Abstract
Patients with chronic wounds may experience persistent, debilitating pain that cannot be adequately managed with analgesics and that negatively impacts their quality of life. In this case series, three painful chronic and ischemic wounds that were caused by polyarteritis nodosa vasculitis (n = 1) and peripheral arterial disease (PAD) (n = 2) were successfully treated with cryopreserved umbilical cord tissue and/or amniotic membrane and umbilical cord particulate, resulting in notable reduction in pain within 7 days followed by expedited wound closure. No adverse events related to these tissue products were observed. These preliminary data demonstrate its safety and efficacy in reducing pain and promoting wound healing in painful chronic and ischemic wounds.
Keywords: Amniotic tissue, chronic wound, skin ulcer, umbilical cord, wound healing
Introduction
Chronic wounds on the lower extremities are a major cause of morbidity and disability, affecting 1%–2% of the world’s population and up to 5% of the elderly population.1 These wounds are particularly difficult to treat and require constant medical attention, resulting in significant healthcare costs.2 The healing process for a chronic wound can take 6–8 months,3 and some wounds last years or even decades.4 Living with a chronic wound has a large impact on the patient’s quality of life,5–7 with pain being one of the symptoms that patients find particularly distressing.8–10 In fact, many patients find pain to be the worst aspect of ulceration, and at times consider pain relief to be more important than wound healing.11–13 The prevalence of pain with chronic wounds is 54%–87%,14–16 with up to 67% of patients reporting severe pain.14 Pain can further delay wound healing due to raised stress levels and compromised immune responses.17
The standard of care for chronic wounds entails debridement, pain management, dressing changes, and re-establishment of circulation, although these mechanisms have limited success in wound healing. Furthermore, opioids are commonly used to treat severe pain in chronic wound cases; however, they have a high abuse potential and limited alternatives.18 Thus, there is an unmet clinical need for a wound care treatment that aids in both pain control and wound healing. Cryopreserved amniotic membrane (AM) and umbilical cord (UC) allograft tissue, which has been shown to promote wound closure in complex, non-healing foot ulcers,19–21 may be one potential solution. The therapeutic value of these tissues is derived from their anti-inflammatory and anti-scarring properties.22 These properties not only promote a regenerative environment to facilitate wound healing, but they may also alleviate nociceptive wound pain indirectly by reducing pro-inflammatory mediators.23 Herein, we report the safe use of UC in promoting wound healing and pain alleviation in three cases with chronic and ischemic wounds. The clinical significance in managing the associated pain is further discussed.
Case presentation
Case 1
The patient is a 60-year-old African American female (body mass index (BMI): 29 kg/m2) with a long-standing history of rheumatoid arthritis (RA) that was managed with methotrexate without success. She was eventually put on etanercept (Enbrel; Bristol Meyers Squibb, New York, NY) 1 year previously and developed painful lumps on her lower extremities that turned necrotic. The patient was subsequently placed on oral prednisone (20 mg per day); nonetheless, the RA remained “active” as demonstrated by high levels of rheumatoid factor and C-reactive protein. The patient is also a former smoker and suffers from non-diabetic peripheral neuropathy (treated with oral gabapentin (500 mg BID)) with numbness and paresthesia in the lower extremity (motor function: normal). The patient also suffers from hypertension and hypothyroidism managed with daily oral levothyroxine (0.1 mg daily).
The patient presented to the wound clinic with a chronic (>3.5 months) painful wound over the anterior tibia measuring 6.7 × 4.5 × 0.1 cm3 on the left leg (Figure 1(a)). At presentation, the wound was thought to be caused by RA-associated vasculitis, etanercept-associated vasculitis, or polyarteritis nodosa. The diagnosis was confirmed to be polyarteritis nodosa about 2 months into treatment. The patient’s chief complaint was wound pain, which was being managed with 5–325 mg of oral oxycodone every 6 h. The wound was tender to touch and had minimal non-viable tissue on the edges. The wound was initially treated with irrigation and curette-based debridement followed by application of lidocaine jelly to reduce pain. In addition, negative pressure wound therapy (NPWT) (KCI Medical, San Antonio, TX) with continuous −125 mmHg pressure was performed. Dressings were changed every third day under home care, and silver dressings (ACTICOAT 7; Smith & Nephew, Memphis, TN) were placed under the NPWT foam in contact with the wound. The patient returned to the clinic every 7 days.
