Abstract
A domain-general monitoring mechanism is proposed to be involved in overt speech monitoring. This mechanism is reflected in a medial frontal component, the error negativity (Ne), present in both errors and correct trials (Ne-like wave) but larger in errors than correct trials. In overt speech production, this negativity starts to rise before speech onset and is therefore associated with inner speech monitoring. Here, we investigate whether the same monitoring mechanism is involved in sign language production. Twenty deaf signers (ASL dominant) and sixteen hearing signers (English dominant) participated in a picture-word interference paradigm in ASL. As in previous studies, ASL naming latencies were measured using the keyboard release time. EEG results revealed a medial frontal negativity peaking within 15 ms after keyboard release in the deaf signers. This negativity was larger in errors than correct trials, as previously observed in spoken language production. No clear negativity was present in the hearing signers. In addition, the slope of the Ne was correlated with ASL proficiency (measured by the ASL Sentence Repetition Task) across signers. Our results indicate that a similar medial frontal mechanism is engaged in pre-output language monitoring in sign and spoken language production. These results suggest that the monitoring mechanism reflected by the Ne/Ne-like wave is independent of output modality (i.e. spoken or signed) and likely monitors pre-articulatory representations of language. Differences between groups may be linked to several factors including differences in language proficiency or more variable lexical access to motor programming latencies for hearing than deaf signers.
Keywords: language monitoring, sign language, error negativity
1. Introduction
Healthy adult speakers only err about once every 1,000 words under natural speech conditions (Levelt, 1999). The same has been shown in sign language (Hohenberger et al., 2002). Such highly efficient behavior is enabled in part by language monitoring processes, which are responsible for controlling our linguistic production as it is being output. While the cognitive and neuronal mechanisms underlying speech monitoring have received some attention in the past few years, these mechanisms have been understudied in sign language production.
Various cognitive models of language monitoring have been proposed (Nozari et al., 2011; Postma, 2000; Postma & Oomen, 2005), and all of these models make a distinction between monitoring processes involved before versus after language output. These monitoring processes have been referred to as the inner and outer loops of speech monitoring respectively. The role of the inner loop is to monitor internal linguistic representations, whereas the outer loop relies on auditory feedback (in overt speech). Differences can be expected between sign and speech monitoring concerning the implementation of the outer loop as the auditory system should not be engaged when signing, and there is evidence that signers do not rely on visual feedback when monitoring sign production for errors (Emmorey, Bosworth, & Kraljic, 2009; Emmorey, Korpics, & Petronio, 2009; Emmorey, Gertsberg, Korpics, & Wright, 2009). However, it is unclear whether or not differences between the inner loop monitoring mechanisms engaged in sign versus speech production would be observed. The way internal linguistic representations are monitored has been conceptualized in different ways. In particular, speech monitoring models differ in terms of whether the inner loop depends on the language comprehension system (Levelt, Roelofs, & Meyer, 1999) or on the language production system (Nozari et al., 2011; Postma, 2000). They also differ in terms of whether or not a domain-general monitoring mechanism is involved in inner speech monitoring (Acheson & Hagoort, 2014; Riès et al., 2011), and whether or not this domain-general monitoring mechanism is conflict-based (Zheng et al., 2018). Therefore, clarifying whether or not a similar brain mechanism is involved in sign language monitoring before signs are actually produced is a necessary step in furthering the understanding of sign language monitoring. Moreover, finding a similar inner loop mechanism in sign and speech production would be of interest in furthering the understanding of language monitoring more generally, as this would suggest the representations involved are not dependent of language output modality.
Similar brain regions have been shown to be engaged in sign and in speech production at the single word level but also at the phrase and narrative levels, including left temporal and left inferior frontal regions (Blanco-Elorrieta et al., 2018; Braun et al., 2001; Emmorey et al., 2007). This common neuronal substrate has been argued to underlie common semantic, lexical, and syntactic properties between sign and spoken language. However, sign and spoken language differ in several ways, particularly in how they are perceived (visual versus auditory), and in the modality of output during production (manual and facial movements versus a phonatory system). Such differences could arguably lead to differences in how sign and spoken languages are monitored. For example, somatosensory feedback in speech monitoring is linked to the movement of speech articulators (Tremblay et al., 2003), but will include manual and facial movements in sign language monitoring (Emmorey et al., 2009, 2014).
Indeed, brain regions known to be associated with speech perception, in particular the superior temporal cortex, have been found to be sensitive to manipulations affecting speech output monitoring (more specifically the outer loop mentioned above) such as speech distortion or delayed auditory feedback (Fu et al., 2006; Hashimoto & Sakai, 2003; McGuire et al., 1996; Tourville et al., 2008). These results have been interpreted as supporting the idea that overt language output monitoring occurs through the language comprehension system, as proposed by (Levelt, 1983).
In sign language, linguistic output is visual, and not auditory. Hence, this could imply that visual brain areas could be involved in sign language monitoring, mirroring the involvement of the auditory system in overt speech monitoring. However, the monitoring of sign language production has been proposed to rely more heavily on proprioceptive feedback than visual feedback (Emmorey et al., 2009). In agreement with this proposal, several studies have reported that parietal regions and not visual regions are more active in sign than in speech production (Emmorey et al., 2014, 2007), including the left supramarginal gyrus (SMG) and the left superior parietal lobule (SPL). Activation of the SPL, in particular, has been associated with proprioceptive monitoring during motoric output (Emmorey et al., 2016). Finally, activation of the superior temporal cortex has not been associated with language output monitoring in sign language production, which is likely due to the different modality of output in sign and speech production.
Speech monitoring has also been shown to rely on the activation of medial frontal regions, such as the anterior cingulate cortex (ACC) and supplementary motor area (SMA) (Christoffels et al., 2007). Activation of the ACC has been shown to be associated with conflict monitoring in and outside language (Barch et al., 2000; Botvinick et al., 1999; Piai et al., 2013). Therefore, speech monitoring has been proposed to depend not only on the perception of one’s own speech through brain mechanisms associated with speech comprehension (see Indefrey, 2011, for a review), but also through the action of a domain-general action monitoring mechanism in medial frontal cortex (Christoffels et al., 2007).
Electroencephalographic studies of speech monitoring have focused on a component referred to as the error negativity (i.e., Ne) or error-related negativity (ERN) (Acheson & Hagoort, 2014; Ganushchak & Schiller, 2008a, 2008b; Masaki, Tanaka, Takasawa, & Yamazaki, 2001; Riès, Fraser, McMahon, & de Zubicaray, 2015; Riès et al., 2011; Riès, Xie, Haaland, Dronkers, & Knight, 2013a; Zheng et al., 2018). This component has a fronto-central distribution (maximal at electrode FCz), and peaks within 100 ms following vocal onset. This component was initially only reported following erroneous utterances and was therefore interpreted as reflecting an error detection mechanism (Masaki et al., 2001). However, this component was more recently also found in correct trials only with a smaller amplitude, suggesting it reflects a monitoring mechanism operating prior to error detection (Riès et al., 2011). Because of the similar topography, time-course, and origin of this component in correct trials and in errors, the component in correct trials has been referred to as the Ne-like wave (Bonini et al., 2014; Roger et al., 2010; Vidal et al., 2003). In speech production, this medial frontal monitoring mechanism also starts to be engaged before the onset of verbal responses, suggesting that it reflects the monitoring of inner speech (i.e., the inner loop mentioned above) rather than that of overt speech production (Riès et al., 2011; 2015; 2013). Combining a neuropsychological and computational approach, Nozari and colleagues have suggested that accurate speech production relies more heavily on this domain-general monitoring mechanism operating prior to speech onset than on the speech comprehension-based monitor (Nozari, Dell, & Schwartz, 2011), which would be hosted in the superior temporal cortex. Whether or not a domain-general monitor in medial frontal cortex is also engaged in sign monitoring before signs are produced is unknown.
