Abstract
Scope:
The fatty acid profile of dietary lipids is reflected in mammary adipose tissue and may influence mammary gland biology and cancer risk. To determine the effects of fish consumption on breast adipose tissue fatty acids, we conducted a study of fish versus n-3 PUFA supplements in women at increased risk of breast cancer.
Methods and results:
High risk women were randomized to comparable doses of marine n-3 PUFAs as canned salmon + albacore or capsules for 3 months. Pre- and posttreatment fatty acid profiles were obtained by GC. Dietary fish (n = 12) and n-3 PUFA capsules (n = 13) yielded increased eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in plasma (p < 0.0001), erythrocyte membranes (p < 0.0001), and breast fat (p < 0.01) at 3 months. Women taking capsules had higher plasma and erythrocyte membrane EPA changes (~four versus twofold, p = 0.002), without significant differences in DHA. Increases in breast adipose EPA, DHA were similar for both groups. Higher BMI correlated with smaller changes in plasma, erythrocyte membrane EPA, and breast adipose EPA, DHA. Adherence was excellent at 93.9% overall and higher in the fish arm (p = 0.01).
Conclusion:
Fish provides an excellent source of n-3 PUFAs that increases breast adipose EPA, DHA similar to supplements and represents a well-tolerated intervention for future studies of the impact of n-3 PUFAs and dietary patterns on breast cancer.
Keywords: Breast adipose tissue, DHA, Dietary fish, EPA, n-3 PUFAs
1. Introduction
The long-chain n-3 PUFAs known as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are bioactive nutrients hypothesized to impact cancer risk [1, 2]. Extensive laboratory-based research has revealed the anti-inflammatory effects of EPA and DHA, as mediated through diverse mechanisms that include modulation of eicosanoid biosynthesis as well as alteration of plasma membrane lipid composition and resultant effects on signal transduction and gene expression [3, 4]. Dietary fat sources of EPA and DHA, which include seafood and especially cold water fish such as salmon, tuna, and sardines, have potential for ameliorating diseases associated with chronic inflammation, such as atherosclerosis, rheumatoid arthritis, and cancer [5]. Currently, nutritional guidelines for dietary fish and n-3 PUFAs exist for cardiovascular disease prevention, with recommendations for consumption of two servings of fish, and preferably fatty fish, per week for the general population[6].
While laboratory investigations in cell culture and rodent models provide compelling evidence of the inhibitory effects of fish oil and EPA, DHA on mammary tumorigenesis [7–9], human studies to date yield mixed results on the preventive benefits of dietary fish/n-3 PUFAs [10]. Imprecision and biases of dietary questionnaires used in epidemiologic studies, coupled with the heterogeneity of breast cancer and recognition of specific molecular subtypes with unique etiologic and prognostic profiles, may contribute to the difficulty of defining the role of dietary n-3 PUFAs in human breast carcinogenesis. Genetic polymorphisms leading to variations in bioavailability, metabolism, and biological responses also complicate the delineation of causal relationships by epidemiologic approaches alone [11–13]. Yet, the emergence of studies examining dietary patterns does suggest that diets enriched in fish may contribute to the benefits of a healthy dietary pattern in reducing the risk of developing breast cancer [14].
Clinical trials to elucidate the benefits and risks of dietary n-3 PUFAs for human diseases have typically employed a pharmacologic approach for ease of administration and adherence, with very few studies examining the impact of food sources. For example, clinical trials of n-3 PUFAs have utilized doses of 5.2 g EPA+DHA/day in 18 capsules in rheumatoid arthritis patients [15] and 7.5 g EPA+DHA/day in 11 capsules in subjects with cancer cachexia [16], which are not achievable by fish consumption. In contrast, prospective secondary prevention trials support dietary intake of fatty fish as well as lower doses of n-3 PUFA supplements to significantly reduce mortality from heart disease with ~1–3 g EPA+DHA/day [17–19]. In a dose finding study of n-3 PUFA supplements in women at high risk of breast cancer, we defined the dynamic changes in fatty acid profiles in breast adipose tissue and serum in response to four different doses (i.e. 0.84, 2.52, 5.04, 7.56 g/d of EPA + DHA), demonstrating a nonlinear response with superiority of ≥ 2.52 g EPA + DHA/day versus 0.84 g/day to increase serum and breast adipose tissue EPA+DHA content over 6 months [20]. Higher DHA, EPA content in breast adipose tissue at baseline was associated with a smaller incremental increase in these fatty acids [20]. As subjects were not on a controlled diet, the baseline differences likely reflect the impact of usual dietary intake, as modulated by individual genetically defined variation in absorptive and metabolic pathways. The present 3 month study was designed to directly compare the impact of increased dietary intake of fish (salmon, albacore tuna) to baseline diet with an oral EPA+DHA supplement on breast adipose tissue and plasma, erythrocyte membrane fatty acids.
2. Materials and methods
2.1. Subjects
Subjects ≥ 18 years old and at high risk for breast cancer were recruited from the outpatient breast clinics of the James Cancer Hospital at The Ohio State University Wexner Medical Center. Determinants of high risk status included having at least one of the following criteria: (1) one or more first-degree relatives with breast cancer, with at least one under the age of 60, (2) two or more second-degree relatives with breast cancer, with at least one under the age of 50, (3) prior biopsy diagnosing atypical lobular hyperplasia, atypical ductal hyperplasia, lobular carcinoma in situ, or ductal carcinoma in situ in the last 10 years, (4) Gail risk assessment with a 5 year Gail ≥1.7 or 10 year Gail ≥3.4% [21], (5) known genetic mutation associated with hereditary breast cancer; or (6) personal diagnosis of T1 or T2 breast cancer within the last 10 years and completion of chemotherapy or antiestrogen therapy for over 6 months.
All participants had a benign/stable mammogram within the past year, Eastern Cooperative Oncology Group performance status of 0 (i.e. fully active, able to carry out usual activities without restrictions), and negative pregnancy test when applicable. Exclusion criteria included BMI greater than 35 (increased from BMI >30 to broaden eligibility following enrollment of the first 20 subjects), less than 1 year from pregnancy or lactation, history of bleeding tendency, diabetes mellitus, hypertension, heart disease, liver disease or stroke, concurrent malignancy or metastatic malignancy, ongoing cancer-related treatment, use of anticoagulant medication, inability to undergo breast fat aspirations, known sensitivity or allergy to fish, and chronic use of full dose aspirin (≥325 mg/day) or nonsteroidal antiinflammatory drugs. Subject who used fish oil or n-3 fatty acid supplements on a chronic basis or regularly consumed more than 2 meals/servings of fish per week within 3 months of study enrollment were not eligible to participate.