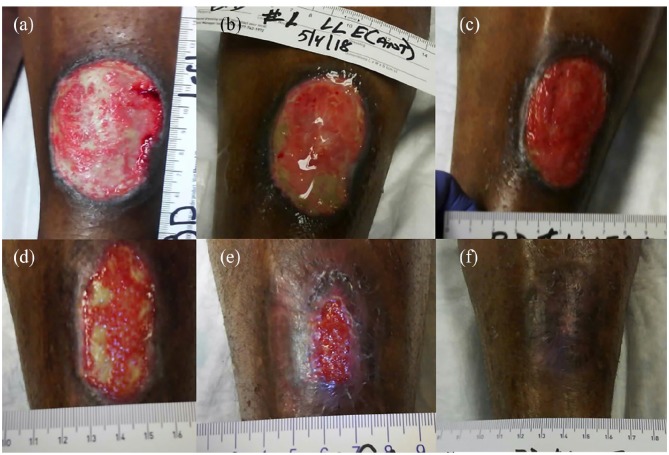
Figure 1.
Chronic painful vasculitic wound due to polyarteritis nodosa. (a) Painful wound on the left leg measured 6.7 × 4.5 × 0.1 cm3 at initial presentation and was treated with negative pressure wound therapy (NPWT) and dressing changes. (b) At week 2, there was lack of epithelialization and pain was still severe (8–9/10), so umbilical cord (UC) allograft was applied. Pain resolved overnight (0/10), and the patient discontinued opioid use. NPWT was discontinued at week 3. (c) At week 4, a second UC graft was placed. (d) By week 11, progressive wound healing was evident along with remnant UC graft. (e) At week 12, an injection of 25 mg of AM/UC particulate was administered. (f) Complete wound closure with return of pigmentation was evident by week 19, following a second AM/UC particulate injection 2 weeks prior.
Within the first 14 days, the wound had formed extreme exudate (Figure 1(b)), and the patient still suffered from severe pain, reporting an 8–9 out of 10 on the Numeric Rating Scale (NRS).24 At this point, a piece of UC allograft (NEOX CORD; Amniox Medical, Miami, FL) was placed on the wound with non-adherent silicone dressing (Adaptic; Acelity, San Antonio, TX). Interestingly, the pain resolved overnight (score of 0/10 on the NRS), and she stopped taking opioids thereafter. At 3 weeks, the wound had drastically decreased in size with evidence of granulation, so NPWT was discontinued. At 4 weeks (Figure 1(c)), another UC allograft was placed on the wound. Over the next 7 weeks (Figure 1(d)), the wound was covered by granulation tissue along with allograft remnant. At 12 weeks (Figure 1(e)), the wound measured 4.0 × 2.0 × 0.1 cm3, and an injection of 25 mg of AM/UC particulate (NEOX FLO; Amniox Medical, Miami, FL) in 2 mL preservative-free saline was given (Figure 2), which resulted in reduction of the wound size and depth. At week 17, another AM/UC injection was administered, which ultimately resulted in complete wound healing 2 weeks later (Figure 1(f)). Upon healing, there was a return of pigmentation at the wound site without pain, scarring, or abnormal sensation. In summary, complete closure of a chronic painful and ischemic vasculitic wound was accomplished in 19 weeks with two UC allografts, two injections of AM/UC particulate, and NPWT for 3 weeks.
Figure 2.