Several arguments suggest that the medial frontal cortex should be similarly engaged in sign and in spoken language monitoring. One of these arguments is that the representations that this monitoring mechanism operates on are likely to be pre-articulatory. Evidence for this proposal comes from the finding that the amplitude of the Ne is modulated by variables that have been tied to stages that precede articulation such as semantic relatedness, lexical frequency, or interference from another language in bilinguals (Ganushchak & Schiller, 2008a, 2008b, 2009; Riès et al., 2015). Such internal representations are likely to be commonly engaged in spoken and sign language production. Another argument is the domain-general nature of the monitoring mechanism hosted in the medial frontal cortex. Indeed, the Ne and Ne-like waves have been shown to be present in overt speech production, in typing (Kalfaoglu et al., 2018; Pinet & Nozari, 2019), but also in other actions such as in manual button-press tasks (Burle et al., 2008; Roger et al., 2010; Vidal et al., 2003, 2000). The source of the Ne and Ne-like waves has been localized to the medial frontal cortex and in particular the anterior cingulate cortex (ACC) (Debener et al., 2005; Dehaene et al., 1994) and/or the supplementary motor area (SMA), as shown through intracranial investigations with depth electrodes inserted in the medial frontal cortex (Bonini et al., 2014). These brain regions are associated with action monitoring generally, and are therefore also likely to be engaged in sign language monitoring.
The present study
In this study, we hypothesized that the domain-general monitoring mechanism hosted in the medial frontal cortex and reflected in the Ne and Ne-like wave is similarly engaged during signing and speaking. This study used a picture-naming task and scalp electroencephalography (EEG) to examine the error (Ne) and error-like (Ne-like) negativities time-locked to the initiation of sign production (as measured through manual key-release, as in (Emmorey et al., 2013). In particular, we used data gathered during a picture-word interference (PWI) paradigm, which has been shown to elicit more errors than simple picture naming. In the PWI task, used extensively in the field of psycholinguistics, pictures are preceded by or presented with superimposed distractor words (e.g., Bürki, 2017; Costa, Alario, & Caramazza, 2005; Piai, Roelofs, Acheson, & Takashima, 2013; Piai, Roelofs, Jensen, Schoffelen, & Bonnefond, 2014; Piai, Roelofs, & Schriefers, 2015; Roelofs & Piai, 2015, 2017). In the semantic version of the task (used here), the distractor words can be semantically related to the picture (e.g., picture of a dog, distractor word: “cat”) or unrelated (e.g., picture of a dog, distractor word: “chair”). Typically in this task, naming the picture takes longer and error rates are higher in the semantically-related, compared to the unrelated condition, although the presence of this effect appears to depend on the language input and output modalities (Giezen & Emmorey, 2016, Emmorey et al., under review). Nevertheless, error rates are expected to be higher in this task than in simpler picture naming, which made this paradigm of interest for the present study.
We tested both deaf and hearing signers as they named pictures by signing the picture names in American Sign Language (ASL). In addition, we investigated whether or not ASL proficiency, as measured through the ASL Sentence Repetition Task (SRT), had an effect on the medial frontal monitoring mechanism. Language proficiency has been found to be a potential factor affecting this mechanism, as suggested by several studies (Ganushchak & Schiller, 2009; Sebastian-Gallés et al., 2006). We note however that these studies used button-press responses and not overt speech. Therefore, more direct investigations involving overt language production are needed. We had reasons to believe ASL proficiency may be different between the deaf and hearing group because, although the hearing signers participating in these experiments are selected to be highly proficient in ASL, their use of ASL in their everyday lives is typically less frequent than that of deaf signers (see Paludneviciene, Hauser, Daggett, and Kurz, 2012), and hearing signers are also surrounded by spoken English in the environment.
Finding similar components in sign language production would provide strong evidence for the universal nature of inner language monitoring. Indeed, it would suggest that the mechanism reflected by the Ne and Ne-like waves is involved in inner language monitoring irrespective of the language output modality. This would constitute a further argument in support of the idea that the representations monitored by this medial frontal monitoring mechanism are pre-articulatory.
2. Methods
The data analyzed in this study was initially collected for another study focusing on the effect of the picture-word interference manipulation on event-related potentials time-locked to stimulus presentation (Emmorey et al., under review). In the present study, we focused on the Ne and Ne-like wave time-locked to keyboard release, which reflected the point at which sign production began. There were not enough errors per participant to investigate the effect of the PWI manipulation on the Ne, so we averaged across conditions to increase the number of trials per component.
2.1. Participants
A total of 26 deaf signers (15 women, mean age: 34 years old, SD: 9 years) and 21 hearing signers (17 women, mean age: 36 years old, SD: 10 years) participated in this study. They were recruited through the San Diego area (California, USA), and gave informed consent in accordance with the San Diego State University Institutional Review Board. They received monetary compensation for their time. All had normal or corrected to normal vision and no history of neurological impairment. Thirteen deaf participants and 5 hearing participants were excluded from our analyses because they had less than 5 error trials remaining after artifact rejection, or because they did not follow instructions. Our analyses were therefore conducted on the remaining 11 deaf (8 women, mean age: 35 years old, SD: 12 years) and 15 hearing participants (12 women, mean age: 37 years old, SD: 12 years). Of these remaining participants, 7 out of the 11 deaf participants acquired ASL from birth from their deaf signing families, and 4 acquired ASL in early childhood (before age 6). Of the included 15 hearing participants, 4 acquired ASL from birth from their deaf signing families, and 11 acquired ASL later, at a mean age of 15 years (SD=7 years), 7 were interpreters, and all had been signing for at least 7 years prior to the experiment (mean = 24 years, SD = 10 years). All included participants were right handed. English proficiency was objectively measured using the Peabody Individual Achievement Test reading comprehension subtest (PIAT; Markwardt, 1998) and a spelling test from Andrews and Hersch (2010). ASL proficiency was objectively measured using the extended (35 sentence) version of the ASL Sentence Repetition Task (ASL-SRT) (Supalla, Hauser, & Bavelier, 2014). In this task, participants view an ASL sentence and then sign back what was just viewed. Sentence complexity and length increased after each trial. The ASL-SRT task has been shown to differentiate deaf from hearing users of sign language, as well as native from non-native users (Supalla et al., 2014).
2.2. Materials and Design
The stimuli consisted of 200 words representing common nouns and 100 pictures (i.e., line drawings) selected from various sources (Snodgrass & Vanderwart, 1980), presented on a white background. Name agreement in English for the pictures was high (average: 90% SD: 14.4)1. The average length in letters for the words was 5.05 letters (SD: 1.87). The words were presented in capital letters in Arial font (size 60 in height by 30 in width). Fifty of the pictures were presented in an identity condition (e.g. the word “house” followed by a picture of a house), and the other 50 were presented in the semantically related condition (e.g., the word “paper” followed by a picture of scissors). All of the pictures were also presented in an unrelated condition (e.g. the word “ring” followed by a picture of scissors). Therefore, each picture appeared twice (once in a related and once in an unrelated condition).
Lists were constructed so that they only contained each target item once, half (50 pictures) in the unrelated condition, ¼ or 25 pictures in the semantically related condition, and ¼ or 25 pictures in the identity condition. Lists were counter-balanced across participants so that any target item was presented first in the related condition to half of the participants and first in the unrelated condition to the rest of the participants.