2.2. Trial design
The study was conducted with approval of the Institutional Review Board ofThe James Cancer Hospital, The Ohio State University Comprehensive Cancer Center, and The Ohio State University (2010C0045) and in accordance with the ethical standards of the institution and the Helsinki Declaration of 1975 as revised in 1983. This trial was registered at clincaltrials.gov as NCT01282580. Eligible subjects were recruited from July 2010 to July 2012 to meet target accrual, and the last subject completed the study in September 2012. Enrollment and study visits were conducted at the Stefanie Spielman Comprehensive Breast Center of the James Cancer Hospital. After obtaining informed consent, subjects were randomized to receive either 4 six ounce servings of canned/pouched fish per week or two capsules per day of an n-3 fatty acid supplement that provided ~1.68 g per day of EPA + DHA (Lovazaۚ, GlaxoSmithKline, Philadelphia, PA) using a block design with two blocks of 15 study participants. The random allocation sequence as generated by Research Randomizer (www.research.randomizer.org) was implemented through the pharmacy of the James Cancer Hospital/Stefanie Spielman Comprehensive Breast Center, with concealment of the sequence until interventions were assigned. The study interventions were designed for equivalent EPA + DHA intake, balancing the practical constraints of fish consumption with a capsule dose likely to alter n-3 PUFAs in breast adipose tissue. Each one gram capsule of the supplement provided at least 0.9 g of the ethyl esters of n-3 fatty acids, predominantly a combination of EPA (~465 mg) and DHA (~ 375 mg) per package insert. We selected 2 capsules/day as the lowest dose likely to elicit a change in breast adipose tissue EPA and DHA during the 3 month study period, based on the results of our previous study of 1, 3, 6, and 9 capsules per day at 3 or 6 months in which a lower dose of one capsule per day did not significantly increase EPA or DHA in breast adipose tissue [20]. In addition, doses up to 9 capsules/day were well tolerated without toxicity [20]. The dietary fish arm was designed to match the combined EPA + DHA content of the capsule dose per week through a mix of canned wild sockeye and pink salmon (Bumblebee) and pouched albacore (Starkist) based on reported n-3 PUFA content. Subjects were advised to consume no more than 1 serving of albacore per week to remain within the recommended limits of tuna/albacore for pregnant women to ensure consumption of safe amounts of mercury (http://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/Metals/UCM400358.pdf). Adverse events that occurred during the treatment period were monitored by telephone calls and monthly study visits. Canned fish was provided in monthly allotments as canned wild sockeye and pink salmon and pouched albacore to yield an estimated 11–12 g/week of n-3 fatty acids, along with sample recipes for preparation of fish meals. Unused cans were counted at study visits. Pills were provided in monthly supplies for an estimated 11.76 g/week as two capsules per day, with collection of pill bottles/remaining capsules at each monthly visit for pill counts. Participants taking capsules were instructed to follow their usual diet. At enrollment and three months, patients underwent anthropometric measurements for BMI and waist hip circumference ratio calculations. While participants and clinic personnel were not blinded to the treatment assignment, all laboratory personnel involved in sample processing and analysis were blinded to the allocation.
Breast adipose tissue samples were obtained by fine needle aspiration at enrollment and 3 months of study treatment and immediately frozen and stored at −80°C. Fasting blood samples collected at enrollment and monthly were stored as plasma and erythrocyte membrane samples at −80°C for subsequent analysis. Biospecimens were labeled with coded identifiers, and the code was not accessible to laboratory personnel conducting the analyses.
2.3. Fatty acid analyses
Total lipids were extracted from plasma and breast adipose samples with 2:1 v/v chloroform/methanol and a 0.88% KCL wash [22]. The fatty acid methyl esters were then prepared using tetramethylguandine at 100°C [23]. Erythrocyte lipids were extracted and methyl esters were prepared using boron-trifluoride in methanol [24, 25]. Analysis of fatty acid methyl esters was completed by GC with column and conditions as previously described [26]. Retention times were compared to standards (Matreya, LLC, Pleasant Gap, PA, Supelco, Bellefonte, PA, and Nu-Check Prep Inc, Elysian, MN) and fatty acids are reported as percent of total identified.
2.4. Gene expression analyses
Breast adipose tissue samples collected by fine needle aspiration at baseline and 3 months of study treatment were frozen and stored at −80°C until analysis. Expression of messenger RNA levels was analyzed by quantitative real-time PCR (Prism 7300 sequence detection system, Applied Biosystems, Foster City, CA). Total RNA from breast adipose tissue was isolated by use of an RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA) and reversed transcribed with random hexamers using MultiScribe reverse transcriptase (Applied Biosystems). After complementary DNA synthesis, real-time PCR analysis was performed with predesigned primers and probes for IL-6, prostaglandin-endoperoxide synthase 2 (PTGS2), cluster of differentiation 68 (CD68), and housekeeping control ornithine decarboxylase antizyme 1 (OAZ1) of Applied Biosystems (TaqMan Gene Expression Assays) for samples in triplicate. Target gene expression was expressed as fold change 2−ΔΔCT by the comparative CT method [27], normalized to the expression of OAZ1 RNA where ΔΔCt is the CT (posttreatment) – CT (pretreatment).
2.5. Statistical analyses
The primary objectives of the study were to determine the effects of increased fish consumption on plasma and breast fat tissue fatty acids in women at high risk for developing breast cancer relative to an n-3 PUFA supplement. A secondary objective of the study was to assess adherence and tolerability of increased dietary intake of fish relative to an n-3 PUFA supplement. The sample size was based on detecting a significant difference in breast adipose tissue fatty acid composition between the two groups (dietary fish, capsules), with the assumption of a two-sided 5% significance level. We hypothesized an effect size of Cohen’s d = 1.2 for this pilot study, which required a sample of 12 women in each arm for 80% power.
Means and SDs were used to summarize fatty acid composition of plasma, erythrocyte membrane, and breast adipose tissue, overall, and separately by group. Change scores were calculated for EPA, DHA, total n-3, and n-3/n-6 ratio as month 3 minus month 0; one-sample t-tests were used to test for overall change from months 0 to 3, pooling groups. Two-sample t-tests on these change scores were used to test for group differences in the change in EPA, DHA, total n-3, and n-3/n-6 ratio from month 0 to month 3. For secondary objectives of evaluating correlations between physical factors and target tissue effects, Pearson’s correlation was used to summarize and test the association between changes in adipose EPA and DHA and baseline BMI and age. A two-sample t-test was used to test for an association between adipose EPA and DHA and menopausal status. Secondary analyses using PTGS2 (also known as cyclooxygenase 2 or COX-2), CD68, and IL-6 gene expression levels in breast adipose tissue as the outcomes in place of fatty acid composition were performed in a similar manner. Data are presented as means ± SDs with p < 0.05 considered significantly different. Statistical analyses were performed using SAS version 9.3 (Cary, North Carolina).
3. Results
3.1. Dietary intervention with fish and capsule supplements
Women (n = 26) were randomized to either dietary fish (n = 13) or n-3 PUFA capsules at ~11.76 g EPA + DHA/wk as daily doses of 2 capsules/day (n = 13). Women in the dietary fish group consumed ~ 4 servings/wk of canned wild salmon ± pouched albacore tuna for weekly intake of EPA + DHA of ~11–12 g/wk (Table 1). One enrollee randomized to the dietary fish arm was ineligible because of ongoing endocrine therapy for breast cancer and was subsequently removed from the study. There were no dropouts. The average age was 51.9 years, without significant differences in demographic characteristics including age, menopausal status, or BMI between groups (Table 2). Most subjects were overweight (BMI>25<30) with mean BMI of 28 ± 1.9 (n = 15) and only two were obese (BMI >30) at 31 and 34.5, respectively.
Table 1.
