Abstract
Objective
Prenatal opioid exposure has been linked with impaired cognitive development, with boys potentially at elevated risk. In the present study, we examined cognitive and language development of children prenatally exposed to opioids, with an additional focus on sex differences.
Methods
A sample of 378 children (n = 194 girls and n = 184 boys) aged 1.2–42.8 months was drawn from the Danish Family Outpatient Clinic database. Developmental outcomes were assessed using the Bayley-III cognitive and language scales, and substance exposure was determined with urine screening and/or verbal report. Children exposed to opioids (n = 94) were compared to children with no prenatal substance exposure (n = 38), and children exposed to alcohol (n = 131) or tobacco (n = 115). Group and sex differences were investigated with separate linear mixed models for each Bayley scale, controlling for concurrent cannabis exposure.
Results
There were significantly reduced scores in opioid-exposed boys compared to boys with no prenatal substance exposure, but no difference between opioid-exposed and nonexposed girls. Additionally, alcohol-exposed boys had lower cognitive scores than nonexposed boys, and alcohol-exposed girls had lower scores on both scales compared to opioid-exposed girls. There were otherwise no significant differences according to group, sex, or scale.
Conclusions
The present findings indicate poorer cognitive and language development in boys after prenatal opioid exposure. As academic performance is rooted in cognitive functioning, long-term follow-up might be necessary for exposed children.
Keywords: cognitive development, language development, prenatal opioid exposure, sex differences
Introduction
Opioid maintenance treatment (OMT) has played a large part in improving outcomes for opioid-dependent patients, and current guidelines recommend maintenance treatment with methadone or buprenorphine for pregnant opioid-dependent women (World Health Organization, 2014). Yet, there is increasing evidence of poorer health and development in children prenatally exposed to opioids. Opioids cross the placenta and the blood–brain barrier of the developing fetus (Rosen & Pippenger, 1975; Syme, Paxton, & Keelan, 2004). In the neonate, prenatal methadone or buprenorphine exposure is associated with lower birth weight (e.g., Nørgaard, Nielsson, & Heide-Jørgensen, 2015; Patrick et al., 2015), lower average gestational age at birth (e.g., Nørgaard et al., 2015; Sarfi, Martinsen, Bakstad, Røislien, & Waal, 2009), and smaller head circumference (e.g., Chasnoff, Hatcher, & Burns, 1980; Lifschitz, Wilson, Smith, & Desmond, 1985) compared to nonexposed groups. Neonatal abstinence syndrome (NAS) occurs in around 50% of exposed infants (Reddy, Davis, Ren, & Greene, 2017), although the reported prevalence varies between studies.
Children exposed to illicit opioids and polysubstances in utero also demonstrate lower levels of general cognitive (Baar & Graaff, 1994; Nygaard, Moe, Slinning, & Walhovd, 2015) and fine-motor functioning (Bunikowski et al., 1998; Nygaard, Slinning, Moe, & Walhovd, 2017) throughout childhood and adolescence, as well as regulatory problems (Nygaard, Slinning, Moe, & Walhovd, 2016; Ornoy, Segal, Bar-Hamburger, & Greenbaum, 2001). Existing evidence suggests that toddlers prenatally exposed to methadone or buprenorphine are at risk for some of the same neurodevelopmental impairments, including poorer gross and fine-motor functioning (Hans & Jeremy, 2001; Strauss, Starr, Ostrea, Chavez, & Stryker, 1976), language abilities (Baar & Graaff, 1994; Beckwith & Burke, 2015; Salo et al., 2009), and cognitive functioning (Baar & Graaff, 1994; Bauman & Levine, 1986; Beckwith & Burke, 2015; Hans & Jeremy, 2001; Salo et al., 2010). In spite of these differences, opioid-exposed children usually score within the range of age-appropriate norms (Nygaard et al., 2017; Strauss et al., 1976). Moreover, in utero exposure to opioids is associated with less severe outcomes compared to in utero exposure to alcohol, which has been consistently linked with impaired cognitive functioning (Behnke, Smith, Committee on Substance Abuse, & Committee on Fetus and Newborn, 2013; Mattson & Riley, 1998). Prenatal tobacco exposure may also have detrimental effects on development, though to a lesser extent than alcohol (Behnke et al., 2013), thus the high prevalence of cigarette smoking reported for women in OMT (e.g., Mactier, Shipton, Dryden, & Tappin, 2014) may confound outcomes associated with in utero opioid exposure.
Prenatal opioid exposure may affect boys and girls differently (Alaedini, Haddadi, & Asadian, 2017). One early study of children exposed to methadone in utero showed that boys scored significantly lower than girls on the Bayley-II mental development index, which assesses early cognitive and language development jointly, at 1 and 2 years of age (Suffet & Brotman, 1984). In a more recent article (Nygaard et al., 2015), differences in cognitive abilities between opioid-exposed and nonexposed children (lower in exposed children) were substantially larger for boys than for girls the first few years of life, but group differences increased over time for girls, while they remained relatively stable for boys. Boys also appear to be more adversely impacted than girls after prenatal exposure to cocaine (Kestler, Bennett, Carmody, & Lewis, 2012). Thus, boys could be at greater risk after in utero opioid exposure during the first few years of life, although further evidence is needed to evaluate this claim.
Prescription opioid abuse among pregnant women is on the rise (Krans & Dunn, 2014; Reddy et al., 2017), with rates as high as 29% in some populations (Epstein et al., 2013). This is particularly the case for pain-management opioids, though these are seldom included in studies of the effects of prenatal exposure. Therefore, the aim of the present study was to compare the performance of children exposed to opioid analgesics and/or maintenance medication in utero to that of comparison groups with other or no in utero substance exposure on the Bayley-III cognitive and language scales. Children with additional exposure to heroin were also included, but constituted a very small minority of the sample. We hypothesized that opioid-exposed children would perform worse than children with no prenatal substance exposure, as well as tobacco-exposed children, but better than alcohol-exposed children. Group differences between opioid-exposed and nonexposed children were expected to be larger for boys than girls. The current study adds to the literature on cognitive outcomes after prenatal opioid exposure by including opioid analgesics, in addition to maintenance medication and heroin. Moreover, our sample size exceeds that of most previous studies, increasing our power to detect small group differences. Our results will thus aid our understanding of sex differences in cognitive and language development after prenatal opioid exposure.