2.3. Procedure
The stimuli were presented within a 2° to 3° visual angle at the center of an LCD computer screen at a viewing distance of approximately 150 cm from the participant’s eyes. This insured the participants did not have to make large eye movements to fully perceive the stimuli. The participants were seated in a dimly lit, sound attenuated room, and were asked to hold down the spacebar of a keyboard and only lift their hands when they were ready to produce the sign corresponding to the picture, marking the naming onset. They were asked to name the pictures as fast and as accurately as possible and ignore the words. They were each given 1 practice round that consisted of 6 trials (these stimuli were not used in the experiment). During the practice, they were instructed to blink during the breaks between stimuli and to minimize facial movements while signing to avoid producing artifacts in the EEG recordings.
Each trial of the experiment began with a fixation cross that was presented in the center of the screen. The cross remained on the screen until the participant placed their hands on the spacebar. The word was then presented for 200ms and was replaced by the picture that was presented for 2000ms. Participants were asked to produce the sign corresponding to the picture name as quickly and as accurately as possible, without hesitating. After signing the picture name, the participants were asked to place their hands back on the spacebar. The fixation cross replaced the picture after 2000ms and the next trial would start only after the participant placed their hands back on the spacebar. Participants were video-recorded during the experiment so that their sign accuracy could be analyzed off-line. The task was self-paced by use of the space bar. Participants were instructed to rest during the fixation periods before placing their hands back on the keyboard. The whole experiment lasted around 20 minutes with some variability in time depending on how many breaks each participant took.
2.4. EEG Recordings
EEG was continually recorded from a 32-channel tin electrode cap (Electro-cap International, Inc., Eaton, OH; using a 10–20 electrode placement). The EEG signal was amplified by a SynAmpsRT amplifier (Neuroscan-Compumedics, Charlotte, NC), and data were collected by Curry Data Acquisition software at a sampling rate of 500 Hz with a band-pass filter of DC to 100 Hz. To monitor for eye blinks and movements, electrodes were placed under the left eye and on the outer canthus of to the right eye. The reference electrode was placed on the left mastoid, and an electrode was placed on the right mastoid for monitoring differential mastoid activity. Impedances were measured before the experiment started and kept below 2.5 kΩ.
2.5. Data Processing
2.5.1. Behavioral data processing:
Reaction times (RTs) were defined as the time separating the picture onset from the release of the spacebar to initiate sign production. The accuracy of sign production was determined offline by visual inspection of the video recordings from the experiment, and all hesitations were discarded from the analysis. Accuracy and hesitation coding was done by two raters, a deaf native signer and a hearing highly proficient ASL signer. Correct trials were those in which an accurate sign was produced at the time of keyboard release with no hesitations. Error trials were trials in which the participant produced an off-target sign (e.g., LION instead of TIGER). Trials in which the participant produced an UM sign or where there was a perceptible pause between the keyboard liftoff and the initiation of the sign were excluded from analysis (see Emmorey et al., 2012). Trials in which the participant did not respond were also excluded from the behavioral and EEG analyses.
2.5.2. EEG Processing:
After acquisition, vertical eye movements (i.e., eye blinks) were removed using independent component analysis (ICA) as implemented in EEGLAB (Delorme & Makeig, 2004). Additional artifacts caused by electromyographic activity associated with facial movements were reduced using a Blind Source Separation algorithm based on Canonical Correlation Analysis (BSS-CCA) (De Clercq et al., 2006), previously adapted to speech production (De Vos et al., 2010), and as used successfully in previous studies investigating speech monitoring processes (Riès et al., 2015, 2011, 2013). Finally, any remaining artifacts were removed through manual inspection in Brain Vision Analyzer ™(Brain Products, Munich). Laplacian transformation (i.e., current source density, C.S.D., estimation), as implemented in Brain Analyser ™, was applied to each participant’s averages and on the grand averages (as in Riès, Janssen, Burle, & Alario, 2013b; Riès et al., 2015, 2011, 2013a); (degree of spline: 3, Legendre polynomial: 15◦ maximum). We assumed a radius of 10 cm for the sphere representing the head. The resulting unit was μV/cm2. Grand averages were created for correct and incorrect trials in both the deaf and hearing groups for the participants with more than 5 error trials remaining after artifact rejection.
Data Analysis
Proficiency scores for English and ASL as measured by the above-listed tests were compared between groups using two-tailed Students t-tests.
Behavioral data were analyzed using linear (for reaction times, RTs) and generalized mixed effect models (for accuracy rates). We tested for main effects of Accuracy and Group (Deaf vs. Hearing), and the interaction between Accuracy and Group on RTs, and tested for a main effect of Group on accuracy rates, and controlled for random effects of subject and items. P-values were obtained using type-II analyses of-deviance tables providing the Wald chi-square tests for the fixed effects in the mixed effect models. For all models, we report the Wald χ2-values and p-values from the analyses of deviance tables as well as raw β estimates (βraw), 95% confidence intervals around these β estimates (CI), standard errors (SE), t-values for reaction times, and Wald Z and associated p-values for accuracy rates.
EEG data were analyzed using 2 types of EEG measures following methods described in previous studies (Riès et al., 2011, 2013a, 2013b). The first measure was the slope of the waveforms on a 150ms time window preceding the key release (the onset of sign production). To find the slope, a linear regression was fitted to the data, and then non-parametric exact Wilcoxon signed-rank tests were used to compare the slopes to 0 for both the errors and correct trials in the deaf and hearing group as the number of error trials was low and the data could not be assumed to be normally-distributed. The second measure was peak-to-peak amplitude (i.e., the difference between the amplitude of two consecutive peaks of activity). Peak-to-peak amplitude was calculated by first determining the peak latencies of the Ne and Ne-like wave as well as the preceding positive peak (also referred to as the start of the rise of the negativity) on the by-participant averages. Latencies were measured on smoothed data to minimize the impact of background noise (the length of the smoothing window was 40 ms) and within 100-ms time windows centered around the latency of the peak on grand averages). Then, for each subject, the surface area was calculated between the waveform and the baseline on a 50ms time-window centered around each peak latency as measured in each participant’s average waveform. Finally, the difference between the surface areas measured around the Ne or Ne-like wave and around the preceding positivity was considered the peak-to-peak amplitude, and is hence independent from the baseline. Again, non-parametric exact Wilcoxon signed-rank one-sided tests (Wilcoxon t-tests) were used to compare peak-to-peak amplitudes in errors versus correct trials because the measures were based on few error trials and the normality of the data could not be assumed (as in Riès et al., 2011, 2013). The use of one-sided tests was justified as the direction of the difference was expected based on several preceding studies in language (Riès et al., 2015, 2011, 2013a), and outside of language (Vidal et al., 2003, 2000). For each test, we report the W statistic for Wilcoxon signed-rank tests, general Z statistic, associated p-value, and effect size r-value. In addition, the effect of Group (deaf vs. hearing) on these differences between correct and error trials were tested using an ANOVA.
Finally, we tested for a correlation between the slope of the Ne and Ne-like wave, and the ASL proficiency score as measured with the ASL-SRT, using Spearman’s rank correlation coefficient rho. We report the rho correlation coefficients, S, and associated p-values.
All statistical analyses were conducted using R (R Core Team, 2014).