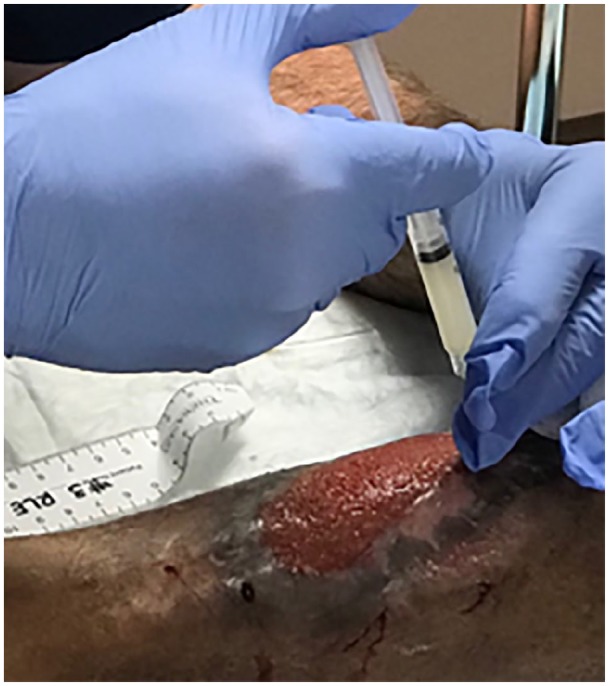
Representative example of AM/UC application. AM/UC particulate was reconstituted in preservative-free saline and injected around the wound parameter.
Case 2
The patient is a 66-year-old female (BMI: 21.5 kg/m2) with a chronic full-thickness wound on the lateral, left extremity below the knee that developed after an arthropod bite 8 months prior. The patient has a medical history of hypertension, hyperlipidemia, depression, anxiety, and idiopathic neuropathy. The patient also has PAD and has undergone multiple procedures including percutaneous revascularization on the involved leg.
The patient complained of sharp pain localized near the wound that was being managed with 300-30 mg of oral acetaminophen-codeine as directed, 81 mg of oral aspirin QD, and 20 mg of oral oxycodone as directed. Neurological examination revealed decreased sensation with the monofilament test. Physical examination revealed the initial wound measured 3.5 × 2.4 × 0.8 cm3 with minimal non-viable tissue on the wound edge. The wound was initially treated with irrigation, 2% lidocaine spray, and debridement, followed by NPWT with a Sorbact® liner with continuous pressure of −125 mmHg and dressing changes every third day. One week later, the wound size decreased to 3 × 1.5 × 0.3 cm3. At 2 weeks, the wound slightly decreased to 2.8 × 1.4 × 0.3 cm (Figure 3(a)), and a 3 × 2 cm2 UC allograft (NEOX CORD) was placed over the wound with non-adherent silicone dressing (Adaptic). One week later (Figure 3(b)), the allograft remained over the wound bed, and the patient reported no wound pain (score of 0/10 on the NRS). Intense granulation tissue was seen by week 5 (Figure 3(c)), followed by closure of the wound by week 7 (Figure 3(d)).
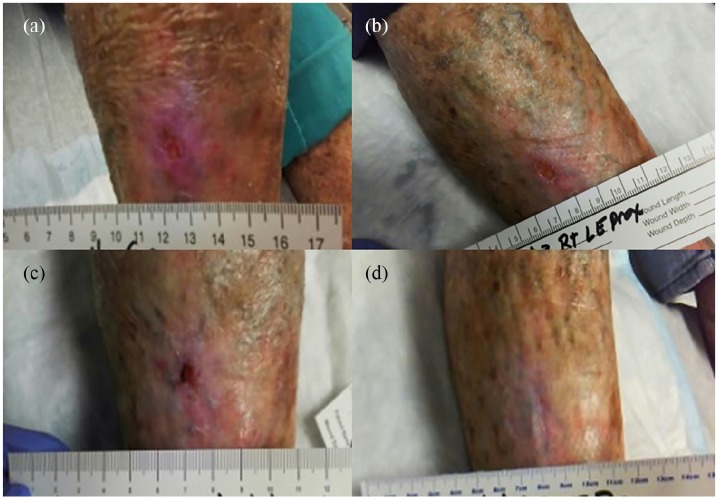
Figure 3.