Methods
Setting
This is a historical cohort study where we analyzed data collected by the regional Danish Family Outpatient Clinics (FOCs) over the course of 7 years. The FOCs provide medical care and guidance primarily for pregnant women, who either have or have had (within 2 years of pregnancy) a substance abuse problem, and their children (Sundhetsstyrelsen, 2015). Care is provided through an interdisciplinary approach by a team consisting of medical specialists (e.g., pediatricians and obstetricians), midwives, psychologists, social workers, and secretaries, on an outpatient basis. Such care may include regular checkups during pregnancy, detoxification or maintenance treatment, treatment and care of the neonate, and family support during pregnancy and after birth. The child may attend regular examinations by a doctor and a psychologist until it reaches school age. Children without prenatal substance exposure, but where the mother has or has had problems related to substance use, are also eligible for follow-up if deemed necessary by the FOCs. As the FOCs’ aim is to be the main health care providers for pregnant women with substance abuse problems in Denmark, the database is likely to include the majority of this population, thus constituting a highly representative sample. Data collection for the database was approved by the Danish Data Protection Agency. The usual requirement of informed consent was waived by both the Danish Data Protection Agency and the Norwegian Regional Committees for Medical and Health Research Ethics. For in-depth information on the work of the FOCs, please see Sundhetsstyrelsen (2015).
Participants
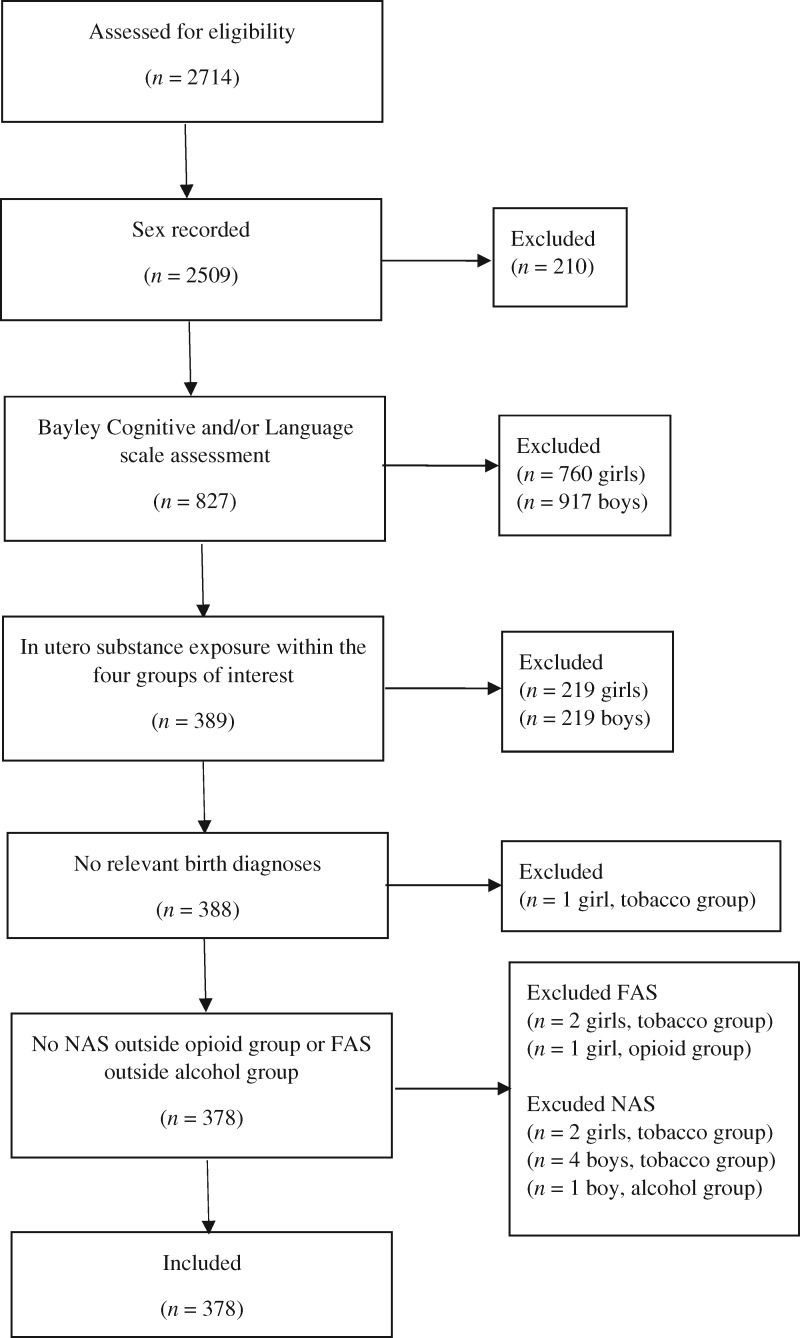
The present sample of 378 mother–child dyads (including 1 twin pair) was drawn from the FOC database, which at the time of analysis contained information about the families who had been assisted by the regional FOCs between 2010 and 2016. Participants were selected based on the following inclusion criteria: (a) A recorded child sex, (b) child assessment on the Bayley-III cognitive and/or language subscales, (c) information indicating either absence of prenatal substance exposure or exposure to tobacco, alcohol, and/or opioids, and (d) no registered medical diagnoses that could affect cognitive outcomes (see Figure 1). Moreover, children diagnosed with NAS were excluded from all but the opioid exposure group, and children diagnosed with fetal alcohol syndrome (FAS) were excluded from all but the alcohol exposure group. While NAS can occur in response to a variety of substances, it is most commonly and consistently reported after prenatal opioid exposure. Children were divided into four groups according to exposure: (a) prenatal exposure to opioids (no further exclusion criteria), (b) prenatal exposure to alcohol (and not opioids), (c) prenatal exposure to tobacco (and not alcohol or opioids), and (d) no prenatal substance exposure, but mothers who had been in contact with the FOCs about substance use problems within 2 years of the pregnancy.
Figure 1.
Selection process. Abbreviations: FAS=fetal alcohol syndrome; NAS=neonatal abstinence syndrome. Numbers are referring to children in the database. The child excluded due to relevant birth diagnoses was diagnosed with sepsis.
Substance exposure was determined by verbal report and/or urine screening (Table I). As most exposure was determined by the former, it is likely that substance use was underreported. Neonatal abstinence syndrome was diagnosed according to Finnegan’s Neonatal Abstinence Scoring System (Finnegan, Connaughton, Kron, & Emich, 1975) and treated with morphine (n = 13) and phenobarbital (n = 3). For nine children in the opioid group, the specific type of opioid could not be identified, but these were not excluded from analyses. Fetal alcohol syndrome (FAS) was diagnosed by specialist pediatricians, and five children in the alcohol-exposed group were diagnosed with FAS. Median age at testing was 10.4 months (range 1.2–42.8 months) for the cognitive scale and 9.4 months (range 1.2–35.7 months) for the language scale.
Table I.