3. Results
3.1. Behavioral results
3.1.1. Language Proficiency
English:
Raw PIAT scores for the deaf group ranged from 42 – 99 (M = 79, SD= 13), and spelling scores ranged from 62 – 80 (M=73, SD=6). PIAT scores from the hearing group ranged from 67 – 99 (M = 92, SD=7), and spelling test scores ranged from 62 – 85 (M=78, SD=5). There was a marginal difference between groups on the spelling test (t(21.06)=−2.0352, p= 0.055), and a significant difference between groups on the PIAT (t(14.56)= −3.016, p= 0.0089): the hearing participants showed higher performance on these tests of English proficiency than the deaf participants.
ASL:
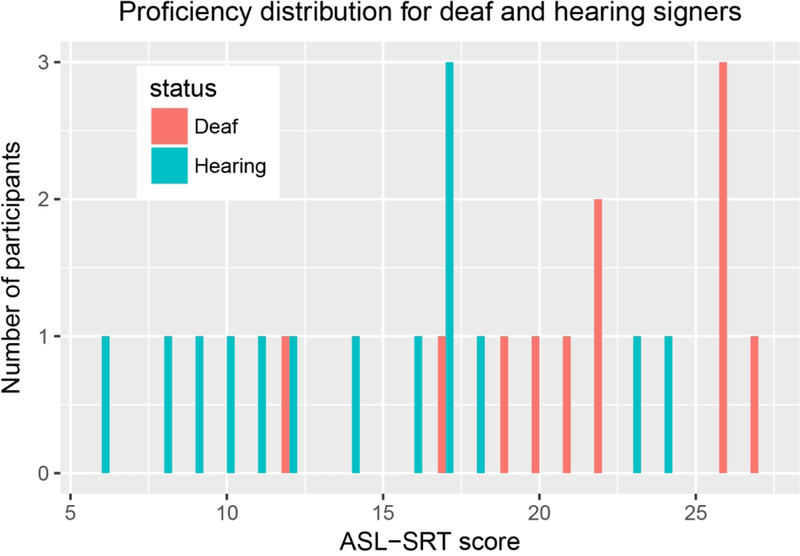
ASL-SRT scores ranged from 12–27 (M=22, SD=5) for deaf signers, and ranged from 6–24 (M= 14, SD= 5) for hearing signers. An ASL-SRT score was not collected from one of the hearing participants because of her familiarity with the test. There was a significant difference in ASL-SRT scores between the deaf and the hearing signers (t(22.82) = 3.60, p = 0.0015): the deaf signers had higher ASL-SRT scores than the hearing signers. Figure 1 provides an illustration of the distribution of the ASL-SRT scores.
Figure 1:
ASL proficiency scores as measured through the ASL-SRT in deaf (blue) and hearing signers (orange).
3.1.2. Reaction times and error rates
The average reaction time was 742 ms (σ = 270 ms) for correct trials, and 848 ms (σ = 376 ms) for errors. There was a significant main effect of Accuracy on RTs (Wald χ2 = 21.08, p < .001), but no effect of Group (Wald χ2 = 0.71, p = .398), and no interaction between Group and Accuracy (Wald χ2 = 0.40, p = .530). Reaction times were shorter in correct than in incorrect trials (βraw =−120.20, CI= [−191.81 −48.59], SE = 36.54, t =−3.29). The median error rate was 5.6% (IQR = 4.6%−12.4%) and there was no effect of Group on accuracy rates (Wald χ2 = 0.91, p= .341; βraw=−0.29, CI= [−0.88 0.30], SE= 0.30, Z=0.34, p = .341). Mean RT, median error rates and number of errors are reported in Table 1 (see Tables S1 and S2 for full fixed effect results from the mixed effect models). On average, 76% (σ= 17%) of correct trials and 74% (σ= 20%) of errors were left after artifact rejection.
Table 1:
Mean reaction times (RTs) per Group (deaf vs. hearing) for errors and correct trials with standard deviations around the mean (σ), median error rates, range and median (M) number of errors per Group with inter quartile ranges (IQR).
| Deaf | Hearing | |||
|---|---|---|---|---|
| Correct | Errors | Correct | Errors | |
| Mean RTs | 688 ms (σ = 242 ms) | 796 ms (σ = 394 ms) | 781 ms (σ = 290 ms) | 886 ms (σ = 372 ms) |
| Median Error rates | 5.08% (IQR=4.12–11.81%) | 6.70% (IQR=4.94–11.52%) | ||
| Range and median number of errors | Range= 5–26, M= 8, IQR=7–10 | Range= 5–33, M=11, IQR=9–13 | ||
3.2. EEG results
3.2.1. Deaf signers
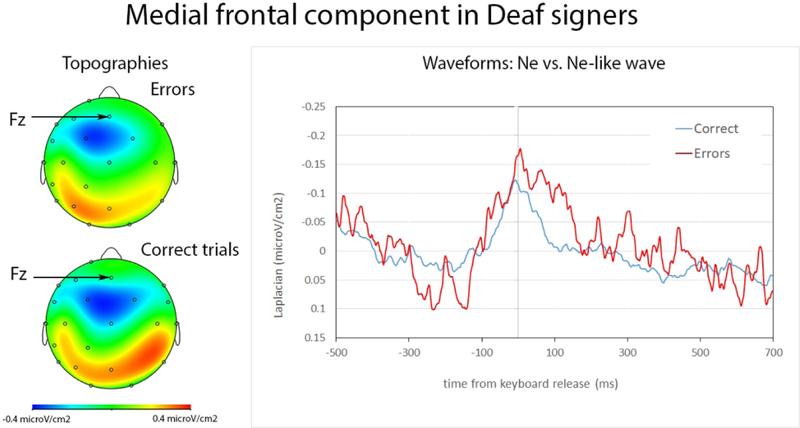
We observed negativities for correct and incorrect trials. The negativity began to rise on average 209 ms (SD=64 ms) before keyboard release in correct trials, and –212 ms (SD=60 ms) in errors. There was no statistical difference between the latency of the positive peak in errors and correct trials (t(10)<1) (W=30.5, Z=−.22, p=.852, r=.04). The negativity reached its maximum on average 13 ms (SD= 31 ms) before keyboard release in correct trials, and 32 ms (SD= 59 ms) after keyboard release in errors. The negativity peaked significantly later in errors than correct trials (t(10)=3.10, p=0.011) (W=63, Z=−2.67, p=.005, r=.56). The negativities reached their maximum at frontocentral electrodes, just posterior to Fz and anterior to Cz (see Figure 2, the used montage did not include electrode FCz which is typically used to study the Ne and Ne-like waves). Slopes measured from –150ms-0ms were significantly different from 0 in correct trials (t(10)= −2.31; p= 0.022) (W=7, Z=−2.60, p=.009, r=.55) and incorrect trials (t(10)= −2.52; p=0.015) (W=7, Z= −2.60, p=.009, r=.55). The amplitude of the negativity for incorrect trials was significantly larger than for correct trials (t(10)=4.03, p=0.001) (W=66, Z= −3.49, p<.001, r=.74).
Figure 2:
Ne and Ne-like wave in deaf participants. On the left, topography of Ne at the peak latency. On the right, waveforms for the Ne (in red, errors) and Ne-like waves (in blue, correct trials) time-locked to keyboard release (in ms). The Ne and Ne-like wave reach their maxima around keyboard release time and the Ne is marginally larger than the Ne-like wave.