Chronic painful wound healing associated with PAD. (a) Umbilical cord allograft was applied to the wound at week 2. Progressive wound healing was seen thereafter at (b) week 3, (c) week 5, and (d) week 7.
Case 3
The patient is an 83-year-old female (BMI: 28.9 kg/m2) with a chronic (>5 months) wound on the proximal, lower right leg following surgical excision of skin carcinoma. After excision, only dressings were applied, and the patient was told the wound would heal on its own. The patient has a medical history of insulin-dependent diabetes mellitus, hypertension, varicose veins, former smoker, arthritis, osteoporosis, and diabetic neuropathy. The patient also had previous triple bypass surgery and radiofrequency ablation to the heart.
Physical examination of the neck revealed bilateral carotid bruits. The patient also had faint femoral pulses, and both feet were cold to touch with prolonged capillary filling. Additional work-up suggested that the patient had severe carotid artery disease and PAD. A wound (1.0 × 1.2 × 0.1 cm3) was located on the proximal part of the right leg and diagnosed as an arterial ulcer (Figure 4(a)). The patient also complained of pain localized near the wound. Curette debridement was performed on the wound after topical application of 2% lidocaine gel. Medihoney (Derma Sciences, Toronto, Canada) and Mepilex Ag antimicrobial foam dressing were applied and changed weekly. The wound demonstrated healing at week 2 (Figure 4(b)) and week 3 (Figure 4(c)). At week 4, a 2 × 2 cm2 UC allograft (NEOX CORD 1K) was applied. Pain decreased to 0/10 on the NRS within 3 days, and the allograft remnant was still present 1 week later. Complete wound closure was achieved at week 6 (Figure 4(d)).
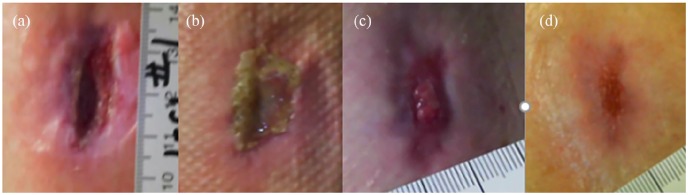
Figure 4.
Chronic painful arterial wound. (a) Initial wound was treated with honey gel and antimicrobial foam dressing. Wound showing healing at (b) week 2 and (c) week 3. (c) Umbilical cord allograft was applied at week 4, and wound pain resolved 3 days later. (d) Complete wound closure was seen at week 6.
Discussion
Herein, we presented three patients with painful chronic and ischemic wounds that were caused by polyarteritis nodosa vasculitis (n = 1) and/or PAD (n = 2). All three patients complained of severe, localized wound pain that was managed with pain medication, including opioids in two cases. After treatment with UC allograft in all three patients and injection of AM/UC particulate in one patient, we observed rapid reduction of pain followed by wound closure. There were no adverse events related to the AM/UC used.
The pain associated with chronic wounds is often debilitating, and consequently can limit daily activities, disrupt sleep, and affect quality of life.25,26 In 40%–57% of these cases, opioids are routinely administered to manage wound pain.27 However, opioid exposure is associated with reduced wound healing due to altered keratinocyte biology28 and insufficient pain relief in many patients.29 In addition, opioids are associated with harmful side effects (such as drowsiness, dizziness, nausea, vomiting, and constipation),30 have a high abuse potential, and are notoriously associated with overdose and death.18 In this case series, patients had pain alleviation within 1–7 days after application of UC allograft, and the two patients discontinued use of opioids. The former is consistent with a case series of cervical necrotizing fasciitis that reported absence of pain 4 days after debridement and AM application.31 Burn patients have also reported significantly less pain on days 1–4 of AM application, further supporting the ability of AM to rapidly relieve wound pain.32 Several studies have also reported significantly less analgesic or narcotic usage in patients treated with AM following penile implant procedures33 and cesarean section,34 supporting the notion that AM may also be useful in reducing opiate usage.
