whether this reduction in AMPK expression is specific to AgRP neurons. Interestingly, Claret et al. (62) reported that deletion of AMPK in AgRP neurons leads to an age-dependent lean phenotype, with significantly lower body weight from 3-months of age. Taken together, we speculate that the reduced AMPK in hypothalamus of AgRP-cre;Ghsrf/f mice under CR could reflect the lower body weight; whether it is part of the compensatory mechanisms driving feeding or locomotor activity in the face of hypoglycemia requires more detailed studies.
More recent studies have demonstrated that carnitine acetyltransferase (Crat) in AgRP neurons regulates metabolic flexibility, peripheral nutrient partitioning and homeostatic adaptation to restricted feeding (60, 63). Crat is an enzyme involved in carnitine metabolism, which regulates intracellular pools of acyl-coenzyme A (CoA) esters. Reichenbach et al. reported that deletion of Crat in AgRP neurons leads to increased fatty acid utilization, with a lower respiratory exchange ratio (RER) in both light and dark phases. Furthermore, liver triglyceride levels and hepatic gluconeogenic genes Pck1, Pck2, and G6pc were significantly increased in fasted AgRP Crat KO mice, while norepinephrine turnover was decreased in fasted AgRP Crat KO mice compared to fasted control mice. In contrast, our data showed that RER was increased in AgRP-cre;Ghsrf/f mice under CR, while hepatic gluconeogenic genes were decreased. AgRP Crat KO mice showed a slower recovery of body weight during adaptation to restricted feeding, but no change in blood glucose levels. However, these mice showed increased FAA in the later phase of adaptation, which is suggested by the authors to reflect the greater energy deficit and body weight loss in KO mice. Taken together, these data suggest that Crat in AgRP neurons could be one of the intracellular mechanisms downstream of GHS-R signaling that regulates metabolic flexibility and adaptation to restricted feeding. Further characterization of isolated AgRP neuron populations from AgRP-Cre;Ghsrf/f and littermate control mice is warranted in order to dissect the molecular mechanisms involved.
In summary, we report that specific deletion of GHS-R in AgRP neurons resets the metabolism to a lower threshold, thus reducing fat mass, blood glucose and basal metabolic rates during 40% mild CR. AgRP-Cre;Ghsrf/f mice exhibited lower blood glucose levels under mild CR, showing decreased hepatic glucose production and reduced glucagon. The calorie-restricted AgRP-Cre;Ghsrf/f mice also showed increased locomotion and enhanced carbohydrate fuel usage when food becomes available. Together, these data suggest that GHS-R in AgRP neurons is an integral part of the complex neurocircuitry involved in metabolic adaptation to energy deficiency. This further supports the concept that ghrelin is a survival hormone, serving as a mediator of CR-associated physiology (15).
Supplementary Material
Acknowledgments
Measurements of body composition, food intake and energy balance were performed in the Mouse Metabolic Research Unit at the USDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine, which is supported by funds from the USDA/ARS. We also thank Michael R. Honig at Houston’s Community Public Radio Station KPFT for his editorial assistance. This study was supported by American Diabetes Association #1–15-BS-177 (YS), R56DK118334 (YS), R01DK118334 (YS), and the United States Department of Agriculture National Institute of Food and Agriculture Hatch project 1010840 (YS). This study was also supported by Robert A. Welch Foundation AU-1731–20160319 (ZC) and the National Institute on Aging at NIH R01AG045828 (ZC). Study was also supported by NIH P30 DK56338, 1T32HD071839 (PI: Morey W. Haymond).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402(6762): 656–60. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004; 101(13): 4679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006; 494(3): 528–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011; 121(4): 1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012; 488(7410): 172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Molecular metabolism. 2014; 3(1): 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CS, Bongmba OYN, Yue J, Lee JH, Lin L, Saito K, Pradhan G, Li DP, Pan HL, Xu A, Guo S, Xu Y, Sun Y. Suppression of GHS-R in AgRP Neurons Mitigates Diet-Induced Obesity by Activating Thermogenesis. International journal of molecular sciences. 2017; 18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Eshghjoo S, Davis J, Alaniz RC, Sun Y. Insights on neuronal functions of ghrelin receptor GHS-R in obesity. J Neurol Neuromed. 2018; 3(4): 69–74. [Google Scholar]