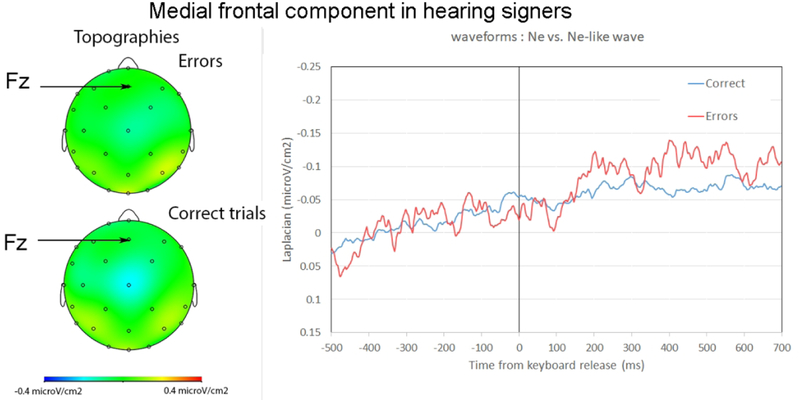
3.2.2. Hearing signers
Slopes measured from –150ms-0ms were significantly different from zero in correct trials (t(14)=−2.14, p=0.025) (W=24, Z=−2.06, p=.039, r=.38). Slopes were not significantly different from zero on the same time-window for errors (t(14)<1) (W=56, Z=−.53, p=.596, r=.10). This indicated there was no reliable Ne in errors for the hearing signers at the same recording site as for deaf signers (i.e., Fz). We note however that a negativity seemed present at Cz (see Figure 3), although this activity was much smaller than the one reported in deaf participants (see Figure 2, the same scale was used in Figures 2 and 3).
Figure 3:
Ne and Ne-like wave in hearing signers. Left: topography around the peak of the negativity in errors and correct trials. Right: Waveforms for the Ne (in red, errors), and Ne-like waves (in blue, correct trials) time-locked to the keyboard release (in ms). For ease of comparison, the same scales were used as for deaf signers (Figure 2).
3.2.3. Deaf vs. hearing signers and effect of ASL proficiency
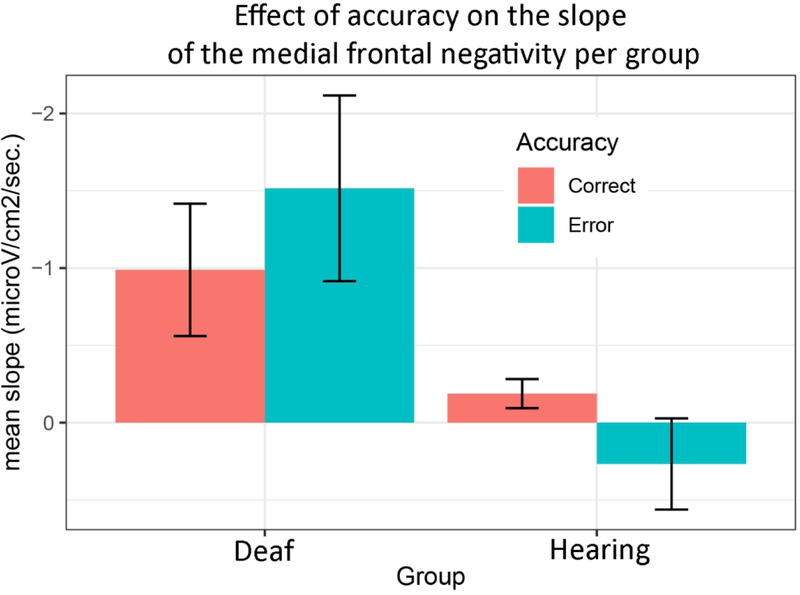
We tested for effects of Accuracy, Group, and ASL-SRT score on the slope of the medial frontal component (the data of the participant for whom we did not have an ASL-SRT score was excluded). We found a significant effect of Group (F(1,21)= 7.14, p=.014), and an interaction between Group and Accuracy (F(1,21)=4.35, p= .050). There was no significant main effect of Accuracy (F(1,21)<1), ASL-SRT score (F(1,21)<1), nor any interaction between Group and ASL-SRT score (F(1,21)<1), Accuracy and ASL-SRT score (F91,21)=1.21, p=.284), and no three-way interaction (F(1,21)<1).
The significant interaction between Group and Accuracy suggests the difference between the slope of the medial frontal negativity in errors versus correct trials was larger in the deaf than in the hearing signers (Figure 4). When tested independently, we however did not find a significant difference between the slope in errors versus correct trials in deaf signers (W=19, Z=−1.55, p=.120, r=.33), although we note that the amplitudes were significantly different as reported in the previous section. In the hearing group, there was no indication of a difference between errors and correct trials (W= 76, Z=−.08, p=.932, r=.02).
Figure 4:
Histogram plotting the mean slope of the Ne and Ne-like wave (with standard deviations fromthe mean) in deaf and hearing signers.
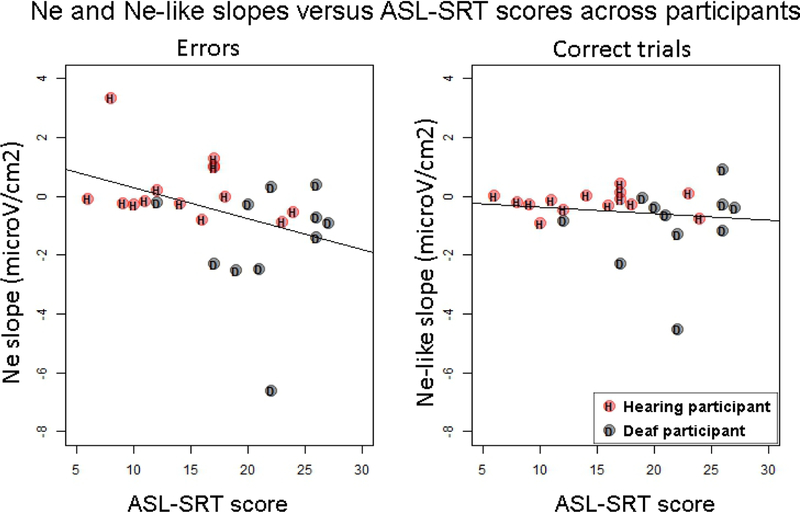
Because the effect of hearing status may have been confounded with ASL proficiency, we tested for a correlation between the slope of the Ne and Ne-like wave (as calculated between –150 ms and keyboard release time) and ASL proficiency scores on the ASL-SRT. The slope of the Ne (in errors) was negatively correlated with ASL proficiency scores across deaf and hearing participants (rho=−.41, S=3677.3, p=.039), meaning that steeper slopes for the Ne (the steeper the slope, the more negative) were associated with higher ASL proficiency (see Figure 5). No significant correlation was observed between ASL proficiency and the slope of the Ne-like wave (in correct trials, rho=−.16, S=3019.3, p=.441).
Figure 5:
Slopes of the Ne (left) and Ne-like wave (right) versus ASL-SRT scores across participants. Hearing participants are indicated in red and with the letter “H”, and Deaf participants are indicated in grey with the letter “D”.
4. Discussion
Our results showed that a medial frontal component is present in sign production for both correct responses and errors when deaf signers name pictures in ASL (time-locked to keyboard release). This component is larger in errors than in correct trials. In addition, the slope of the Ne was correlated with proficiency levels in ASL across hearing and deaf signers. The slope was steeper with increasing proficiency, as measured through the ASL-SRT. In hearing signers, this medial frontal component was not larger in errors than correct trials and was absent in errors at the same recording site as for deaf signers, but was present in correct trials.