Nociceptive pain is caused by inflammatory mediators in the wound micro-environment in response to tissue damage. Inflammatory responses following tissue damage sensitize peripheral nociceptors in the skin,33 and this inflammatory response is often prolonged in chronic wounds as demonstrated by elevated levels of cytokines (e.g. tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), transforming growth factor β (TGF-β)), proteases, and matric metalloproteinases (MMPs).34–36 The presence of wound infection further stimulates inflammatory responses and neutrophil infiltration, which can exacerbate pain levels experienced.37 Other sources of wound pain could be friction/shear, moisture-related damage, nerve damage, blood vessel injury, and ischemia which leads to hypoxia and can increase infection rates.38 To manage wound pain, the underlying nature of the patient’s pain must be assessed. For example, changes in body position and movement are beneficial for predictable pain. Dressings with non-aggressive adhesives and moisture control are important to provide an appropriate physical environment for healing.39
AM tissue has been shown to improve burn and wound-related pain and increase patient tolerance during wound dressing changes.31,32,40,41 Several prospective, randomized controlled trials have also shown that AM significantly reduces post-operative pain and can even lower analgesic usage as soon as 24 h post-op.42–44 AM and UC are also valuable in especially providing biological factors to accelerate the healing process by managing the cellular and molecular environment. AM and UC have been shown to promote apoptosis and phagocytosis of neutrophils while reducing secretion of inflammatory cytokines.23,45,46 In addition, AM and UC tissues have been shown to suppress TGF-β signaling, which directly suppresses scar formation and favors keratinocyte proliferation and migration.35,47–49 The presence of neuropeptides within the UC tissue may also modulate the function of immunocompetent and inflammatory cells to promote cutaneous innervation that is vital for successful wound healing.50 Based on the pathophysiology of the wounds with background pain39 presented in this case series, the aforementioned properties are likely responsible for the notable reduction in wound pain following AM/UC application and promote an adequate environment for wound healing. Overall, these cases suggest AM and UC may be a safe treatment option in reducing wound pain and promoting closure of chronic and ischemic wounds.
Conclusion
This case series highlights the utilization of AM and UC tissue to rapidly alleviate pain and promote closure in painful chronic and ischemic wounds. Pain reduction may be an early clinical surrogate of successful wound healing that physicians should monitor in their patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient or legally authorized representative for their anonymized information to be published in this article.
ORCID iD: Pablo Acevedo  https://orcid.org/0000-0001-8878-6404
https://orcid.org/0000-0001-8878-6404
References
- 1. Mekkes JR, Loots MA, Van Der Wal AC, et al. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003; 148(3): 388–401. [DOI] [PubMed] [Google Scholar]
- 2. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018; 21(1): 27–32. [DOI] [PubMed] [Google Scholar]
- 3. Moffatt CJ, Franks PJ, Doherty DC, et al. Sociodemographic factors in chronic leg ulceration. Br J Dermatol 2006; 155(2): 307–312. [DOI] [PubMed] [Google Scholar]
- 4. Abbade LP, Lastoria S, de Almeida Rollo H, et al. A sociodemographic, clinical study of patients with venous ulcer. Int J Dermatol 2005; 44(12): 989–992. [DOI] [PubMed] [Google Scholar]
- 5. Newbern S. Identifying pain and effects on quality of life from chronic wounds secondary to lower-extremity vascular disease: an integrative review. Adv Skin Wound Care 2018; 31: 102–108. [DOI] [PubMed] [Google Scholar]