Substance Exposure per Exposure Group/Method of Confirming Positive Exposure
| Prenatal opioid exposure n (% of group)(n = 94) | Prenatal alcohol exposure n (% of group)(n = 131) | Prenatal tobacco exposure n (% of group)(n = 115) | Verbal confirmation n (% of substance) | Positive urine screening n (% of substance) | |
|---|---|---|---|---|---|
| Alcohol | 3 (3.2) | 131 (100.0) | NA | 134 (100.0) | NA |
| Tobacco | 29 (30.9) | 58 (44.3) | 115 (100.0) | 203 (100.0) | NA |
| Opioids | |||||
| Buprenorphine | 5 (5.3) | NA | NA | 5 (100.0) | 1 (20.0) |
| Codeine | 6 (6.4) | NA | NA | 6 (100.0) | 1 (16.7) |
| Fentanyl | 2 (2.1) | NA | NA | 2 (100.0) | 0 (0.0) |
| Heroin | 1 (1.1) | NA | NA | 1 (100.0) | 1 (100.0) |
| Ketobemidone | 2 (2.1) | NA | NA | 2 (100.0) | 0 (0.0) |
| Methadone | 29 (30.9) | NA | NA | 24 (82.8) | 11 (37.9) |
| Morphine | 24 (25.5) | NA | NA | 24 (100.0) | 5 (20.8) |
| Oxycodone | 5 (5.3) | NA | NA | 5 (100.0) | 3 (60.0) |
| Tramadol | 27 (28.7) | NA | NA | 27 (100.0) | 2 (7.4) |
| Other substances | |||||
| Amphetamine | 3 (3.2) | 14 (10.7) | 5 (4.3) | 19 (86.4) | 3 (13.6) |
| Antidepressants | 8 (8.5) | 1 (0.8) | 4 (3.5) | 13 (100.0) | NA |
| Antipsychotics | 2 (2.1) | 0 (0.0) | 0 (0.0) | 2 (100.0) | NA |
| Benzodiazepines (prescribed and illicit) | 10 (10.6) | 1 (0.8) | 6 (5.2) | 13 (76.5) | 6 (35.3) |
| Cannabis | 15 (16.0) | 36 (27.5) | 49 (42.6) | 84 (84.0) | 50 (50.0) |
| Cocaine | 1 (1.1) | 6 (4.6) | 0 (0.0) | 6 (85.7) | 1 (14.3) |
| Ecstasy/MDMA | 2 (2.1) | 2 (1.5) | 0 (0.0) | 3 (75.0) | 1 (25.0) |
| Psychotropic medication (prescribed) | 1 (1.1) | 2 (1.5) | 4 (3.5) | 7 (100.0) | NA |
Abbreviations: MDMA=3.4-methylenedioxymethamphetamine; NA–not applicable. Note. Urine screening was performed for amphetamine, benzodiazepines, cannabis, cocaine, ecstasy, and opioids (including separate tests for buprenorphine, methadone, and tramadol). The no-exposure group is not included, as no children in this group had any evidence of prenatal substance exposure.
Outcome
The cognitive and language scales of the Bayley Scales of Infant and Toddler Development (Bayley-III; Albers & Grieve, 2007) were used to assess child cognitive and language development, respectively. The Bayley-III consists of a standard variety of measurements intended to evaluate development in infants and toddlers and is one of the most widely used tools for this purpose. Both the cognitive and language scales are demonstrated to have good reliability and validity across age groups (Albers & Grieve, 2007). In terms of predictive validity, Bode, D’Eugenio, Mettelman, and Gross (2014) found that Bayley-III cognitive and language scores at 2 years of age were predictive of Weschler Preschool and Primary Scale of Intelligence-III scores at 4 years of age, with correlations of .81 and .78, respectively. However, other studies find limited predictive validity of the Bayley-III (Månsson, Stjernqvist, Serenius, Ådén, & Källén, 2019; Spencer-Smith, Spittle, Lee, Doyle, & Anderson, 2015).
In the present analysis, we present age-corrected standardized scores (mean = 100, SD = 15), which have a possible range between 55 and 145 for the cognitive scale and 47 and 153 for the language scale. Most of the children (n = 218) were tested at least twice, up to a maximum of 7 times (n = 1), yielding a total of n = 747 test administrations on the cognitive scale and n = 633 test administrations on the language scale.
Analyses
Effects of in utero substance exposure were examined separately for Bayley-III cognitive and language scores in linear mixed models, with factors Exposure Group, Sex, Group × Sex, and Prenatal Cannabis Exposure, and covariates Age and Maternal Education as fixed effects regressors. Prenatal cannabis exposure was added as a control variable due to the potential association between in utero cannabis exposure and adverse neonatal and cognitive outcomes (Calvigioni, Hurd, Harkany, & Keimpema, 2014; Huizink, 2014). The mixed model allows for controlling for variable recurrences of repeated measures. As 175 children lacked data on maternal education, and as age was lacking for eight test administrations, multiple imputation was used to provide estimates for the missing values, and subsequent analyses were performed with pooled estimates for 200 imputations. This was preferred to conducting the analyses without controlling for these additional variables, as maternal socioeconomic status may be an important factor for child development (McLoyd, 1998). The main objective was to compare the exposure groups with the nonexposed group, thus the latter was set to 0 in the models. Analyses were also repeated with the opioid group set to 0, in order to explore differences between this and other exposure groups. Effects were evaluated at α = .05. Imputation and analyses were performed in IBM SPSS Statistics 25.
Results
Sample characteristics are displayed in Table II, and group means and SDs for Bayley cognitive and language scores by sex are displayed in Figures 2 and 3, respectively (exact means and SDs in Supplementary Table 1). Linear mixed models revealed that children with recorded maternal education were, on average, 2.6 months (95% CI [1.6, 3.7]) and 1.7 months (95% CI [.6, 2.8]) younger at cognitive and language assessment, respectively, compared to children without recorded maternal education. Otherwise, there were no significant differences between these groups on any measure listed in Table II. Twelve children (four in the opioid group, five in the alcohol group, and three in the tobacco group) scored at least two SDs below the mean on at least one test administration, of which eight children scored below 70 on the cognitive scale, and four children scored below 70 on the language scale.
Table II.