4.1. Fronto-medial monitoring mechanism in sign language production
The first important result is that a medial frontal component is present in sign language production in both correct (Ne-like wave) and error trials (Ne). This component had a fronto-central distribution, started to rise before naming onset (as indexed by keyboard release), and peaked just after keyboard release in errors and slightly earlier in correct trials. In addition, it had a larger amplitude in errors than in correct trials. This result suggests that a similar medial frontal mechanism is engaged in pre-output language monitoring in both signed and spoken language production. Indeed, a similar medial frontal negativity was previously reported in overt speech production (Acheson & Hagoort, 2014; Riès et al., 2015, 2011, 2013a; Zheng et al., 2018). Because this activity starts to rise before vocal onset, it was associated with inner speech monitoring. The topography and time course of this component in sign language production is very similar to that observed in overt speech. In overt speech, the Ne was reported to peak between 30 and 40 ms after vocal onset (Riès et al., 2011, 2013a), which corresponds to what we observed in the present study (mean latency of negative peak: 32 ms after keyboard release). In addition, the preceding positive peak was found to precede the onset of language output (speech and sign) across studies even though there were some differences in the latency of this preceding positive peak: 166 ms (SD=80 ms) pre-vocal onset in Ries et al. (2013), 46 ms (SD=52 ms) pre-vocal onset in Riès et al., (2011), and 212 ms (SD= 60 ms) before keyboard release in the present study. One possible explanation for these differences in latencies for the positive peak could be that the task used here was more complex as it required participants to ignore the distractor word and to release the spacebar to start signing. The relatively small difference in amplitude between the Ne and Ne-like wave in the present study would be in agreement with this interpretation. Indeed, the difference in amplitude between the Ne and the Ne-like wave has been shown to be smaller with increasing task difficulty (leading to higher error rates) (due for example to decreased response certainty or in time-pressure situations; Ganushchak & Schiller, 2006 2009; Sebastian Gallés et al., 2006). We note that the reaction times were on average longer (742 ms, SD= 270 ms, for correct trials) and that the error rates were higher (median= 5.6%, IQR=4.6%−12.4%) in the present study compared to experiment 2 of Ries et al. (2011), which used simple overt picture naming (mean RT for correct trials: 651 ms, SD=72 ms; mean error rate: 1.31%, SD=0.96%). In addition, in the present study, the requirement to release the spacebar before signing constitutes an additional step in response programming that may have caused the increased delay between the latency of the peak of the Ne and that of the preceding negativity. However, the similarity in the latency of the negative peak and the fact that it starts to rise before articulation onset, as well as the similar topographies associated with this component in speech and in sign language production suggest that the medial frontal component we report here is similar to the medial frontal component reported in overt speech (e.g., Ries et al., 2011, 2013). This suggests that this medial frontal component is involved in the inner loop of language output monitoring irrespective of the output modality, which would be in line with the idea that the representations that are monitored by this mechanism are pre-articulatory.
In addition to specifying the role of the medial frontal monitoring mechanism in language production, our results also shed light on sign language monitoring more specifically. Indeed, based on the perceptual loop theory of self-monitoring (Levelt, 1983; Levelt, 1993), previous studies had investigated the role of visual feedback in sign language monitoring. Emmorey et al. (2009b) found that preventing visual feedback with a blindfold had little impact on sign production (i.e., there is no Lombard effect for sign language). Emmorey et al. (2009a) showed that blurring or completely masking visual feedback did not alter how well novel signs were learned, suggesting that signers do not rely on visual feedback to fine-tune articulation during learning. In fact, production performance of hearing non-signers was slightly worse with than without visual feedback. This led the authors to suggest that sign language monitoring may rely more heavily on proprioceptive feedback than on visual feedback (see also Emmorey et al., 2009c). What the present results suggest is that a medial frontal monitoring mechanism may also be involved in sign language monitoring and that this monitoring mechanism is engaged before proprioceptive feedback is available (i.e., before beginning to move the hand(s) to sign). Evidence for this claim comes from the time-course of the Ne and Ne-like waves, which start to rise before sign production onset (i.e., prior to key-release). In addition, Allain, Hasbroucq, Burle, Grapperon, and Vidal, (2004) reported Ne and Ne-like waves in a completely deafferented patient. This rare clinical case was tested using a two-response reaction time task and a go-no go task and showed the expected Ne and Ne-like wave patterns in both tasks. Our results therefore indicate yet another similarity in the processing of sign and speech production, and implies that current models of speech monitoring should be adapted to sign language production (Levelt, 1983; Nozari et al., 2011; Postma & Oomen, 2005).
4.2. Difference between deaf and hearing signers
At the group level, no clear Ne was visible in the hearing signers at the same recording site as for deaf signers. Although the hearing signers were highly proficient in ASL (many worked as interpreters), their scores on the ASL-SRT were significantly lower than for the deaf signers we tested. Therefore, we tested for an effect of proficiency on the slope of the Ne and found that the slope of the Ne was negatively correlated with ASL proficiency scores across deaf and hearing signers, meaning that the higher the ASL-SRT score, the more negative-going the slope of the Ne was. Nevertheless, we note that there was no significant effect of ASL proficiency when tested along with the effect of Group. It therefore appeared that Group and ASL proficiency were confounded in our study. Consequently, more detailed examinations of the possible effect of ASL proficiency on the medial frontal monitoring mechanism are needed in future studies.
Previous studies investigating the Ne and/or Ne-like wave in overt speech monitoring have been performed in bilinguals (Acheson et al., 2012; Ganushchak & Schiller, 2009; Sebastian-Gallés et al., 2006). In particular, (Ganushchak & Schiller, 2009) compared German-Dutch bilinguals to Dutch monolingual participants as they performed a phoneme monitoring ‘go-no go’ task (i.e., they were asked to press a button if the Dutch name of the presented picture contained a specific phoneme) under time-pressure versus not. In the time-pressure condition, the stimuli were presented for a shorter duration than in the control condition, and this duration was adapted on an individual basis. The results showed differential effects of time-pressure on the amplitude of the Ne (referred to as the ERN in their study) as a function of group. German-Dutch bilinguals, who were performing the task in their non-native language, showed a larger Ne in the time-pressure condition than in the control condition, and Dutch monolingual speakers showed the reverse effect. Importantly, the bilingual individuals tested in this study were not balanced bilinguals and had lower proficiency in the language in which they were tested (i.e., Dutch) than in their native language (i.e., German). Although the task we used was very different from the task used in (Ganushchak & Schiller, 2009), their results suggest that the Ne may be sensitive to language proficiency. Interestingly and similarly to our results, the mean amplitude of the Ne in the control condition, which is more comparable to the set-up of our study, appeared to be lower in the German-Dutch bilinguals than in the Dutch monolinguals, although this difference was not directly tested.
Relatedly, (Sebastian-Gallés et al., 2006) compared Spanish-Catalan bilinguals who were Spanish-vs. Catalan-dominant in a lexical decision task in Catalan. For Catalan-dominant bilinguals, they observed the expected pattern of a larger negativity in errors than correct trials. However, for Spanish-dominant bilinguals, the amplitude of the negativity was not larger in errors than in correct trials. These results suggest language dominance is an important variable influencing inner speech monitoring abilities. However, we did not test for an effect of language dominance independently from language proficiency. Typically, English is considered the dominant language for hearing signers because English is the language of schooling and the surrounding community, while ASL is considered the dominant language for deaf signers (for discussion see Emmorey, Giezen, and Gollan, 2016). Interestingly, (Sebastian-Gallés et al., 2006) reported a negativity in correct trials (our Ne-like wave) in both groups of bilinguals, which was larger when lexical decision was more difficult (i.e., for non-word trials versus word trials). This finding is in line with our results as we also found a significant Ne-like wave in the hearing signers, even though the Ne was not statistically reliable at the group level.