- 6. Green J, Jester R, McKinley R, et al. The impact of chronic venous leg ulcers: a systematic review. J Wound Care 2014; 23(12): 601–612. [DOI] [PubMed] [Google Scholar]
- 7. Do HT, Edwards H, Finlayson K. Identifying relationships between symptom clusters and quality of life in adults with chronic mixed venous and arterial leg ulcers. Int Wound J 2016; 13(5): 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Price P, Fogh K, Glynn C, et al. Managing painful chronic wounds: the wound pain management model. Int Wound J 2007; 4(Suppl. 1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson AB. Quality of life and leg ulceration from the patient's perspective. Br J Nurs 2004; 13(11): S17–20. [DOI] [PubMed] [Google Scholar]
- 10. Ribu L, Wahl A. Living with diabetic foot ulcers: a life of fear, restrictions, and pain. Ostomy Wound Manage 2004; 50(2): 57–67. [PubMed] [Google Scholar]
- 11. Hyland ME, Ley A, Thomson B. Quality of life of leg ulcer patients: questionnaire and preliminary findings. J Wound Care 1994; 3(6): 294–298. [DOI] [PubMed] [Google Scholar]
- 12. Husband L. Venous Ulceration: the pattern of pain and the paradox. Clin Effect Nurs 2001; 5: 35–40. [Google Scholar]
- 13. Persoon A, Heinen MM, van der Vleuten CJ, et al. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004; 13(3): 341–354. [DOI] [PubMed] [Google Scholar]
- 14. Phillips T, Stanton B, Provan A, et al. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994; 31(1): 49–53. [DOI] [PubMed] [Google Scholar]
- 15. Szor JK, Bourguignon C. Description of pressure ulcer pain at rest and at dressing change. J Wound Ostomy Continence Nurs 1999; 26(3): 115–120. [DOI] [PubMed] [Google Scholar]
- 16. Eriksson E, Hietanen H, Asko-Seljavaara S. Prevalence and characteristics of pressure ulcers. A one-day patient population in a Finnish city. Clin Nurse Spec 2000; 14(3): 119–125. [DOI] [PubMed] [Google Scholar]
- 17. Woo KY. Exploring the effects of pain and stress on wound healing. Adv Skin Wound Care 2012; 25(1): 38–44, quiz 45. [DOI] [PubMed] [Google Scholar]
- 18. Office HP. HHS Acting Secretary Declares Public Health Emergency to Address National Opioid Crisis, 2017, https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html
- 19. Caputo WJ, Vaquero C, Monterosa A, et al. A retrospective study of cryopreserved umbilical cord as an adjunctive therapy to promote the healing of chronic, complex foot ulcers with underlying osteomyelitis. Wound Repair Regen 2016; 24(5): 885–893. [DOI] [PubMed] [Google Scholar]
- 20. Couture M. A single-center, retrospective study of cryopreserved umbilical cord for wound healing in patients suffering from chronic wounds of the foot and ankle. Wounds 2016; 28(7): 217–225. [PubMed] [Google Scholar]
- 21. Raphael A, Gonzales J. Use of cryopreserved umbilical cord with negative pressure wound therapy for complex diabetic ulcers with osteomyelitis. J Wound Care 2017; 26(Sup. 10): S38–S44. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Sheha H, Fu Y, et al. Update on amniotic membrane transplantation. Expert Rev Ophthalmol 2010; 5(5): 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tseng SC. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci 2016; 57(5): ORSFh1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005; 14(7): 798–804. [DOI] [PubMed] [Google Scholar]
- 25. Kirsner RS, Sabolinski ML, Parsons NB, et al. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen 2015; 23(5): 737–744. [DOI] [PubMed] [Google Scholar]
- 26. Hampton S. An introduction to various types of leg ulcers and their management. Br J Nurs 2006; 15(11): S9–13. [DOI] [PubMed] [Google Scholar]