Sample Characteristics per Exposure Group
| Prenatal opioid exposure (n = 94) | Prenatal alcohol exposure (n = 131) | Prenatal tobacco exposure (n = 115) | No prenatal exposure (n = 38) | p | |
|---|---|---|---|---|---|
| Girls, n (%) | 49 (52.1%) | 66 (50.4%) | 62 (53.9%) | 17 (44.7%) | .79 |
| Age at testing in months (Bayley cognitive scale), mean (SD) | 12.58 (8.02) | 13.32 (8.25) | 11.60 (6.61) | 12.84 (7.68) | .10 |
| n = 176 (test administrations) | n = 264 (test administrations) | n = 235 (test administrations) | n = 67 (test administrations) | ||
| Age at testing in months (Bayley language scale), mean (SD) | 11.42 (6.94) | 12.29 (7.75) | 10.57 (5.98) | 12.29 (7.35) | .07 |
| n = 149 (test administrations) | n = 222 (test administrations) | n = 202 (test administrations) | n = 57 (test administrations) | ||
| Birth weight in milligrams, mean (SD) | 3,177.93 (597.23) | 3,127.41 (658.45) | 3,078.43 (632.85) | 3,093.05 (736.55) | .73 |
| n = 94 | n = 131 | n = 113 | n = 37 | ||
| Birth head circumference in centimeters, mean (SD) | 34.39 (1.66) | 34.06 (2.00) | 34.01 (2.15) | 33.56 (3.03) | .32 |
| n = 83 | n = 110 | n = 98 | n = 25 | ||
| Gestational age in weeks, mean (SD) | 38.67 (2.28) | 39.10 (2.55) | 39.13 (2.69) | 38.63 (3.40) | .46 |
| n = 94 | n = 124 | n = 113 | n = 38 | ||
| SGA, n (%) | 7 (7.4%) | 13 (10.5%) | 11 (10.0%) | 4 (10.5%) | .88 |
| n = 94 | n = 124 | n = 110 | n = 38 | ||
| NAS, n (%) | 13 (31.7%) | NA | NA | NA | NA |
| n = 41 | |||||
| Not discharged to biological parent(s), n (%) | 7 (12.7%) | 13 (17.8%) | 9 (12.0%) | 3 (17.6%) | .73 |
| n = 55 | n = 73 | n = 75 | n = 17 | ||
| Maternal education, median (IQR) | 2 (2) | 2 (1) | 2 (1) | 2 (0) | .08 |
| n = 48 | n = 71 | n = 76 | n = 8 |
Abbreviations: NA = not applicable; NAS = neonatal abstinence syndrome; SGA = small for gestational age. Note. Age at testing was calculated across all test administrations. Maternal education was divided into six levels (1 = less than middle school education, 2 = middle school education or equivalent, 3 = high school education and/or non-university education, 4 = university education < 3 years, 5 = university education 3-4 years, 6 = university education > 4 years). SGA was defined as having a birth weight in the 22nd percentile or lower. Statistical significance of group differences were calculated with analyses of variance (birth weight, head circumference, gestational age, and maternal education), chi-square tests (sex, SGA, and not discharged to biological parents), and linear mixed models (age at testing).
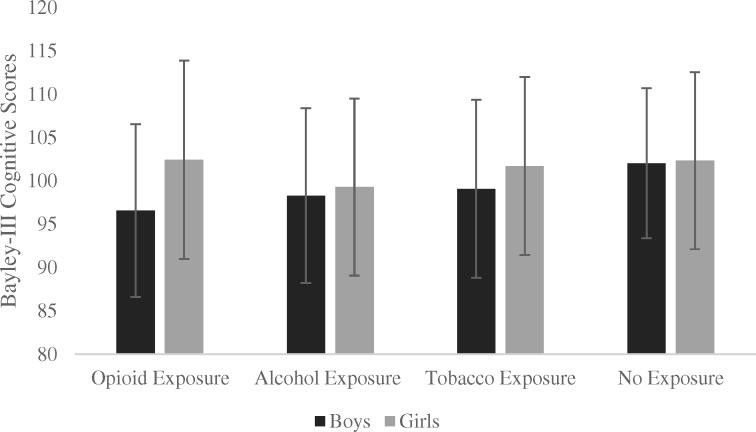
Figure 2.
Bayley-III Cognitive group means per exposure group and sex.
Note. For n = 358 administrations for boys and n = 389 administrations for girls. Error bars represent +/- one standard deviation.
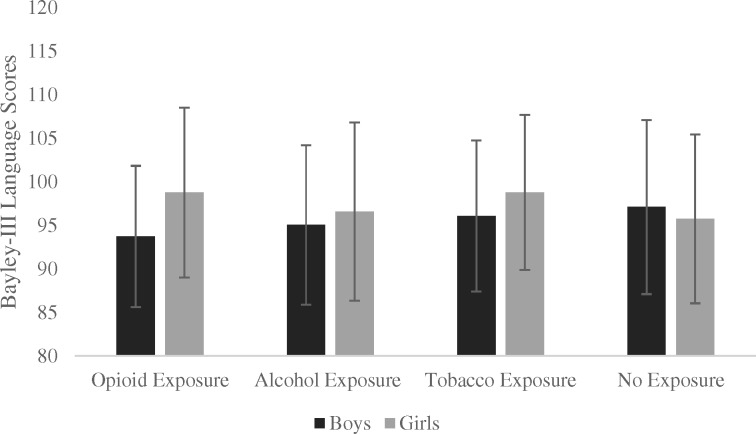
Figure 3.
Bayley-III Language group means per exposure group and sex.
Note. For n = 305 administrations for boys, and n = 328 administrations for girls. Error bars represent +/- one standard deviation.
Results of the linear mixed models are presented in Tables III and IV for cognitive and language scores, respectively. There were no significant main effects of Age, Maternal Education, or Sex for either scale. The main effect of prenatal cannabis exposure was significant for the language scale, in the direction of higher scores with prenatal exposure (mean difference 2.46, 95% CI [0.73, 4.19]). There was evidence of a Opioid × Sex interaction effect for both the cognitive (mean difference ±5.78 [girls/boys], 95% CI [−11.64, 0.07]) and language (mean difference ±6.63 [girls/boys], 95% CI [−12.41, −0.86]) scales, in the direction of lower scores for opioid-exposed boys. Boys in the opioid group had lower cognitive scores compared to boys in the nonexposed group (mean difference −5.82, 95% CI [−9.92, −1.47]), with the corresponding comparison for the language scale falling just within the set level of significance (mean difference −3.98, 95% CI [−7.96, 0.00]). Alcohol-exposed boys also had lower cognitive scores as compared to nonexposed boys (mean difference −3.92, 95% CI [−7.83, −0.01]). There was no evidence of reduced scores in tobacco-exposed boys, and no significant differences between any groups for girls.
Table III.