Fatty acid content of study interventions
| Fatty acid, % total | Red Salmona) |
Pink Salmonb) |
Albacorec) | Lovaza capsule | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Light | Dark | All | Skin | Light | Dark | All | All | ||
| C18:2n6 | 2.23 ± 0.3 | 1.77 ± 0.35 | 2.05 ± 0.26 | 2.04 ± 0.19 | 1.82 ± 0.2 | 1.28 ± 0.35 | 1.87 ± 0.42 | 1.66 ± 0.09 | 2.66 ± 1.28 | 0.15 |
| C18:3n6 | 0.16 ± 0.02 | 0.13 ± 0.02 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.2 ± 0.01 | 0.18 ± 0.07 | 0.17 ± 0.003 | 0.21 ± 0.03 | 0.40 ± 0.04 | 0.18 |
| C18:3n3 | 1.08 ± 0.21 | 0.94 ± 0.18 | 1.04 ± 0.24 | 1.07 ± 0.13 | 1.18 ± 0.1 | 0.92 ± 0.16 | 1.31 ± 0.07 | 1.19 ± 0.07 | 0.37 ± 0.16 | 0.05 |
| C18:4n3 | 2.19 ± 0.87 | 1.8 ± 0.66 | 2.11 ± 0.93 | 2.10 ± 0.56 | 2.25 ± 0.54 | 1.47 ± 0.24 | 2.66 ± 0.22 | 2.09 ± 0.35 | 0.13 ± 0.02 | 2.21 |
| C20:2n6 | 0.38 ± 0.05 | 0.34 ± 0.06 | 0.40 ± 0.08 | 0.39 ± 0.04 | 0.62 ± 0.09 | 0.56 ± 0.19 | 0.54 ± 0.16 | 0.65 ± 0.03 | 0.26 ± 0.03 | |
| C20:3n6 | 0.13 ± 0.03 | 0.10 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.03 | 0.35 ± 0.06 | 0.30 ± 0.18 | 0.23 ± 0.06 | 0.32 ± 0.1 | 0.18 ± 0.02 | 0.06 |
| C20:4n6 | 0.53 ± 0.02 | 0.55 ± 0.02 | 0.52 ± 0.05 | 0.55 ± 0.06 | 1.17 ± 0.05 | 1.15 ± 0.15 | 0.92 ± 0.05 | 1.08 ± 0.14 | 4.45 ± 0.39 | 2.54 |
| C20:4n3 | 1.47 ± 0.43 | 1.31 ± 0.27 | 1.38 ± 0.41 | 1.43 ± 0.30 | 1.54 ± 0.32 | 1.26 ± 0.12 | 1.59 ± 0.27 | 1.5 ± 0.18 | 0.19 ± 0.02 | 0.97 |
| C20:5n3 | 9.05 ± 0.45 | 10.03 ± 0.92 | 9.07 ± 0.98 | 9.43 ± 0.2 | 11.65 ± 0.28 | 11.22 ± 1.36 | 11.17 ± 1.47 | 11.59 ± 0.69 | 3.27 ± 0.20 | 51.03 |
| C22:4n6 | 0.13 ± 0.03 | 0.09 ± 0.05 | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.21 ± 0.08 | 0.14 ± 0.03 | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.42 ± 0.03 | 0.35 |
| C22:5n6 | 0.11 ± 0.03 | 0.14 ± 0.04 | 0.12 ± 0.01 | 0.12 ± 0.04 | 0.23 ± 0.005 | 0.26 ± 0.01 | 0.27 ± 0.03 | 0.23 ± 0.01 | 3.73 ± 0.29 | 0.91 |
| C22:5n3 | 2.26 ± 0.37 | 2.39 ± 0.41 | 2.28 ± 0.26 | 2.30 ± 0.30 | 3.21 ± 0.36 | 3.04 ± 0.3 | 2.95 ± 0.41 | 3.27 ± 0.22 | 0.79 ± 0.12 | 2.88 |
| C22:6n3 | 11.74 ± 1.74 | 19.18 ± 2.2 | 14.05 ± 2.31 | 15.02 ± 2.17 | 22.17 ± 1.82 | 30.98 ± 11.44 | 26.40 ± 1.87 | 22.98 ± 1.07 | 35.03 ± 2.68 | 38.15 |
| All SFAd) | 26.56 ± 2.18 | 26.98 ± 2.35 | 27.56 ± 1.94 | 26.97 ± 1.52 | 28.56 ± 2.37 | 27.04 ± 1.21 | 27.45 ± 1.88 | 28.02 ± 1.67 | 29.71 ± 0.58 | 0.05 |
| All MUFA | 41.44 ± 2.40 | 33.81 ± 1.51 | 38.48 ± 1.42 | 37.67 ±1.93 | 24.46 ± 1.96 | 19.90 ± 7.15 | 21.92 ± 0.9 | 24.67 ± 1.14 | 17.96 ± 1.60 | 0.25 |
| All PUFA | 32 ± 3.60 | 39.21 ± 2.18 | 33.96 ± 3.33 | 35.36 ± 3.44 | 46.98 ± 2.36 | 53.06 ± 8.32 | 50.63 ± 0.98 | 47.30 ± 0.84 | 52.32 ± 1.86 | 99.70 |
| Total n-3 | 27.78 ± 3.19 | 35.64 ± 2.41 | 29.92 ± 2.94 | 31.34 ± 3.13 | 42.00 ± 2.40 | 48.89 ± 9.30 | 46.07 ± 1.37 | 42.62 ± 1.14 | 39.78 ± 2.36 | 95.29 |
| Total n-6 | 3.68 ± 0.41 | 3.13 ± 0.52 | 3.50 ± 0.36 | 3.50 ± 0.34 | 4.59 ± 0.08 | 3.89 ± 0.91 | 4.17 ± 0.42 | 4.32 ± 0.36 | 12.11 ± 0.77 | 4.19 |
| n3/n6e) | 7.57 ± 0.61 | 11.64 ± 2.07 | 8.56 ± 0.46 | 8.98 ± 0.68 | 9.16 ± 0.66 | 13.50 ± 5.92 | 11.11 ± 1.43 | 9.95 ± 1.16 | 3.31 ± 0.39 | 22.76 |
Values derived from three separate cans, one to three samples per can of each component plus all contents homogenized, mean ± SD.
Values derived from two separate cans, one to three samples per can of each component plus all contents homogenized, mean ± SD.
Values derived from three separate pouches, two to three samples per pouch, all contents homogenized, mean ± SD.
Total saturated, monounsaturated, polyunsaturated, omega 3, and omega 6 fatty acids as the sum of % of total fatty acids per group.
n-3 to n-6 ratio calculated as the ratio of the sum of n-3 to the sum of n-6 fatty acids, [c18:3n3 + c18:4n3 + c20:4n3 + c20:5n3 + c22:5n3 + c22:6n3]/[c18:2n6 + c18:3n6 + c20:2n6 + c20:3n6 + c20:4n6 + c22:4n6 + c22:5n6].
Table 2.
Demographics at baselinea)
| Fish (n = 12) | Capsule (n = 13) | ||
|---|---|---|---|
| Age | Mean (SD) | 51.8 (14.6) | 52.1 (13.5) |
| Range | 23–73 | 24–82 | |
| Premenopausal | N (%) | 6 (50%) | 7 (54%) |
| Race | Caucasian | 11 | 13 |
| African American | 1 | 0 | |
| BMI (kg/m2) | Mean (SD) | 26.8 (3.3) | 25.3 (5.0) |
| Range | 21.2–31.0 | 18.7–34.5 | |
| Weight (lb) | Mean (SD) | 159 (24) | 149 (35) |
| Range | 131–207 | 111–220 | |
| Height (in) | Mean (SD) | 65 (3.8) | 64 (2.6) |
| Range | 59–72.5 | 59.5–68.5 | |
| Waist-Hip ratio | Mean (SD) | 0.82 (0.07) | 0.82 (0.06) |
| Range | 0.72–0.94 | 0.73–0.91 | |
| Waist (cm) | Mean (SD) | 87.1 (8.7) | 82.8 (13.9) |
| Range | 73–100 | 63.5–103 | |
| Family history of breast cancer | N (%) | 9 (75%) | 12 (92%) |
| High risk histologyb) | N (%) | 2 (17%) | 1 (8%) |
| Personal history breast cancer | N (%) | 1 (8%) | 0 (0%) |
| Gail score>1.67, n = 20 evaluable | N | 8 | 9 |
There were no significant differences between groups, as expected due to randomization.