- 9.Chen SR, Chen H, Zhou JJ, Pradhan G, Sun Y, Pan HL, Li DP. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. Journal of neurochemistry. 2017; 142(4): 512–20. [DOI] [PubMed] [Google Scholar]
- 10.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001; 50(8): 1714–9. [DOI] [PubMed] [Google Scholar]
- 11.Verbaeys I, Tolle V, Swennen Q, Zizzari P, Buyse J, Epelbaum J, Cokelaere M. Scheduled feeding results in adipogenesis and increased acylated ghrelin. Am J Physiol Endocrinol Metab. 2011; 300(6): E1103–11. [DOI] [PubMed] [Google Scholar]
- 12.Zizzari P, Hassouna R, Longchamps R, Epelbaum J, Tolle V. Meal anticipatory rise in acylated ghrelin at dark onset is blunted after long-term fasting in rats. J Neuroendocrinol. 2011; 23(9): 804–14. [DOI] [PubMed] [Google Scholar]
- 13.Henry FE, Sugino K, Tozer A, Branco T, Sternson SM. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife. 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014; 20(1): 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mani BK, Zigman JM. Ghrelin as a Survival Hormone. Trends Endocrinol Metab. 2017; 28(12): 843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008; 149(2): 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers NH, Walsh H, Alvarez-Garcia O, Park S, Gaylinn B, Thorner MO, Smith RG. Metabolic Benefit of Chronic Caloric Restriction and Activation of Hypothalamic AGRP/NPY Neurons in Male Mice Is Independent of Ghrelin. Endocrinology. 2016; 157(4): 1430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010; 107(16): 7467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009; 9(6): 489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton DF, Mistlberger RE. Circadian adaptations to meal timing: neuroendocrine mechanisms. Frontiers in neuroscience. 2013; 7 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Saha PK, Ma X, Henshaw IO, Shao L, Chang BH, Buras ED, Tong Q, Chan L, McGuinness OP, Sun Y. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011; 10(6): 996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Molecular metabolism. 2018; 7 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Garcia JM, Smith RG. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology. 2007; 148(3): 1323–9. [DOI] [PubMed] [Google Scholar]
- 24.Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Molecular metabolism. 2014; 3(7): 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015; 160(6): 1222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas MA, Xue B. Mechanisms for AgRP neuron-mediated regulation of appetitive behaviors in rodents. Physiol Behav. 2018; 190 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009; 164(2): 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2011; 21(5): 384–92. [DOI] [PubMed] [Google Scholar]
- 29.Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav. 2011; 103(1): 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westbrook R, Bonkowski MS, Arum O, Strader AD, Bartke A. Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. J Gerontol A Biol Sci Med Sci. 2014; 69(1): 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84(1): 277–359. [DOI] [PubMed] [Google Scholar]
- 32.Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014; 76 225–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Molecular endocrinology. 2011; 25(9): 1600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velasquez DA, Martinez G, Romero A, Vazquez MJ, Boit KD, Dopeso-Reyes IG, Lopez M, Vidal A, Nogueiras R, Dieguez C. The Central Sirtuin 1/p53 Pathway Is Essential for the Orexigenic Action of Ghrelin. Diabetes. 2011; 60(4): 1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, Deoliveira RM, Castaneda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschop MH, Dieguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008; 7(5): 389–99. [DOI] [PubMed] [Google Scholar]
- 36.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing Res Rev. 2017; 39 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zigman JM, Bouret SG, Andrews ZB. Obesity Impairs the Action of the Neuroendocrine Ghrelin System. Trends Endocrinol Metab. 2016; 27(1): 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunapala KM, Gallardo CM, Hsu CT, Steele AD. Single gene deletions of orexin, leptin, neuropeptide Y, and ghrelin do not appreciably alter food anticipatory activity in mice. PLoS One. 2011; 6(3): e18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma X, Lin L, Qin G, Lu X, Fiorotto M, Dixit VD, Sun Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS One. 2011; 6(1): e16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin L, Lee JH, Bongmba OY, Ma X, Zhu X, Sheikh-Hamad D, Sun Y. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging (Albany NY). 2014; 6(12): 1019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982; 215(4538): 1415–8. [DOI] [PubMed] [Google Scholar]