Previous reports have also shown modulations of the Ne-like wave outside of language as a function of response uncertainty (Pailing & Segalowitz, 2004), and the accuracy of the following trial (Allain, Carbonnell, Falkenstein, Burle, & Vidal, 2004). In particular, the amplitude of the Ne-like wave has been shown to increase with response uncertainty, whereas the amplitude of the Ne has been shown to decrease with response uncertainty (Pailing & Segalowitz, 2004). Hence, one possible interpretation of our results could be that the hearing signers experienced greater response uncertainty compared to deaf signers. This hypothesis would also be in line with the proposal that hearing signers are less aware of their sign errors compared to deaf signers, as suggested by (Nicodemus & Emmorey, 2015). Another (possibly related) reason for the lack of an Ne in hearing signers could be linked to a time-alignment issue with the event used to mark sign production onset, namely the keyboard release. Even though we carefully rejected all trials containing a perceptible pause between the keyboard release time and the onset of the sign (e.g., when the dominant hand reached the target location of the sign; see Caselli, Sehyr, Cohen-Goldberg, and Emmorey (2017) for a detailed description of how sign onsets are determined), naming onset may not have been as closely aligned to keyboard release time in hearing signers as compared to deaf signers. That is, hearing signers may have been more likely to pre-maturely release the spacebar before they had completely encoded the sign for articulation. This could explain why a later, though not strongly reliable, negativity was observed in the subgroup of proficient hearing signers. Indeed, for these signers, the sign onset itself, occurring after keyboard release, might be a better marker to use for the observation of an Ne. Future studies are needed to clarify this issue.
4.3. Conclusion
In sum, our study reports for the first time the presence of a medial frontal negativity associated with inner language output monitoring in sign language production. The presence of this negativity in sign language production strongly suggests that a similar medial frontal mechanism is involved in language monitoring before response initiation irrespective of language output modality, and suggests the representations that are monitored by this mechanism are pre-articulatory. In addition, in line with previous studies using phonological monitoring and lexical decision tasks, our results showed that this mechanism was modulated by language proficiency in sign language production, suggesting similar factors affect medial frontal language output monitoring across modalities.
Supplementary Material
Acknowledgements
This research was supported by an award to S.K.R. and K.E. from the Collaborative Pilot Grant Program from the Center for Cognitive and Clinical Neuroscience at San Diego State University and by NIH Grant DC010997 (K.E.). We are very thankful to the participants who took part in this study.
Footnotes
ASL name agreement was available for 61 of the stimuli (from an on-going ASL picture naming study in the Emmorey lab), and agreement was also high for these stimuli (average: 83.0% SD: 21.6%).
References
- Acheson DJ, Ganushchak LY, Christoffels IK, & Hagoort P. (2012). Conflict monitoring in speech production: Physiological evidence from bilingual picture naming. Brain and Language, 123(2), 131–136. 10.1016/j.bandl.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Acheson DJ, & Hagoort P. (2014). Twisting tongues to test for conflict-monitoring in speech production. Frontiers in Human Neuroscience, 8, 206 10.3389/fnhum.2014.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain S, Carbonnell L, Falkenstein M, Burle B, & Vidal F. (2004). The modulation of the Ne-like wave on correct responses foreshadows errors. Neuroscience Letters, 372(1), 161–166. 10.1016/j.neulet.2004.09.036 [DOI] [PubMed] [Google Scholar]
- Allain S, Hasbroucq T, Burle B, Grapperon J, & Vidal F. (2004). Response monitoring without sensory feedback. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(9), 2014–2020. 10.1016/j.clinph.2004.04.013 [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, & Noll DC (2000). Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. Journal of Cognitive Neuroscience, 12(2), 298–309. [DOI] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, Kastner I, Emmorey K, & Pylkkänen L. (2018). Shared neural correlates for building phrases in signed and spoken language. Scientific Reports, 8(1), 5492 10.1038/s41598-018-23915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini F, Burle B, Liégeois-Chauvel C, Régis J, Chauvel P, & Vidal F. (2014). Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science (New York, N.Y.), 343(6173), 888–891. 10.1126/science.1247412 [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, & Cohen JD (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402(6758), 179–181. 10.1038/46035 [DOI] [PubMed] [Google Scholar]
- Braun AR, Guillemin A, Hosey L, & Varga M. (2001). The neural organization of discourse: an H2 15O-PET study of narrative production in English and American sign language. Brain: A Journal of Neurology, 124(Pt 10), 2028–2044. [DOI] [PubMed] [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, & Hasbroucq T. (2008). Error Negativity Does Not Reflect Conflict: A Reappraisal of Conflict Monitoring and Anterior Cingulate Cortex Activity. Journal of Cognitive Neuroscience, 20(9), 1637–1655. 10.1162/jocn.2008.20110 [DOI] [PubMed] [Google Scholar]
- Caselli NK, Sehyr ZS, Cohen-Goldberg AM, & Emmorey K. (2017). ASL-LEX: A lexical database of American Sign Language. Behavior Research Methods, 49(2), 784–801. 10.3758/s13428-016-0742-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels IK, Formisano E, & Schiller NO (2007). Neural correlates of verbal feedback processing: an fMRI study employing overt speech. Human Brain Mapping, 28(9), 868–879. 10.1002/hbm.20315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq W, Vergult A, Vanrumste B, Van Paesschen W, & Van Huffel S. (2006). Canonical correlation analysis applied to remove muscle artifacts from the electroencephalogram. IEEE Transactions on Bio-Medical Engineering, 53(12 Pt 1), 2583–2587. 10.1109/TBME.2006.879459 [DOI] [PubMed] [Google Scholar]
- De Vos M, Riès S, Vanderperren K, Vanrumste B, Alario F-X, Van Huffel S, Huffel VS, & Burle B. (2010). Removal of muscle artifacts from EEG recordings of spoken language production. Neuroinformatics, 8(2), 135–150. 10.1007/s12021-010-9071-0 [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, Cramon DY von, & Engel AK. (2005). Trial-by-Trial Coupling of Concurrent Electroencephalogram and Functional Magnetic Resonance Imaging Identifies the Dynamics of Performance Monitoring. Journal of Neuroscience, 25(50), 11730–11737. 10.1523/JNEUROSCI.3286-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, & Tucker DM (1994). Localization of a Neural System for Error Detection and Compensation. Psychological Science, 5(5), 303–305. JSTOR. [Google Scholar]