- 27. Purcell A, Buckley T, King J, et al. Eutectic mixture of local anaesthetics (EMLA(R)) as a primary dressing on painful chronic leg ulcers: a pilot randomised controlled trial. Pilot Feasibility Stud 2018; 4: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shanmugam VK, Couch KS, McNish S, et al. Relationship between opioid treatment and rate of healing in chronic wounds. Wound Repair Regen 2017; 25(1): 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nemeth KA, Harrison MB, Graham ID, et al. Pain in pure and mixed aetiology venous leg ulcers: a three-phase point prevalence study. J Wound Care 2003; 12(9): 336–340. [DOI] [PubMed] [Google Scholar]
- 30. Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Phys 2008; 11(2 Suppl.): S105–120. [PubMed] [Google Scholar]
- 31. Nanda S, Chakraborty S, Ray A, et al. Healing of cervical necrotizing fasciitis using amniotic membrane as a dressing material. Natl J Maxillofac Surg 2011; 2(2): 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adly OA, Moghazy AM, Abbas AH, et al. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns 2010; 36(5): 703–710. [DOI] [PubMed] [Google Scholar]
- 33. Widgerow AD, Kalaria S. Pain mediators and wound healing–establishing the connection. Burns 2012; 38(7): 951–959. [DOI] [PubMed] [Google Scholar]
- 34. Albino FP, Koltz PF, Gusenoff JA. A comparative analysis and systematic review of the wound-healing milieu: implications for body contouring after massive weight loss. Plast Reconstr Surg 2009; 124: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 35. Kiritsi D, Nystrom A. The role of TGFbeta in wound healing pathologies. Mech Ageing Dev 2018; 172: 51–58. [DOI] [PubMed] [Google Scholar]
- 36. Grellner W, Georg T, Wilske J. Quantitative analysis of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int 2000; 113(1–3): 251–264. [DOI] [PubMed] [Google Scholar]
- 37. White RJ. Wound infection-associated pain. J Wound Care 2009; 18(6): 245–249. [DOI] [PubMed] [Google Scholar]
- 38. Frescos N. What causes wound pain? J Foot Ankle Res 2011; 4: P22–P. [Google Scholar]
- 39. Bechert K, Abraham SE. Pain management and wound care. J Am Col Certif Wound Special 2009; 1: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eskandarlou M, Azimi M, Rabiee S, et al. The healing effect of amniotic membrane in burn patients. World J Plast Surg 2016; 5(1): 39–44. [PMC free article] [PubMed] [Google Scholar]
- 41. Salehi SH, As'adi K, Mousavi SJ, et al. Evaluation of amniotic membrane effectiveness in skin graft donor site dressing in burn patients. Indian J Surg 2015; 77(Suppl. 2): 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Velez I, Parker WB, Siegel MA, et al. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol 2010; 81(12): 1797–1804. [DOI] [PubMed] [Google Scholar]
- 43. Xue SL, Liu K, Parolini O, et al. Human acellular amniotic membrane implantation for lower third nasal reconstruction: a promising therapy to promote wound healing. Burns Trauma 2018; 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mohseni F, Saem J, Sekhavati E, et al. Amniotic membrane for pain control after cesarean section. Crescent J Med Biol Sci 2018; 5: 198–202. [Google Scholar]
- 45. Cooke M, Tan EK, Mandrycky C, et al. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care 2014; 23(10): 465–74476. [DOI] [PubMed] [Google Scholar]
- 46. Kia Tan E, Cooke M, Mandrycky C, et al. Structural and biological comparison of cryopreserved and fresh amniotic membrane tissues. J Biomater Tissue Eng 2014; 4: 1180. [Google Scholar]
- 47. Alcaraz A, Mrowiec A, Insausti CL, et al. Amniotic membrane modifies the genetic program induced by TGFß, stimulating keratinocyte proliferation and migration in chronic wounds. PLoS ONE 2015; 10: e0135324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999; 179(3): 325–335. [DOI] [PubMed] [Google Scholar]
- 49. Witherel CE, Yu T, Concannon M, et al. Immunomodulatory effects of human cryopreserved viable amniotic membrane in a pro-inflammatory environment in vitro. Cell Mol Bioeng 2017; 10(5): 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood) 2005; 230(4): 271–280. [DOI] [PubMed] [Google Scholar]