Group Differences in Bayley-III Cognitive Scores by Sex
| Boys |
Girls |
|||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI [upper, lower] | p | Estimate | 95% CI [upper, lower] | p | |
| Prenatal opioid exposure group | −5.82 | [−9.92, −1.72] | .005 | −0.03 | [−4.48, 4.41] | .99 |
| Prenatal alcohol exposure group | −3.92 | [−7.83, −0.01] | .05 | −3.27 | [−7.57, 1.03] | .14 |
| Prenatal tobacco exposure group | −3.22 | [−7.25, 0.81] | .12 | −0.76 | [−5.12, 3.60] | .73 |
| Prenatal cannabis exposure | 1.38 | [−0.36, 3.13] | .12 | 1.38 | [−0.36, 3.13] | .12 |
| Age | 0.04 | [−0.05, 0.14] | .38 | 0.04 | [−0.05, 0.14] | .38 |
| Maternal education | 0.52 | [−0.47, 1.52] | .30 | 0.48 | [−.047, 1.52] | .30 |
| Sex | −0.31 | [−5.30, 4.69] | .90 | 0.31 | [−4.69, 5.30] | .90 |
| Opioids × Sex | −5.78 | [−11.64, 0.07] | .05 | 5.78 | [−0.07, 11.64] | .05 |
| Alcohol × Sex | −.065 | [−6.21, 4.92] | .82 | 0.65 | [−4.92, 6.22] | .82 |
| Tobacco × Sex | −2.46 | [−8.11, 3.20] | .40 | 2.46 | [−3.20, 8.11] | .40 |
Note. Linear mixed models analysis with pooled group estimates for n = 747 test administrations (n = 375 children) based on 200 imputed datasets. The non-exposed group is set to zero in the analyses. Sex was set to zero for boys and girls, respectively, in two separate analyses, yielding group comparisons for each sex. Estimates are presented as Bayley-III standardized scores.
Table IV.
Group Differences in Bayley-III Language Scores by Sex
| Boys |
Girls |
|||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI [upper, lower] | p | Estimate | 95% CI [upper, lower] | p | |
| Prenatal opioid exposure group | −3.98 | [−7.96, 0.00] | .05 | 2.65 | [−1.66, 6.96] | .23 |
| Prenatal alcohol exposure group | −2.66 | [−6.44, 1.12] | .17 | −0.04 | [−4.24, 4.17] | .99 |
| Prenatal tobacco exposure group | −2.20 | [−6.14, 1.73] | .27 | 1.95 | [−2.27, 6.18] | .37 |
| Prenatal cannabis exposure | 2.46 | [0.73, 4.19] | .005 | 2.46 | [0.73, 4.19] | .005 |
| Age | −0.09 | [−0.19, 0.02] | .11 | −0.09 | [−0.19, 0.02] | .11 |
| Maternal education | 0.10 | [−0.80, 1.01] | .82 | 0.10 | [−0.80, 1.01] | .82 |
| Sex | 1.37 | [−3.55, 6.29] | .59 | −1.37 | [−6.29, 3.55] | .59 |
| Opioids × Sex | −6.63 | [−12.41, −0.86] | .02 | 6.63 | [0.86, 12.41] | .02 |
| Alcohol × Sex | −2.62 | [−8.13, 2.88] | .35 | 2.62 | [−2.88, 8.13] | .35 |
| Tobacco × Sex | −4.16 | [−9.73, 1.41] | .14 | 4.16 | [−1.41, 9.73] | .14 |
Note. Linear mixed models analysis with pooled group estimates for n = 633 test administrations (n = 319 children) based on 200 imputed datasets. The non-exposed group is set to zero in the analyses. Sex was set to zero for boys and girls, respectively, in two separate analyses, yielding group comparisons for each sex. Estimates are presented as Bayley-III standardized scores.
The analyses were then repeated with the opioid-exposed children serving as the comparison group. There was a highly significant effect of Sex, girls having overall higher scores both on the cognitive (mean difference 6.09, 95% CI [3.00, 9.18]) and language (mean difference 5.26, 95% CI [2.23, 8.39]) scales compared to boys. There was also a significant Group × Sex interaction for the alcohol group on both the cognitive (mean difference 5.14, 95% CI [1.19, 9.09]) and language (mean difference 4.01, 95% CI [0.11, 7.91]) scales, with comparisons within each sex revealing lower scores for alcohol compared to opioid-exposed girls (mean difference −3.24, 95% CI [−6.03, −0.44] for the cognitive scale, and mean difference −2.69, 95% CI [−5.42, 0.04] for the language scale), but no differences for boys. There were no significant differences for the tobacco group.
The general pattern remained when the analyses were performed with the unimputed dataset, without maternal education in the model, and the eight cases lacking age at testing excluded (Supplementary Tables 2 and 3). However, when only those participants with full datasets were included (i.e., using unimputed data, and controlling for maternal education), all significant effects disappeared (Supplementary Tables 4 and 5). These two sets of Supplementary tables directly mirror Tables III and IV in the main text, and estimates for main and interaction effects are thus comparable between the two sets of analyses.
Discussion
The present results reveal reduced cognitive and language development only in boys after in utero exposure to opioids. Opioid-exposed boys lagged behind an average of 5.82 points on the Bayley cognitive scale and 3.98 points on the language scale, as compared to nonexposed boys. This represents a difference of roughly a third of the expected standard deviation of 15 for standardized scores on the Bayley scales, thus scores remain within the normal range. There was a significant Group × Sex interaction effect, and group differences between opioid-exposed and nonexposed girls did not reach statistical significance for either scale. Thus, the association of lower cognitive and language scores with in utero opioid exposure appears to be sex specific, with adverse outcomes in boys, and girls not much different from nonexposed groups. These results are of particular importance given the paucity of studies investigating sex differences after in utero opioid exposure. Indeed, to the authors’ knowledge, this is the first study to investigate sex differences after in utero exposure to analgesic opioids, in addition to maintenance medication. The current study thus elaborates on the existing literature on sex differences after in utero opioid exposure, while replicating previous findings on OMT medication and illicit opioids with opioid analgesics.
The present findings are congruent with previous studies investigating the interaction between prenatal opioid exposure and sex on cognitive development. In a study by Suffet and Brotman (1984), opioid-exposed girls had higher cognitive scores than opioid-exposed boys at 1–2 years of age. Both Nygaard et al. (2015) and Moe and Slinning (2001) found elevated group differences on developmental tests for boys after in utero opioid and polysubstance exposure at 1–3 years of age, with girls not much different from children with no known risk. The same pattern was suggested in a recent review by Alaedini et al. (2017). It is unclear why boys appear to be more adversely affected by in utero opioid exposure than girls in some domains. Boys may be more vulnerable to intrauterine factors typically occurring with substance use (Kestler et al., 2012). Additionally, boys may be less adept at emotional self-regulation than girls, thus necessitating greater external help to downregulate (Weinberg, Tronick, Cohn, & Olson, 1999). It could be that mothers with substance abuse problems are less capable of assisting their children in this type of regulation (Salo et al., 2010), resulting in cumulative regulatory risk factors for boys. Children exposed to drugs in utero may in general have impaired behavioral regulation (Lester & Tronick, 1994), and Moe and Slinning (2001) propose that resulting difficulties in achieving a calm alert state is a suboptimal base for learning, thus placing prenatally exposed boys at particular risk.