History of breast biopsy with atypical ductal or lobular hyperplasia or lobular carcinoma in situ.
Based on can and pill counts, the mean adherence rate for all participants was 97.9% ± 3.5. Adherence to the dietary fish regimen was higher at 99.7% by self-report compared to 96.3% by self-report and pill counts in the capsule group (p = 0.01). Women in the dietary fish arm did not report any adverse events. In the capsule group, four participants mentioned sporadic instances of flatulence, headaches, loose stools that were mild (grade 1); one woman had an episode of grade 2 abdominal pain that resolved spontaneously and appeared unrelated to the study intervention.
3.2. Dietary fish and n-3 fatty acid capsules increase plasma, erythrocyte DHA, and EPA
Plasma and erythrocyte membrane fractions of blood samples obtained at baseline and 3 months of study intervention were analyzed for fatty acid composition (Tables 3 and 4). For both treatment arms combined, plasma, and erythrocyte membrane EPA and DHA increased significantly from 0 to 3 months, with also higher total n-3 fatty acids and n-3/n-6 fatty acid ratio (p < 0.0001 for all; Fig. 1). Women taking n-3 fatty acid capsules compared to dietary fish showed greater increases in EPA in plasma (p = 0.002) and erythrocyte membranes (p = 0.04). The increase in total n-3 was also greater in women taking n-3 fatty acid capsules for plasma (p = 0.002) but not significantly greater for erythrocyte membranes (p = 0.10). However, changes in DHA content and n-3/n-6 fatty acid ratio did not differ by treatment arm for either plasma (p = 0.54, 0.06, respectively) or erythrocyte membranes (p = 0.89, 0.12, respectively).
Table 3.
Fatty acid composition of total plasma membrane lipids at baseline and 3 monthsa)
| Fatty acid | Time | Fish (n = 12) Mean ± SD | Capsules (n = 13) Mean ± SD |
|---|---|---|---|
| C14:0 | 0 | 0.86 ± 0.47 | 0.88 ± 0.36 |
| 3 | 0.63 ± 0.23 | 0.73 ± 0.26 | |
| C16:0 | 0 | 22.66 ± 3.19 | 22.68 ± 2.88 |
| 3 | 22.11 ± 2.26 | 22.39 ± 2.08 | |
| C16:1n7 | 0 | 1.78 ± 1.29 | 1.50 ± 0.6 |
| 3 | 1.39 ± 0.53 | 1.39 ± 0.62 | |
| C18:0 | 0 | 8.02 ± 1.03 | 7.66 ± 0.97 |
| 3 | 8.29 ± 0.98 | 7.70 ± 1.05 | |
| C18:1n9 | 0 | 17.41 ± 3.07 | 17.08 ± 2.33 |
| 3 | 16.21 ± 2.36 | 16.46 ± 2.48 | |
| C18:1n7 | 0 | 1.62 ± 0.21 | 1.58 ± 0.32 |
| 3 | 1.55 ± 0.21 | 1.49 ± 0.18 | |
| LA | 0 | 32.01 ± 7.06 | 33.38 ± 5.92 |
| 3 | 32.91 ± 4.54 | 32.65 ± 4.76 | |
| C18:3n6 | 0 | 0.52 ± 0.14 | 0.52 ± 0.19 |
| 3 | 0.46 ± 0.15 | 0.44 ± 0.22 | |
| C18:3n3 | 0 | 0.56 ± 0.14 | 0.58 ± 0.16 |
| 3 | 0.55 ± 0.11 | 0.58 ± 0.18 | |
| C20:2n6 | 0 | 0.25 ± 0.05 | 0.27 ± 0.07 |
| 3 | 0.25 ± 0.05 | 0.24 ± 0.08 | |
| C20:3n6 | 0 | 1.88 ± 0.49 | 1.93 ± 0.37 |
| 3 | 1.86 ± 0.53 | 1.51 ± 0.43 | |
| C20:4n3 | 0 | 0.31 ± 0.19 | 0.23 ± 0.06 |
| 3 | 0.24 ± 0.09 | 0.31 ± 0.23 | |
| AA | 0 | 8.01 ± 1.23 | 8.23 ± 1.46 |
| 3 | 7.41 ± 0.96 | 7.36 ± 1.38 | |
| EPAb) | 0 | 0.73 ± 0.43 | 0.54 ± 0.18 |
| 3 | 1.46 ± 0.88 | 2.15 ± 0.73 | |
| C22:4n6 | 0 | 0.23 ± 0.08 | 0.25 ± 0.05 |
| 3 | 0.17 ± 0.06 | 0.16 ± 0.04 | |
| C22:5n3b) | 0 | 0.72 ± 0.11 | 0.69 ± 0.18 |
| 3 | 0.82 ± 0.20 | 0.91 ± 0.17 | |
| DHAb) | 0 | 1.86 ± 0.97 | 1.52 ± 0.55 |
| 3 | 3.22 ± 1.29 | 3.08 ± 0.74 | |
| n3/n6b)’c) | 0 | 0.10 ± 0.04 | 0.08 ± 0.02 |
| 3 | 0.15 ± 7 | 0.17 ± 5 |
Values are expressed as mean % of total fatty acids ± SD.
3 month values are significantly higher than baseline,
p < 0.001.
The n-3/n-6 ratio for plasma represents the sum of [18:3n3, 20:4n3, 20:5n3, 22:5n3, 22:6n3]/sum of [18:2n6, 18:3n6, 20:2n6, 20:3n6, 20:4n6, 22:4n6].
Table 4.
Fatty acid composition of total erythrocyte membrane lipids at baseline and 3 monthsa)
| Fatty acid | Time | Fish (n = 12) Mean ± SD | Capsules (n = 13) Mean ± SD |
|---|---|---|---|
| C14:0 | 0 | 0.38 ± 0.13 | 0.39 ± 0.11 |
| 3 | 0.33 ± 0.07 | 0.39 ± 0.14 | |
| C16:0 | 0 | 24.47 ± 1.59 | 24.28 ± 2.16 |
| 3 | 23.56 ± 1.56 | 23.80 ± 2.59 | |
| C16:1n7 | 0 | 0.71 ± 0.29 | 0.62 ± 0.18 |
| 3 | 0.58 ± 0.22 | 0.65 ± 0.30 | |
| C18:0 | 0 | 19.88 ± 1.37 | 19.75 ± 1.32 |
| 3 | 19.51 ± 1.07 | 19.39 ± 1.42 | |
| C18:1n9 | 0 | 12.99 ± 0.76 | 12.81 ± 0.98 |
| 3 | 13.31 ± 1.11 | 12.96 ± 0.98 | |
| LA | 0 | 13.24 ± 1.90 | 13.76 ± 2.44 |
| 3 | 13.85 ± 2.18 | 13.61 ± 1.81 | |
| C18:3n3 | 0 | 0.19 ± 0.07 | 0.34 ± 0.44 |
| 3 | 0.18 ± 0.06 | 0.18 ± 0.07 | |
| C20:2n6 | 0 | 0.51 ± 0.35 | 0.45 ± 0.15 |
| 3 | 0.41 ± 0.08 | 0.52 ± 0.40 | |
| C20:3n6 | 0 | 1.89 ± 0.46 | 1.81 ± 0.31 |
| 3 | 1.76 ± 0.40 | 1.55 ± 0.29 | |
| AA | 0 | 15.38 ± 1.35 | 15.75 ± 0.92 |
| 3 | 14.43 ± 1.11 | 14.34 ± 2.07 | |
| EPAb) | 0 | 0.59 ± 0.28 | 0.44 ± 0.15 |
| 3 | 1.21 ± 0.53 | 1.71 ± 0.53 | |
| C22:4n6 | 0 | 3.30 ± 0.80 | 3.64 ±0.52 |
| 3 | 2.50 ± 0.51 | 2.54 ± 0.63 | |
| C22:5n6 | 0 | 0.46 ± 0.16 | 0.46 ± 0.14 |
| 3 | 0.33 ± 0.16 | 0.38 ± 0.16 | |
| C22:5n3b) | 0 | 2.37 ± 0.42 | 2.36 ± 0.64 |
| 3 | 2.56 ± 0.48 | 2.93 ± 0.23 | |
| DHAb) | 0 | 3.64 ± 1.34 | 3.13 ± 0.77 |
| 3 | 5.48 ± 1.44 | 5.03 ± 0.74 | |
| n3/n6b)’c) | 0 | 0.20 ± 0.06 | 0.18 ± 0.03 |
| 3 | 0.29 ± 0.09 | 0.30 ± 0.06 |
Values are expressed as mean % of total fatty acids ± SD.