- Delorme A, & Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Emmorey K, Bosworth R, & Kraljic T. (2009). Visual feedback and self-monitoring of sign language. Journal of Memory and Language, 61(3), 398–411. 10.1016/j.jml.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, McCullough S, Mehta S, & Grabowski TJ (2014). How sensory-motor systems impact the neural organization for language: direct contrasts between spoken and signed language. Frontiers in Psychology, 5, 484 10.3389/fpsyg.2014.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Mehta S, & Grabowski TJ (2007). The neural correlates of sign versus word production. NeuroImage, 36(1), 202–208. 10.1016/j.neuroimage.2007.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Mehta S, McCullough S, & Grabowski TJ (2016). The neural circuits recruited for the production of signs and fingerspelled words. Brain and Language, 160, 30–41. 10.1016/j.bandl.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Petrich JAF, & Gollan TH (2012). Bilingual processing of ASL–English code-blends: The consequences of accessing two lexical representations simultaneously. Journal of Memory and Language, 67(1), 199–210. 10.1016/j.jml.2012.04.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Petrich JAF, & Gollan TH (2013). Bimodal bilingualism and the frequency-lag hypothesis. Journal of Deaf Studies and Deaf Education, 18(1), 1–11. 10.1093/deafed/ens034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Vythelingum GN, Brammer MJ, Williams SCR, Amaro E, Andrew CM, Yágüez L, van Haren NEM, Matsumoto K, & McGuire PK (2006). An fMRI study of verbal self-monitoring: neural correlates of auditory verbal feedback. Cerebral Cortex (New York, N.Y.: 11991), 16(7), 969–977. 10.1093/cercor/bhj039 [DOI] [PubMed] [Google Scholar]
- Ganushchak LY, & Schiller NO (2008a). Motivation and semantic context affect brain error-monitoring activity: an event-related brain potentials study. NeuroImage, 39(1), 395–405. 10.1016/j.neuroimage.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Ganushchak LY, & Schiller NO (2008b). Brain error-monitoring activity is affected by semantic relatedness: an event-related brain potentials study. Journal of Cognitive Neuroscience, 20(5), 927–940. 10.1162/jocn.2008.20514 [DOI] [PubMed] [Google Scholar]
- Ganushchak LY, & Schiller NO (2009). Speaking one’s second language under time pressure: An ERP study on verbal self-monitoring in German–Dutch bilinguals. Psychophysiology, 46(2), 410–419. 10.1111/j.1469-8986.2008.00774.x [DOI] [PubMed] [Google Scholar]
- Giezen MR, & Emmorey K. (2016). Language co-activation and lexical selection in bimodal bilinguals: Evidence from picture-word interference. Bilingualism (Cambridge, England), 19(2), 264–276. 10.1017/S1366728915000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, & Sakai KL (2003). Brain activations during conscious self-monitoring of speech production with delayed auditory feedback: an fMRI study. Human Brain Mapping, 20(1), 22–28. 10.1002/hbm.10119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfaoglu C, Stafford T, & Milne E. (2018). Frontal Theta Band Oscillations Predict Error Correction and Post Error Slowing in Typing. Journal of Experimental Psychology Human Perception & Performance, 44(1). 10.1037/xhp0000417 [DOI] [PubMed] [Google Scholar]
- Levelt WJ (1983). Monitoring and self-repair in speech. Cognition, 14(1), 41–104. [DOI] [PubMed] [Google Scholar]
- Levelt WJM (1993). Speaking: From Intention to Articulation. MIT Press. [Google Scholar]
- Levelt WJ, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. The Behavioral and Brain Sciences, 22(1), 1–38-75. [DOI] [PubMed] [Google Scholar]
- Masaki H, Tanaka H, Takasawa N, & Yamazaki K. (2001). Error-related brain potentials elicited by vocal errors. Neuroreport, 12(9), 1851–1855. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, & Frith CD (1996). Functional neuroanatomy of verbal self-monitoring. Brain: A Journal of Neurology, 119 (Pt 3), 907–917. [DOI] [PubMed] [Google Scholar]
- Emmorey K, Mott M, Meade G, Holcomb PJ, Midgely KJ (under review). Bilingual lexical selection without lexical competition: ERP evidence from bimodal bilinguals and picture-word interference. Language, Cognition and Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus B, & Emmorey K. (2015). Directionality in ASL-English interpreting: Accuracy and articulation quality in L1 and L2. Interpreting: International Journal of Research and Practice in Interpreting, 17(2), 145–166. 10.1075/intp.17.2.01nic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Dell GS, & Schwartz MF (2011). Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cognitive Psychology, 63(1), 1–33. 10.1016/j.cogpsych.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing PE, & Segalowitz SJ (2004). The effects of uncertainty in error monitoring on associated ERPs. Brain and Cognition, 56(2), 215–233. 10.1016/j.bandc.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Piai V, Roelofs A, Acheson DJ, & Takashima A. (2013). Attention for speaking: domain-general control from the anterior cingulate cortex in spoken word production. Frontiers in Human Neuroscience, 7, 832 10.3389/fnhum.2013.00832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinet S, & Nozari N. (2019). Electrophysiological Correlates of Monitoring in Typing with and without Visual Feedback. Journal of Cognitive Neuroscience, 1–18. 10.1162/jocn_a_01500 [DOI] [PubMed] [Google Scholar]
- Postma A. (2000). Detection of errors during speech production: a review of speech monitoring models. Cognition, 77(2), 97–132. [DOI] [PubMed] [Google Scholar]
- Postma A, & Oomen CE (2005). Critical issues in speech monitoring. In Phonological encoding and monitoring in normal and pathological speech (pp. 157–186). [Google Scholar]
- R Core Team. (2014). R: A language and environment for statistical computing. http://www.R-project.org/
- Riès S, Janssen N, Burle B, & Alario F-X (2013b). Response-locked brain dynamics of word production. PloS One, 8(3), e58197. 10.1371/journal.pone.0058197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riès SK, Fraser D, McMahon KL, & de Zubicaray GI (2015). Early and Late Electrophysiological Effects of Distractor Frequency in Picture Naming: Reconciling Input and Output Accounts. Journal of Cognitive Neuroscience, 27(10), 1936–1947. 10.1162/jocn_a_00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riès SK, Janssen N, Dufau S, Alario F-X, & Burle B. (2011). General-purpose monitoring during speech production. Journal of Cognitive Neuroscience, 23(6), 1419–1436. 10.1162/jocn.2010.21467 [DOI] [PubMed] [Google Scholar]
- Riès SK, Xie K, Haaland KY, Dronkers NF, & Knight RT (2013a). Role of the lateral prefrontal cortex in speech monitoring. Frontiers in Human Neuroscience, 7, 703 10.3389/fnhum.2013.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger C, Bénar CG, Vidal F, Hasbroucq T, & Burle B. (2010). Rostral Cingulate Zone and correct response monitoring: ICA and source localization evidences for the unicity of correct-and error-negativities. NeuroImage, 51(1), 391–403. 10.1016/j.neuroimage.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian-Gallés N, Rodríguez-Fornells A, de Diego-Balaguer R, & Díaz B. (2006). First-and second-language phonological representations in the mental lexicon. Journal of Cognitive Neuroscience, 18(8), 1277–1291. 10.1162/jocn.2006.18.8.1277 [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, & Vanderwart M. (1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6(2), 174–215. 10.1037/0278-7393.6.2.174 [DOI] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, & Guenther FH (2008). Neural mechanisms underlying auditory feedback control of speech. NeuroImage, 39(3), 1429–1443. 10.1016/j.neuroimage.2007.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, & Ostry DJ (2003). Somatosensory basis of speech production. Nature, 423(6942), 866–869. 10.1038/nature01710 [DOI] [PubMed] [Google Scholar]
- Vidal F, Burle B, Bonnet M, Grapperon J, & Hasbroucq T. (2003). Error negativity on correct trials: a reexamination of available data. Biological Psychology, 64(3), 265–282. 10.1016/S0301-0511(03)00097-8 [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, & Bonnet M. (2000). Is the “error negativity” specific to errors? Biological Psychology, 51(2–3), 109–128. 10.1016/S0301-0511(99)00032-0 [DOI] [PubMed] [Google Scholar]
- Zheng X, Roelofs A, Farquhar J, & Lemhöfer K. (2018). Monitoring of language selection errors in switching: Not all about conflict. PloS One, 13(11), e0200397. 10.1371/journal.pone.0200397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.