It should be noted that performance among girls with prenatal opioid exposure has been shown to deteriorate as they reach kindergarten or school age (Nygaard et al., 2015), suggesting that even though there were no significant differences for girls in the present sample, these may emerge with age. Additionally, lower cognitive performance among opioid-exposed children as compared to nonexposed children may in general persist as they reach school (Nygaard et al., 2015) and even young adult age (Nygaard et al., 2017). A recent population-based study by Oei et al. (2017) revealed a progressive deficit in academic performance among children diagnosed with NAS at birth, where by grade 7 children with NAS scored lower than a comparison group in grade 5. Increased risk of poorer academic achievement may necessitate close long-term follow-up of opioid-exposed children.
We were also interested in exploring whether there were group and/or sex differences for the alcohol- and tobacco-exposed groups. There were no significant differences between tobacco-exposed and opioid-exposed boys or girls. Our data thus suggest similar outcomes in children with prenatal exposure to tobacco and opioids. However, there was a significant difference between opioid-exposed and nonexposed boys, but not between tobacco-exposed and nonexposed boys, in addition to a larger difference in scores in the former. These results indicate a stronger association between prenatal exposure and cognitive development for opioids as compared to tobacco. It should be noted that the relatively high level of tobacco exposure in the opioid and alcohol groups in the present sample limits conclusions regarding the effects of these substances. Future studies are needed to determine the relative effects of prenatal tobacco and opioids on early cognitive development. Contrary to other studies (e.g., Cornelius & Day, 2009), the tobacco-exposed group did not perform significantly different compared to the nonexposed group. The association between in utero tobacco exposure and adverse cognitive outcomes is inconsistent, and the lack of differences in the present study could have been due to the limited number of participants relative to previous studies of prenatal tobacco exposure (England et al., 2017), or the presence of other risk factors drowning out the effect of tobacco.
Consistent with our hypotheses, the alcohol-exposed girls scored lower on both the cognitive and language scale compared to the opioid-exposed girls. There were, however, no significant differences between alcohol-exposed girls and girls with no prenatal substance exposure. Alcohol-exposed boys scored lower than nonexposed boys on the cognitive scale, with no significant differences between alcohol-exposed and opioid-exposed boys for either scale. The overall pattern of results thus seems to suggest that boys exposed to either opioids or alcohol in utero experience a similar negative impact on cognitive ability, while for girls this is only the case for alcohol exposure. However, this account is speculative, as there were no significant differences between alcohol and nonexposed girls. This was somewhat surprising, given the well-established link between prenatal alcohol exposure and intellectual impairment (Behnke et al., 2013; Mattson & Riley, 1998). A possible explanation for this could be that the overall extent of alcohol exposure may have been relatively low in this group, as group inclusion criterion was any exposure to alcohol during gestation. Finally, we found a positive effect of prenatal cannabis exposure on Bayley language scores. This effect was unexpected, though longitudinal human studies have not consistently revealed adverse outcomes in children with prenatal exposure to cannabis (Huizink, 2014).
It should be noted that all significant differences between nonexposed and substance-exposed children disappeared when the analyses were performed including only those children who had full datasets. These models were performed with unimputed data, and thus had lower sample sizes of n = 203 and n = 201 children for the cognitive and language scale, respectively. Indeed, only eight children in the nonexposed group were included in this set of analyses, and the results should therefore be interpreted with caution. The results of these analyses are displayed in Supplementary Tables 4 and 5 and can be compared with results of the main analyses in Tables III and IV. As is evident, estimated differences between exposure groups change when the analyses are performed with the smaller sample, thus the lack of significant effects is unlikely to be due just to a reduction in power. Instead, these results might indicate that children without recorded maternal education had systematically different Bayley scores compared to children with recorded maternal education. Children with recorded maternal education were assessed on average 1–2 months earlier than children without recorded maternal education, otherwise there were no significant differences between these groups on any measure. Age at assessment was included as a covariate in all models and was not significantly associated with scores in the models including the full sample. Therefore, it might be that children with and without recorded maternal education differed on unmeasured confounding factors, which could account for the differences in the results. It is also unclear why maternal education was unrelated to cognitive and language outcomes in the current sample, as the link between maternal socioeconomic variables and development is well established (Bradley & Corwyn, 2002). This could be due to the somewhat low variability of education levels around a low median, corresponding to less than a high school education.
An important strength of the present study is the relative homogeneity between comparison groups, as all children in the sample were under the care of a FOC. As the FOCs are mainly intended for women with a current or previous substance abuse problem, all children in the present sample were likely born to mothers with such issues, regardless of their exposure status. Moreover, the four exposure groups did not differ significantly on perinatal variables (birth weight, gestational age, etc.) or likelihood of being discharged to foster or institutional care. However, the children included in this study constitute a vulnerable sample with a number of risk factors, any of which may overshadow the possible direct or indirect effects of in utero substance exposure. Multiple studies find that reduced cognitive and/or motor functioning in drug-exposed children is related to social environment factors (Hans & Jeremy, 2001; Lifschitz et al., 1985; Messinger et al., 2004), which might also modulate the effect of substance exposure (Marcus, Hans, & Jeremy, 1984). Moreover, women in maintenance treatment are often of lower socioeconomic status than no-risk groups (Sarfi et al., 2009) and may employ less optimal parenting strategies in interaction with their children (Bauman & Levine, 1986; Salo et al., 2010). Such confounding is inherently difficult to disentangle and adjust for, hence residual confounding is always a potential problem in clinical studies on substance abuse.
One important limitation in the present study is potential confounding by polysubstance exposure. The relatively high rate of tobacco exposure in the opioid and alcohol groups in particular may have confounded outcomes for these groups. However, we believe that a comparison group of tobacco-exposed children, excluding alcohol or opioid exposure, partly addresses this concern, as effects attributable to tobacco use alone should emerge in the comparison between this and the nonexposed group. Exposure to any one substance was <11% for all other substances in all groups, thus we do not believe this had a major impact on the outcomes. The relatively low number of children in the nonexposed group is another limitation of the present study, as this may have limited our power to detect group differences. Moreover, the use of medical records, and thus lack of control over data collection, made it difficult to quantify and control for timing, duration, and extent of prenatal exposure, leaving room for information bias (Kesmodel, 2018). This may have contributed to depressed group differences, insofar as negligible levels of substance exposure (e.g., one cigarette and one alcoholic drink) were combined with and potentially diluted the effects of heavy levels of exposure. This is one potential explanation for why we were not able to clearly replicate previous findings of impaired cognitive functioning in tobacco- and alcohol-exposed children. Similarly, grouping together children exposed to “heavy” opioids (e.g., heroin) and “light” opioids (e.g., codeine) may conceal potential variability attributable to exposure to different kinds of opioids, although only one child in the present sample was exposed to heroin in utero. Further lack of precision in substance exposure estimates may also have been introduced from using maternal self-report as the main indicator of exposure, as this could mean that use was underreported. Finally, the relatively low median age at assessment of 9.4 months for the Bayley language scale could have reduced the sensitivity of this measure, as problems with language development may be difficult to detect this early in life.