3 month values are significantly higher than baseline,
p < 0.0001.
The n-3/n-6 ratio represents the sum of [18:3n3, 20:5n3, 22:5n3, 22:6n3]/sum of [18:2n6, 20:2n6, 20:3n6, 20:4n6, 22:4n6, 22:5n6].
Figure 1.
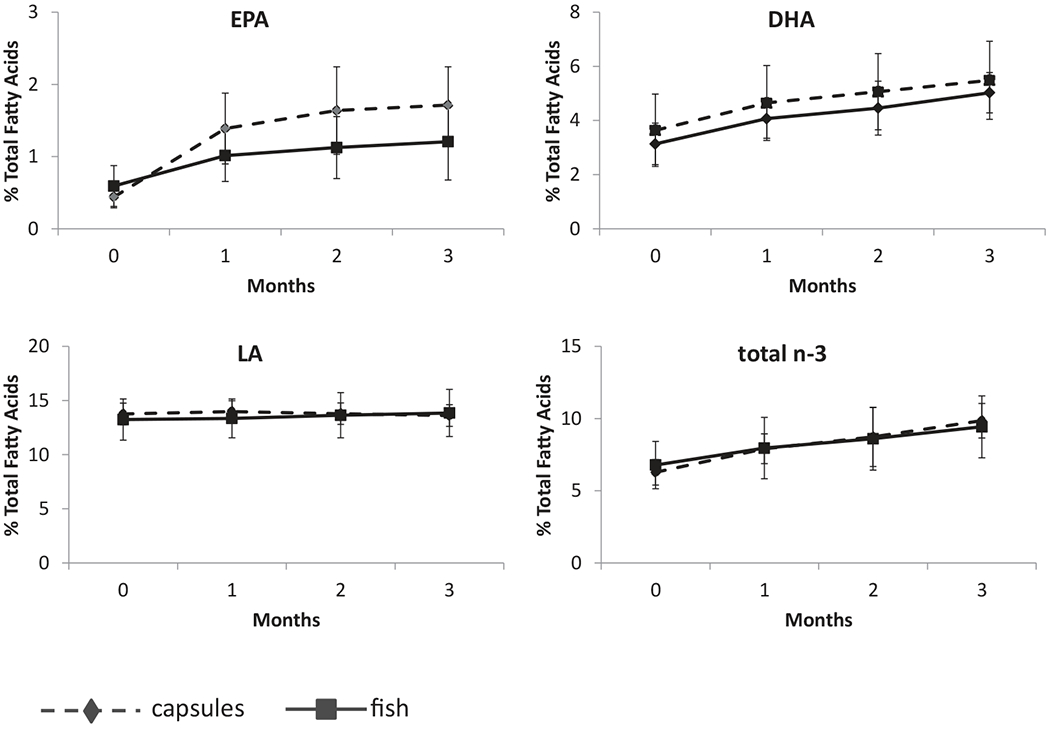
Erythrocyte membrane EPA, DHA content by treatment arm from 0 to 3 months. Mean values for DHA, EPA, and total n-3 PUFAs as a percent of total fatty acids increased each month for particpants in each study arm (n = 12 fish, n = 13 capsules) with stable linoleic acid (LA) content.
Changes in plasma and erythrocyte EPA correlated significantly with weight (p = 0.02 for both) and BMI (p = 0.03, 0.009, respectively), with higher weight and BMI associated with smaller changes in EPA (Table 6). However, changes in plasma and erythrocyte DHA were not significantly correlated with weight or BMI (p > 0.05). Neither age nor menopausal status was significantly associated with changes in EPA or DHA in either plasma or erythrocytes (p > 0.13).
Table 6.
Pearson correlations of changes in fatty acid composition of total lipids in plasma, erythrocyte membranes, and breast adipose from 0 to 3 months with baseline weight, BMI, and age
| Correlation (p-value) |
||||
|---|---|---|---|---|
| Weight | BMI | Age | ||
| Plasma | EPA Change | −0.46 | −0.42 | −0.17 |
| (0.02) | (0.03) | (0.43) | ||
| DHA Change | −0.36 | −0.30 | −0.06 | |
| (0.07) | (0.14) | (0.79) | ||
| Erythrocyte | EPA Change | −0.47 | −0.51 | −0.31 |
| (0.02) | (0.009) | (0.13) | ||
| DHA Change | −0.29 | −0.16 | 0.16 | |
| (0.16) | (0.44) | (0.46) | ||
| Adipose | EPA Change | −0.48 | −0.44 | −0.19 |
| (0.02) | (0.03) | (0.36) | ||
| DHA Change | −0.43 | −0.44 | −0.15 | |
| (0.03) | (0.03) | (0.48) | ||
Bolded values indicate statistically significant associations (p < 0.05).
3.3. Dietary fish and omega 3 fatty acid capsules increase breast adipose tissue n-3 fatty acids
Fatty acid profiling demonstrated increased EPA and DHA content of breast adipose tissue, as well as total n-3 fatty acids and n-3/n-6 fatty acid ratio, from 0 to 3 months of study intervention for both study arms (p < 0.01 for all, Table 5), without significant differences by treatment arm (p > 0.15 for all). Changes in breast adipose tissue EPA and DHA correlated significantly with weight (p = 0.02, 0.03, respectively) and BMI (p = 0.03 for both) with higher weight and BMI associated with smaller changes in EPA, DHA (Table 6). Breast adipose tissue EPA and DHA changes at 3 months were not associated with age (p = 0.36, 0.48, respectively) or menopausal status (p = 0.49, 0.69, respectively). One participant had a very large change in both EPA and DHA in adipose tissue, and exclusion of this individual from the analyses showed that the correlation with change in EPA persisted for weight (correlation = −0.51, p = 0.01) and BMI (correlation = −0.53, p = 0.01) but the change in DHA was no longer significantly associated with weight (correlation = −0.33, p = 0.11) or BMI (correlation = −0.39, p = 0.06).
Table 5.