- 42.Kirchner H, Hofmann SM, Fischer-Rosinsky A, Hembree J, Abplanalp W, Ottaway N, Donelan E, Krishna R, Woods SC, Muller TD, Spranger J, Perez-Tilve D, Pfluger PT, Tschop MH, Habegger KM. Caloric restriction chronically impairs metabolic programming in mice. Diabetes. 2012; 61(11): 2734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin SC, Li P. CIDE-A, a novel link between brown adipose tissue and obesity. Trends Mol Med. 2004; 10(9): 434–9. [DOI] [PubMed] [Google Scholar]
- 44.Sharma BK, Patil M, Satyanarayana A. Negative regulators of brown adipose tissue (BAT)-mediated thermogenesis. J Cell Physiol. 2014; 229(12): 1901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007; 104(12): 5223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belgardt BF, Okamura T, Bruning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009; 587(Pt 22): 5305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes. 2010; 59(2): 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab. 2014; 307(10): E859–71. [DOI] [PubMed] [Google Scholar]
- 49.Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. The Journal of biological chemistry. 2012; 287(22): 17942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004; 53(12): 3142–51. [DOI] [PubMed] [Google Scholar]
- 51.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab. 2017; 26(1): 267–77 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girardet C, Mavrikaki M, Southern MR, Smith RG, Butler AA. Assessing interactions between Ghsr and Mc3r reveals a role for AgRP in the expression of food anticipatory activity in male mice. Endocrinology. 2014; 155(12): 4843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodpaster BH, Sparks LM. Metabolic Flexibility in Health and Disease. Cell Metab. 2017; 25(5): 1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith RL, Soeters MR, Wust RCI, Houtkooper RH. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr Rev. 2018; 39(4): 489–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dos-Santos RC, Reis LC, Perello M, Ferguson AV, Mecawi AS. The actions of ghrelin in the paraventricular nucleus: energy balance and neuroendocrine implications. Annals of the New York Academy of Sciences. 2019. [DOI] [PubMed] [Google Scholar]
- 56.Heppner KM, Piechowski CL, Muller A, Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi R, Biebermann H, Tschop MH, Sandoval DA, Perez-Tilve D. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2014; 63(1): 122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JH, Lin L, Xu P, Saito K, Wei Q, Meadows AG, Bongmba OY, Pradhan G, Zheng H, Xu Y, Sun Y. Neuronal Deletion of Ghrelin Receptor Almost Completely Prevents Diet-Induced Obesity. Diabetes. 2016; 65(8): 2169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cansell C, Denis RG, Joly-Amado A, Castel J, Luquet S. Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne). 2012; 3 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, Prola A, Flamment M, Foufelle F, Magnan C, Luquet S. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. The EMBO journal. 2012; 31(22): 4276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reichenbach A, Stark R, Mequinion M, Denis RRG, Goularte JF, Clarke RE, Lockie SH, Lemus MB, Kowalski GM, Bruce CR, Huang C, Schittenhelm RB, Mynatt RL, Oldfield BJ, Watt MJ, Luquet S, Andrews ZB. AgRP Neurons Require Carnitine Acetyltransferase to Regulate Metabolic Flexibility and Peripheral Nutrient Partitioning. Cell reports. 2018; 22(7): 1745–59. [DOI] [PubMed] [Google Scholar]
- 61.Lockie SH, Stark R, Mequinion M, Ch’ng S, Kong D, Spanswick DC, Lawrence AJ, Andrews ZB. Glucose Availability Predicts the Feeding Response to Ghrelin in Male Mice, an Effect Dependent on AMPK in AgRP Neurons. Endocrinology. 2018; 159(11): 3605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007; 117(8): 2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichenbach A, Mequinion M, Bayliss JA, Lockie SH, Lemus MB, Mynatt RL, Stark R, Andrews ZB. Carnitine Acetyltransferase in AgRP Neurons Is Required for the Homeostatic Adaptation to Restricted Feeding in Male Mice. Endocrinology. 2018; 159(6): 2473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.