Conclusions
The above limitations notwithstanding, the present results support the possibility that prescribed opioids could be related to delayed early cognitive and language development in boys. These findings are particularly alarming given the increasing user rates among pregnant women and should assist in informing treatment guidelines for pregnant women in opioid maintenance therapy. Moreover, our results, in connection with previous studies, underline the importance of future research investigating developmental differences after in utero substance exposure for each sex separately. Reduced cognitive functioning may later be associated with deteriorating academic performance in opioid-exposed children, and the children and their families might therefore need additional long-term follow-up.
Ethical Considerations
Requirement of informed consent was waived by the Danish DPA and the Norwegian Regional Committees for Medical and Health Research Ethics.
Conflicts of interest: None declared.
Supplementary Material
References
- Alaedini K., Haddadi K., Asadian L. (2017). A review of neurobehavioral challenges in children exposed prenatally to intrauterine opioid. Journal of Pediatrics Review, 5, e9234.doi:10.5812/jpr.9234 [Google Scholar]
- Albers C. A., Grieve A. J. (2007). Test review: Bayley, N. (2006). Bayley scales of infant and toddler development—Third edition. San Antonio, TX: Harcourt Assessment. Journal of Psychoeducational Assessment, 25, 180–190. doi:10.1177/0734282906297199 [Google Scholar]
- Baar A., Graaff B. M. (1994). Cognitive development at preschool-age of infants of drug-dependent mothers. Developmental Medicine and Child Neurology, 36, 1063–1075. doi:10.1111/j.1469-8749.1994.tb11809.x [DOI] [PubMed] [Google Scholar]
- Bauman P. S., Levine S. A. (1986). The development of children of drug addicts. International Journal of the Addictions, 21, 849–863. doi:10.3109/10826088609027399 [DOI] [PubMed] [Google Scholar]
- Beckwith A. M., Burke S. A. (2015). Identification of early developmental deficits in infants with prenatal heroin, methadone, and other opioid exposure. Clinical Pediatrics, 54, 328–335. doi:10.1177/0009922814549545 [DOI] [PubMed] [Google Scholar]
- Behnke M., Smith V. C; Committee on Substance Abuse, & Committee on Fetus and Newborn. (2013). Prenatal substance abuse: Short- and long-term effects on the exposed fetus. Pediatrics, 131, e1009–e1024. doi:10.1542/peds.2012-3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode M. M., D’Eugenio D. B., Mettelman B. B., Gross S. J. (2014). Predictive validity of the Bayley, at 2 years for intelligence quotient at 4 years in preterm infants. Journal of Developmental & Behavioral Pediatrics, 35, 570–575. doi:10.1097/DBP.0000000000000110 [DOI] [PubMed] [Google Scholar]
- Bradley R. H., Corwyn R. F. (2002). Socioeconomic status and child development. Annual Review of Psychology, 53, 371–399. doi:10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- Bunikowski R., Grimmer I., Heiser A., Metze B., Schäfer A., Obladen M. (1998). Neurodevelopmental outcome after prenatal exposure to opiates. European Journal of Pediatrics, 157, 724–730. doi:10.1007/s004310050923 [DOI] [PubMed] [Google Scholar]
- Calvigioni D., Hurd Y. L., Harkany T., Keimpema E. (2014). Neuronal substrates and functional consequences of prenatal cannabis exposure. European Child & Adolescent Psychiatry, 23, 931–941. doi:10.1007/s00787-014-0550-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff I. J., Hatcher R., Burns W. J. (1980). Early growth patterns of methadone-addicted infants. American Journal of Diseases of Children, 134, 1049–1051. doi:10.1001/archpedi.1980.02130230029009 [DOI] [PubMed] [Google Scholar]
- Cornelius M. D., Day N. L. (2009). Developmental consequences of prenatal tobacco exposure. Current Opinion in Neurology, 22, 121–125. doi:10.1097/WCO.0b013e328326f6dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- England L. J., Aagaard K., Bloch M., Conway K., Cosgrove K., Grana R., Wakschlag L. (2017). Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neuroscience and Biobehavioral Reviews, 72, 176–189. doi:10.1016/j.neubiorev.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R. A., Bobo W. V., Martin P. R., Morrow J. A., Wang W., Chandrasekhar R., Cooper W. O. (2013). Increasing pregnancy-related use of prescribed opioid analgesics. Annals of Epidemiology, 23, 498–503. doi:10.1016/j.annepidem.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan L. P., Connaughton J. J., Kron R. E., Emich J. P. (1975). Neonatal abstinence syndrome: Assessment and management. Addictive Diseases, 2, 141–158. [PubMed] [Google Scholar]
- Hans S. L., Jeremy R. J. (2001). Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Mental Health Journal, 22, 300–315. doi:10.1002/imhj.1003 [Google Scholar]
- Huizink A. (2014). Prenatal cannabis exposure and infant outcomes: Overview of studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 52, 45–52. doi:10.1016/j.pnpbp.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Kesmodel U. S. (2018). Information bias in epidemiological studies with a special focus on obstetrics and gynecology. Acta Obstetricia et Gynecologica Scandinavica, 97, 417–423. doi:10.1111/aogs.13330 [DOI] [PubMed] [Google Scholar]
- Kestler L., Bennett D.S., Carmody D.P., Lewis M. (2012). Gender-dependent effects of prenatal cocaine exposure In Lewis M., Kestler L. (Eds.), Gender differences in prenatal substance exposure (pp.11–29). Washington, DC: American Psychological Association. [Google Scholar]
- Krans E. E., Dunn S. L. (2014). Health care use patterns of opioid-dependent pregnant women. Obstetrics and Gynecology, 123, 61S.doi10.1097/01.AOG.0000447364.47611.7d [Google Scholar]
- Lester B. M., Tronick E. Z. (1994). The effects of prenatal cocaine exposure and child outcome. Infant Mental Health Journal, 15, 107–120. doi:10.1002/1097-0355(199422)15:2<107::AID-IMHJ2280150203>3.0.CO; 2-R [Google Scholar]
- Lifschitz M. H., Wilson G. S., Smith E. B., Desmond M. M. (1985). Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics, 75, 269–274. [PubMed] [Google Scholar]
- Mactier H., Shipton D., Dryden C., Tappin D. M. (2014). Reduced fetal growth in methadone‐maintained pregnancies is not fully explained by smoking or socio‐economic deprivation. Addiction, 109, 482–488. doi:10.1111 /add.12400 [DOI] [PubMed] [Google Scholar]