Fatty acid composition of total lipids in breast adipose at baseline and 3 monthsa)
| Fatty acid | Time | Fish (n = 12) Mean ± SD | Capsules (n = 13) Mean ± SD |
|---|---|---|---|
| C14:0 | 0 | 2.58 ± 0.36 | 2.50 ± 0.48 |
| 3 | 2.53 ± 0.50 | 2.53 ± 0.5 | |
| C16:0 | 0 | 20.9 ± 1.90 | 20.67 ± 1.67 |
| 3 | 20.81 ± 2.33 | 20.25 ± 1.74 | |
| C16:1n7 | 0 | 3.09 ± 1.13 | 2.71 ± 0.73 |
| 3 | 2.9 ± 1.05 | 2.57 ± 0.94 | |
| C18:0 | 0 | 5.19 ± 1.23 | 5.41 ± 1.01 |
| 3 | 5.17 ± 1.28 | 5.73 ± 1.24 | |
| C18:1n9 | 0 | 43.22 ± 1.89 | 43.3 ± 1.95 |
| 3 | 43.41 ± 2.37 | 42.98 ± 2.12 | |
| C18:1n7 | 0 | 2.4 ± 0.25 | 2.27 ± 0.21 |
| 3 | 2.30 ± 0.23 | 2.23 ± 0.27 | |
| LA | 0 | 18.58 ± 2.11 | 19.21 ± 1.87 |
| 3 | 18.82 ± 2.26 | 19.56 ± 2.11 | |
| C18:3n6 | 0 | 0.11 ± 0.06 | 0.09 ± 0.02 |
| 3 | 0.09 ± 0.02 | 0.1 ± 0.02 | |
| C18:3n3 | 0 | 0.93 ± 0.10 | 1.00 ± 0.23 |
| 3 | 0.97 ± 0.15 | 1.01 ± 0.27 | |
| C20:2n6 | 0 | 0.26 ± 0.06 | 0.26 ± 0.05 |
| 3 | 0.26 ± 0.06 | 0.25 ± 0.05 | |
| C20:3n6 | 0 | 0.29 ± 0.1 | 0.27 ± 0.11 |
| 3 | 0.26 ± 0.06 | 0.24 ± 0.1 | |
| C20:4n6 | 0 | 0.45 ± 0.14 | 0.42 ± 0.16 |
| 3 | 0.40 ± 0.09 | 0.45 ± 0.26 | |
| EPAb) | 0 | 0.04 ± 0.02 | 0.04 ± 0.02 |
| 3 | 0.06 ± 0.04 | 0.10 ± 0.12 | |
| C22:4n6 | 0 | 0.16 ± 0.06 | 0.15 ± 0.07 |
| 3 | 0.15 ± 0.04 | 0.15 ± 0.07 | |
| C22:5n3 | 0 | 0.3 ± 0.1 | 0.26 ± 0.11 |
| 3 | 0.31 ± 0.1 | 0.32 ± 0.11 | |
| DHAb) | 0 | 0.16 ± 0.07 | 0.13 ± 0.08 |
| 3 | 0.20 ± 0.11 | 0.21 ± 0.18 | |
| n3/n6b),c) | 0 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| 3 | 0.08 ± 0.01 | 0.08 ± 0.02 |
Values are expressed as mean % of total fatty acids ± SD.
3 month values are significantly higher than baseline, p < 0.01.
The n-3/n-6 ratio represents the sum of [18:3n3, 20:5n3, 22:5n3, 22:6n3]/sum of [18:2n6, 18:3n6, 20:2n6, 20:3n6, 20:4n6, 22:4n6].
At baseline, plasma and erythrocyte membrane and breast adipose tissue samples all significantly correlated for EPA, DHA, total n-3 PFUAs, n-3/n-6 ratio (all r > 0.4, p < 0.05), with the exception of plasma and breast adipose tissue total n-3 PUFAs (correlation = 0.22, p = 0.29). For changes in EPA, DHA, total n-3 PUFAs, and n-3/n-6 ratio, plasma and erythrocyte membranes were significantly correlated (all r > 0.6, p < 0.05), but breast adipose tissue correlated with plasma and erythrocyte membranes for only EPA and n-3/n-6 ratio (not DHA or total n-3 PUFAs). Using the n-3 index (EPA + DHA) as a biomarker of n-3 PUFA exposure, the change in breast adipose tissue and change in plasma n-3 index correlated marginally (r = 0.40, p = 0.05) but not with change in erythrocyte membranes (r = 0.30, p = 0.14) (Table 7). To ensure that these correlations were not driven by the one outlying subject with very large increases in adipose tissue EPA and DHA, we also computed Spearman (nonparametric) correlations (Table 7). The Spearman correlations showed stronger associations between changes in plasma and erythrocyte n-3 index and changes in breast adipose EPA, DHA, and n-3 index. Neither plasma nor erythrocyte n-3 index was significantly correlated with changes in breast adipose tissue total n-3 or n-3/n-6 ratio (Table 7).
Table 7.
Correlations between changes in erythrocyte and plasma n-3 index and changes in breast adipose n-3 index
| Change in adipose: | Pearson correlations |
Spearman correlations |
||
|---|---|---|---|---|
| Change in plasma n-3 index | Change in RBC n-3 index | Change in plasma n-3 index | Change in RBC n-3 index | |
| EPA | 0.43 | 0.33 | 0.50 | 0.41 |
| 0.03 | 0.11 | 0.01 | 0.04 | |
| DHA | 0.36 | 0.27 | 0.57 | 0.49 |
| 0.08 | 0.19 | 0.003 | 0.01 | |
| Total n-3 | 0.21 | 0.17 | 0.20 | 0.22 |
| 0.32 | 0.43 | 0.34 | 0.29 | |
| n-3/n-6 Ratio | 0.25 | 0.21 | 0.20 | 0.19 |
| 0.22 | 0.32 | 0.34 | 0.36 | |
| n-3 Index | 0.40 | 0.30 | 0.68 | 0.56 |
| 0.05 | 0.14 | 0.0002 | 0.004 | |
Values are correlations and p-values. RBC = erythrocyte.
3.4. COX-2, CD68, and IL-6 gene expression levels in breast adipose tissue do not change with dietary ω-3 fatty acids as fish or capsules
Breast adipose tissue samples obtained via fine needle aspiration were processed for total RNA to assess expression of genes related to proinflammatory signaling responsive to dietary n-3 PUFAs, namely COX-2, CD68 as a macrophage marker, and cytokine IL-6 [2, 28, 29]. By quantitative RT-PCR, COX-2, CD68, and IL-6 expression in breast adipose tissue did not significantly change at 3 months of the study intervention; from 0 to 3 months, average fold change values pooling across groups were not significantly different from 1 (p > 0.10 for all tests, Fig. 2). Mean fold change values for COX-2 were 1.4 ± 2.0 and 3.0 ± 5.8 in the dietary fish and n-3 capsule groups, respectively; there was one outlier with a fold change of 21.1 in the capsule group whose exclusion reduced that group mean to 1.5 ± 2.2. Mean fold change values for CD68 were 1.1 ± 0.38 and 1.1 ± 0.43 in the dietary fish and capsule groups, respectively. Mean fold change values for IL-6 were 1.2 ± 0.60 and 3.4 ± 5.9 in the dietary fish and capsule groups, respectively; there was one outlier with a fold change of 22.1 in the capsule group whose exclusion reduced the group mean to 1.8 ± 1.8.
Figure 2.
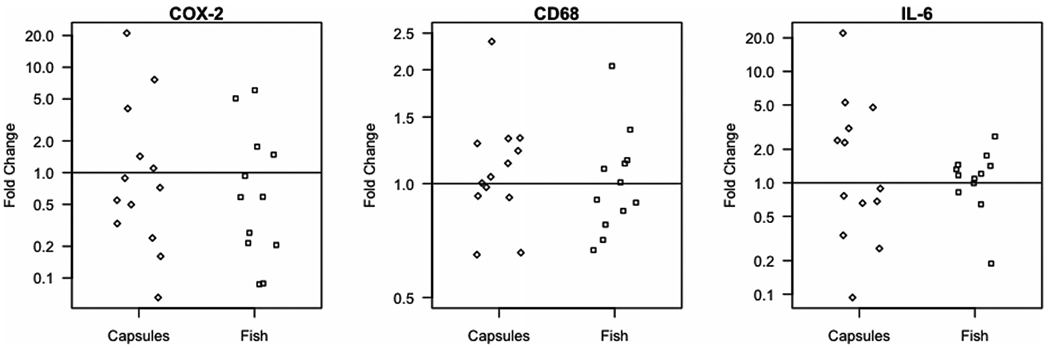
Changes in COX-2, CD68, IL-6 expression in breast adipose tissue from 0 to 3 months. By qRT-PCR, COX-2, CD68, and IL-6 expression in breast adipose tissue samples obtained at baseline and 3 months of study intervention did not differ by study arm. Average fold change values are shown in a log scale; each point represents one participant.
4. Discussion
This study shows that dietary fish and n-3 PUFA capsule supplements both increased EPA and DHA content of breast adipose tissue over 3 months without significant differences between treatment arms. On average, breast adipose EPA and DHA increased 2- and 1.5-fold, respectively, at the end of study. Plasma and erythrocyte EPA and DHA content also increased significantly with both canned fish and n-3 PUFA capsules. Although prior reports have established that consumption of fish and fish oil capsules are generally equivalent means of increasing n-3 fatty acid content of erythrocyte membranes and plasma phospholipids [24, 30], we demonstrate for the first time that both routes of supplementation also significantly raise EPA and DHA in breast fat without clear superiority of capsule or fish consumption at dose levels of ~ 11–12 g/wk.
The strong correlation of plasma, erythrocyte membrane, and breast adipose tissue EPA, DHA, total n-3 PUFAs, n-3/n-6, and n-3 index (except for breast adipose tissue and plasma total n-3 PUFAs) at baseline is consistent with stable dietary and supplement patterns at study entry. Correlation of changes in breast adipose and plasma, erythrocyte membrane might strengthen with larger sample size and/or longer duration of study intervention given the smaller scale changes in n-3 PFUAs in adipose tissue compared to plasma or erythrocyte membranes.
The exploratory analyses of pro-inflammatory gene expression (i.e. IL-6, CD68, and COX-2) in breast adipose tissue samples showed no significant differences by time point or study arm, which may relate to the relatively small sample size of this trial. The short term nature of the intervention and extent of fatty acid changes achievable with the study doses over 3 months may also contribute to the lack of effect on these selected proinflammatory markers, although the study interventions might not elicit changes in expression of the selected genes regardless of the length of time. Additionally, participants were generally healthy without underlying inflammatory conditions, such as diabetes or atherosclerosis, and with variable risk of developing breast cancer. We and others have previously noted a lack of effect of fish oil/n-3 fatty acid capsules or fish diets on serum cytokines in healthy volunteers, which may relate to a low inflammatory status, compared to subjects with inflammation-based diseases [20, 31, 32].
In addition to the relatively small sample size, another limitation of the study relates to the differences in the relative amounts of EPA, DHA provided by each intervention, despite efforts to balance dietary fish and capsule treatments for total EPA + DHA/n-3 PUFAs. The n-3 fatty acid capsules provided a higher EPA to DHA ratio than the dietary fish intervention of salmon and tuna. Indeed, fatty acid analysis of erythrocyte membrane and plasma phospholipids showed ~ twofold higher EPA in participants taking capsules versus dietary fish. However, breast adipose tissue triglycerides did not significantly differ by treatment arm for EPA and/or DHA content at 3 months. These differences in EPA content in breast adipose tissue versus erythrocyte membranes, plasma may relate also to the differential processing of EPA, DHA, with increased incorporation of EPA into cholesterol esters and decreased esterification in triacylglycerol relative to DHA in plasma versus fat [33], as well as greater lag time for changing the fatty acid composition of specific fat depots. The bioavailability of EPA and DHA as ethyl esters (i.e. Lovaza) differs from the triacyglycerol forms provided by fish, although this might favor higher absorption in the dietary fish group [34]. Differences in EPA, DHA uptake may also depend on the tissue or cell type, duration of treatment, and continuous versus intermittent administration. Indeed, a recent study of EPA + DHA supplements daily or twice weekly that showed higher plasma EPA and DHA with continuous dosing for the first months of the study that did not persist to the end of study at 12 months as well as significantly higher platelet and mononuclear cell EPA, DHA at one year [35].
Despite excellent reported adherence to a regimen of four 6 oz servings of a combination of canned salmon and pouched albacore per week to achieve ~ 11–12 g EPA+DHA/wk, consumption of dietary fish at this level and formulation (canned, not fresh) may not be as well accepted on a long-term basis. Although monitoring of adherence to the dietary fish intervention relied on self-report, the study findings support a significant change in dietary intake of marine long chain fatty acids. End of study questionnaires (data not shown) indicated that while a few participants in the dietary fish arm could envision continuing with dietary fish at this high level of consumption, the majority of women in both groups expressed willingness to adhere to a combination of fish and fish oil/n-3 PUFA capsules. We utilized an ambitious regimen of canned fish for this short-term intervention study and envision future studies of dietary fish employing a wider array of fresh and canned options over longer time periods. Fish as a component of interventions examining dietary patterns, rather than single nutrient factors [27], may prove critical to optimizing dietary strategies for breast cancer prevention.
In contrast to fish oil/n-3 fatty acid supplements, fish contains other nutrients including vitamin D and selenium and provides a low fat source of protein with the potential for additional health benefits by additive, synergistic effects on n-3 PUFA-mediated processes or by alternative mechanisms. For example, in healthy volunteers randomized to salmon fillets or salmon oil capsules for eight weeks, increases in erythrocyte long chain n-3 fatty acids were similar for fish and capsule interventions with significantly greater increases in plasma selenium in subjects consuming salmon fillets [36]. The risk of contaminants such as mercury, PCBs, dioxins appears low in general with fish as well as fish oil supplements [37, 38].
Taken together, our study data demonstrate the effectiveness of both dietary fish and n-3 PUFA supplements to increase breast adipose tissue EPA, DHA in women at high risk for developing breast cancer. By showing the feasibility of modulating breast adipose tissue EPA and DHA by a major change in the consumption of fish, this trial serves as the foundation for development of dietary strategies as well as fish oil/n-3 PUFA supplements to increase n-3 fatty acids in mammary tissue for future breast cancer prevention trials.
Acknowledgments
This research was supported by grants from the Seafood Industry Research Fund (L.D.Y.) and Food Innovation Center, The Ohio State University (L.D.Y.). We gratefully acknowledge the commitment of the study participants and support of the Pharmacy and Surgical Oncology Clinic staff at the Stefanie Spielman Comprehensive Breast Center, James Cancer Hospital. We thank Dr. Susan Olivo-Marston for the sample size calculation and randomization scheme.