- Månsson J., Stjernqvist K., Serenius F., Ådén U., Källén K. (2019). Agreement between Bayley-III measurements and WISC-IV measurements in typically developing children. Journal of Psychoeducational Assessment, 37, 603–616. doi: 10.1177/0734282918781431 [DOI] [Google Scholar]
- Marcus J., Hans S. L., Jeremy R. J. (1984). A longitudinal study of offspring born to methadone-maintained women. III. Effects of multiple risk factors on development at 4, 8, and 12 months. American Journal of Drug and Alcohol Abuse, 10, 195–207. doi:10.3109/00952998409002780 [DOI] [PubMed] [Google Scholar]
- Mattson S. N., Riley E. P. (1998). A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism—Clinical and Experimental Research, 22, 279–294. doi:10.1111/j.1530-0277.1998.tb03651.x [DOI] [PubMed] [Google Scholar]
- McLoyd V. C. (1998). Socioeconomic disadvantage and child development. American Psychologist, 53, 185–204. doi:10.1037//0003-066x.53.2.185 [DOI] [PubMed] [Google Scholar]
- Messinger D. S., Bauer C. R., Das A., Seifer R., Lester B. M., Lagasse L. L., Poole W. K. (2004). The maternal lifestyle study: Cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics, 113, 1677–1685. doi:10.1542/peds.113.6.1677 [DOI] [PubMed] [Google Scholar]
- Moe V., Slinning K. (2001). Children prenatally exposed to substances: gender‐related differences in outcome from infancy to 3 years of age. Infant Mental Health Journal, 22, 334–350. doi:10.1002/imhj.1005 [Google Scholar]
- Nørgaard M., Nielsson M. S., Heide-Jørgensen U. (2015). Birth and neonatal outcomes following opioid use in pregnancy: A Danish population-based study. Substance Abuse: Research and Treatment, 9, 5–11. doi:10.4137/SART.S23547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard E., Moe V., Slinning K., Walhovd K. B. (2015). Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatric Research, 78, 330–335. doi:10.1038/pr.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard E., Slinning K., Moe V., Walhovd K. B. (2016). Behavior and attention problems in eight-year-old children with prenatal opiate and poly-substance exposure: a longitudinal study. PLoS One, 11, e0158054.doi:10.1371/journal.pone.0158054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard E., Slinning K., Moe V., Walhovd K. B. (2017). Cognitive function of youths born to mothers with opioid and poly-substance abuse problems during pregnancy. Child Neuropsychology, 23, 159–187. doi:10.1080/09297049.2015.1092509 [DOI] [PubMed] [Google Scholar]
- Oei J. L., Melhuish E., Uebel H., Azzam N., Breen C., Burns L., Wright I. M. (2017). Neonatal abstinence syndrome and high school performance. Pediatrics, 139, e20162651.doi:10.1542/peds.2016-2651 [DOI] [PubMed] [Google Scholar]
- Ornoy A., Segal J., Bar-Hamburger R., Greenbaum C. (2001). Developmental outcome of school-age children born to mothers with heroin dependency: Importance of environmental factors. Developmental Medicine and Child Neurology, 43, 668–675. doi:10.1017/S0012162201001219 [DOI] [PubMed] [Google Scholar]
- Patrick S. W., Dudley J., Martin P. R., Harrell F. E., Warren M. D., Hartmann K. E., Cooper W. O. (2015). Prescription opioid epidemic and infant outcomes. Pediatrics, 135, 842–850. doi:10.1542/peds.2014-3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy U. M., Davis J. M., Ren Z., Greene M. F. (2017). Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: Executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstetrics and Gynecology, 130, 10–28. doi:10.1097/AOG.0000000000002054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen T., Pippenger C. (1975). Disposition of methadone and its relationship to severity of withdrawal in the newborn. Addictive Diseases, 2, 169–178. [PubMed] [Google Scholar]
- Salo S., Kivistö K., Korja R., Biringen Z., Tupola S., Kahila H., Kivitie-Kallio S. (2009). Emotional availability, parental self-efficacy beliefs, and child development in caregiver–child relationships with buprenorphine-exposed 3-year-olds. Parenting, Science and Practice, 9, 244–259. doi:10.1080/15295190902844563 [Google Scholar]
- Salo S., Politi J., Tupola S., Biringen Z., Kalland M., Halmesmäki E., Kivitie-Kallio S. (2010). Early development of opioid‐exposed infants born to mothers in buprenorphine‐replacement therapy. Journal of Reproductive and Infant Psychology, 28, 161–179. doi:10.1080/02646830903219109 [Google Scholar]
- Sarfi M., Martinsen H., Bakstad B., Røislien J., Waal H. (2009). Patterns in sleep–wakefulness in three-month old infants exposed to methadone or buprenorphine. Early Human Development, 85, 773–778. doi:10.1016/j.earlhumdev.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M. M., Spittle A. J., Lee K. J., Doyle L. W., Anderson P. J. (2015). Bayley-III cognitive and language scales in preterm children. Pediatrics, 135, e1258–e1265. doi:10.1542/peds.2014-3039 [DOI] [PubMed] [Google Scholar]
- Strauss M. E., Starr R. H., Ostrea E. M., Chavez C. J., Stryker J. C. (1976). Behavioral concomitants of prenatal addiction to narcotics. The Journal of Pediatrics, 89, 842–846. doi:10.1016/S0022-3476(76)80822-0 [DOI] [PubMed] [Google Scholar]
- Suffet F., Brotman R. (1984). A comprehensive care program for pregnant addicts: Obstetrical, neonatal, and child development outcomes. International Journal of the Addictions, 19, 199–219. doi:10.3109/10826088409057176 [DOI] [PubMed] [Google Scholar]
- Sundhetsstyrelsen. (2015). Evaluering af Etableringen af Familieambulatoriene Retrieved from https://sundhedsstyrelsen.dk/da/sundhed/graviditet/familieambulatorier/∼/media/EF2A0FCD71014488BBBCCE57A6550AA6.ashx
- Syme M. R., Paxton J. W., Keelan J. A. (2004). Drug transfer and metabolism by the human placenta. Clinical Pharmacokinetics, 43, 487–514. doi:10.2165/00003088-200443080-00001 [DOI] [PubMed] [Google Scholar]
- Weinberg M. K., Tronick E. Z., Cohn J. F., Olson K. L. (1999). Gender differences in emotional expressivity and self-regulation during early infancy. Developmental Psychology, 35, 175–188. doi:10.1037//0012-1649.35.1.175 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2014). Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.