Abbreviations:
- CD68
cluster of differentiation 68
- COX-2
cyclooxygenase 2
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- OAZ1
ornithine decarboxylase antizyme 1
- PTGS2
prostaglandin-endoperoxide synthase 2
Footnotes
The authors have declared no conflict of interest.
References
- [1].Yee LD, Young DC, Rosol TJ, VanBuskirk AM et al. , Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neuinduced breast cancer in mice independently of the PPARγ ligand rosiglitazone. J. Nutr. 2005, 135, 983–988. [DOI] [PubMed] [Google Scholar]
- [2].Yee LD, Agarwal D, Rosol TJ, Lehman A et al. , The inhibition of early stages of HER-2/neu-mediated mammary carcinogenesis by dietary n-3 polyunsaturated fatty acids. Mol. Nutr. Food Res. 2012, 57, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Larsson SC, Kumlin M, Ingelman-Simdberg M, Wolk A, Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [DOI] [PubMed] [Google Scholar]
- [4].Calder PC, Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [DOI] [PubMed] [Google Scholar]
- [5].Simopoulos AP, Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [DOI] [PubMed] [Google Scholar]
- [6].Kris-Etherton PM, Harris WS, Appel LJ, AHA scientific statement. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [DOI] [PubMed] [Google Scholar]
- [7].Karmali RA, Marsh J, Fuchs C, Effect of omega-3 fatty acids on growth of a rat mammary tumor. J. Natl. Cancer Inst. 1981, 73, 457–461. [DOI] [PubMed] [Google Scholar]
- [8].Rose DP, Connolly JM, Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J. Natl. Cancer Inst.1993, 85, 1743–1747. [DOI] [PubMed] [Google Scholar]
- [9].Cave WT, Omega-3 polyunsaturated fatty acids in rodent models of breast cancer. Breast Cancer Res.Treat. 1997, 46, 239–246. [DOI] [PubMed] [Google Scholar]
- [10].Iyengar NM, Hudis CA, Gucalp A, Omega-3 fatty acids for the prevention of breast cancer: an update and state of the science. Curr. Breast Cancer Rep. 2013, 5, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simopoulos AP, Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [DOI] [PubMed] [Google Scholar]
- [12].Maki KC, Slavin JL, Tains TM, Kris-Etherton PM, Limitations of observational evidence: implications for evidence-based dietary recommendations. Adv. Nutr 2014, 5, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lattkaa E, Illiga T, Koletzkob B, Heinrich J, Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr. Opin. Lipidol. 2010, 21, 64–69. [DOI] [PubMed] [Google Scholar]
- [14].Buckland G, Travier N, Cottet V, Gonź alez, C. A. et al. , Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [DOI] [PubMed] [Google Scholar]
- [15].Cleland LG, French JK, Betts WH, Murphy GA et al. , Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J. Rheumatol. 1988, 15, 1471–1475. [PubMed] [Google Scholar]
- [16].Burns CP, Halabi S, Clamon G, Kaplan E et al. , Phase II study of high-dose fish oil capsules for patients with cancerrelated cachexia-a Cancer and Leukemia Group B Study. Cancer 2004, 101, 370–378. [DOI] [PubMed] [Google Scholar]
- [17].Burr ML, Fehily AM, Gilbert JF, Rogers S et al. , Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [DOI] [PubMed] [Google Scholar]
- [18].Investigators of the GISSI-Prevenzione trial, Dietary supplementationwith n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [PubMed] [Google Scholar]
- [19].Singh RB, Niaz MA, Sharma JP, Kumar R et al. , Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival–4. Cardiovasc. Drugs Ther. 1997, 11, 485–491. [DOI] [PubMed] [Google Scholar]
- [20].Yee LD, Lester JL, Cole RM, Richardson JR et al. , Omega 3 fatty acid supplements in women at high risk for breast cancer show dose dependent effects on breast adipose fatty acid composition. Am. J. Clin. Nutr. 2010, 91, 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ, Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl. Cancer Inst. 1989, 81, 1879–1886. [DOI] [PubMed] [Google Scholar]
- [22].Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [PubMed] [Google Scholar]
- [23].Shantha NC, Decker EA, Hennig B, Comparison of methylation methods for the quantitation of conjugated linoleic acid. J. AOAC Int. 1993, 76, 644–649. [Google Scholar]
- [24].Harris WS, Pottala JV, Sands SA, Jones PG, Comparison of the effects of fish and fish-oil capsules on the n-3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr. 2007, 86, 1621–1625. [DOI] [PubMed] [Google Scholar]
- [25].Morrison WR, Smith LM, Preparation of fatty acidmethyl esters and dimethylacetals from lipids in boron fluoridemethanol. J. Lipid Res. 1964, 5, 600–608. [PubMed] [Google Scholar]
- [26].Belury MA, Kempa-Steczko A, Dietary conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids 1997, 32, 199–204. [DOI] [PubMed] [Google Scholar]
- [27].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real time quantitative PCR and the 2 –__CT method. Methods 2001, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- [28].Guebre-Egziabher F, Debard C, Drai J, Denis L et al. , Differential dose effect of fish oil on inflammation and adipose tissue gene expression in chronic kidney disease patients. Nutrition 2013, 29, 730–736. [DOI] [PubMed] [Google Scholar]
- [29].Saraswathi V, Gao L, Morrow JD, Chait A et al. , Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J. Nutr. 2007, 137, 1776–1782. [DOI] [PubMed] [Google Scholar]
- [30].Thorsdottir I, Tomasson H, Gunnarsdottir I, Gisladottir E et al. , Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int. J. Obes. 2007, 31, 1560–1566. [DOI] [PubMed] [Google Scholar]
- [31].Grieger JA, Miller MD, Cobiac L, Investigation of the effects of a high fish diet on inflammatory cytokines, blood pressure, and lipids in healthy older Australians. Food Nutr. Res 2014, 58, 20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khorsan R, Crawford C, Ives JA, Walter AR, Jonas WB, The effect of omega-3 fatty acids on biomarkers of inflammation: a rapid evidence assessment of the literature. Military Med. 2014, 179, 2–60. [DOI] [PubMed] [Google Scholar]
- [33].Sadon H, Leger CL, Descomps B, Barjon J-N et al. , Differential incoroporation of fish-oil eicosapentaenoate and docosahexaenoate into lipids of lipoprotein fractions as related to their glyceryl esterifications: a short-term (postprandial) and long-term study in healthy humans. Am. J. Clin. Nutr. 1995, 62, 1193–1200. [DOI] [PubMed] [Google Scholar]
- [34].Dyerberg J, Madsen P, Møller JM, Aardestrup I, Schmidt EB, Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essen. Fatty Acids 2010, 83, 137–141. [DOI] [PubMed] [Google Scholar]
- [35].Browning LM, Walker CG, Mander AP, West AL et al. , Compared with daily, weekly n-3 PUFA intake affects the incorporation of eicosapentaenoic acid and docosahexaenoic acid into platelets and mononuclear cells in humans. J. Nutr. 2014, 144, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stonehouse W, Pauga MR, Kruger R, Thomson CD et al. , Consumption of salmon v. salmon capsules: effects on n-3 PUFA and selenium status. Br. J. Nutr. 2011, 106, 1231–1239. [DOI] [PubMed] [Google Scholar]
- [37].Mozaffarian D, Rimm EB, Fish intake, contaminants, and human health—evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [DOI] [PubMed] [Google Scholar]
- [38].Shim SM, Dorworth LE, Lasrado JA, Santerre CR, Mercury and fatty acids in canned tuna, salmon, and mackerel. J. Food Sci. 2004, 69, c681. [Google Scholar]


