Abstract
Acute myeloid leukemia (AML) is a systemic, heterogeneous hematologic malignancy with poor overall survival. While some malignancies have seen improvements in clinical outcomes with immunotherapy, success of these agents in AML remains elusive. Despite limited progress, stem cell transplantation and donor lymphocyte infusions show that modulation of the immune system can improve overall survival of AML patients. Understanding the causes of immune evasion and disease progression will identify potential immune-mediated targets in AML. This review explores immunosuppressive mechanisms that alter T cell-mediated immunity in AML.
INTRODUCTION
Acute myeloid leukemia (AML) is a genetically diverse hematopoietic malignancy and the most common acute leukemia in adults with an annual incidence of over 20,000 new cases in the United States.1, 2 The overall prognosis remains poor as only a quarter of patients are alive five years following their diagnosis.1 Over the last two years, AML has seen several new drugs come to market with some promising impact on outcomes. However, most of these target mutated or otherwise critical proteins in leukemia.3, 4 While many other neoplastic diseases have seen significant clinical impact of agents targeting the immune system, AML therapy has yet to realize such gains.5, 6
There is, however, a case for the immune system to be harnessed for anti-leukemic therapy. Allogeneic hematopoietic stem cell transplantation and subsequent donor lymphocyte infusions remain the primary forms of immunotherapy for AML and can be curative.7 This “graft-versus-leukemia” (GVL) effect has fueled interest in other modalities of immunotherapy, including the use of chimeric antigen receptor T cells (CAR-T cells), vaccines, immune checkpoint inhibitors, and bispecific T-cell engagers (BiTE), and dual-affinity retargeting (DART) molecules in many malignancies. However, the success of newer immunotherapies has been limited in myeloid malignancies.5, 8, 9 Challenges specific to immunotherapy in AML likely stem, in part, from the ability of the leukemia cells to co-opt a number of intrinsic myeloid mechanisms, either directly or indirectly, to create a suppressive microenvironment that results in reduced anti-leukemic immunity and disease pathogenesis and progression.5, 10–13 It has also been suggested that the relatively low mutational burden in AML results in a lower level of neo-antigen formation and subsequent leukemia immunogenicity, making leukemia cells difficult targets for immunotherapy.14 Furthermore, AML is defined by intra-leukemic genetic heterogeneity. Multiple genetic subclones can possess various phenotypic and functional properties that exert variable influence on nonleukemic immune cell populations.15
Here, we establish the potential importance of anti-leukemic immunity in AML in the pre-transplant setting and explore available evidence for altered T cell states in AML, including T cell exhaustion. We will review the identified and putative mechanisms by which AML impacts the function and distribution of T cells. We have limited our scope to primarily the pre-transplant setting and will focus on mechanisms by which both leukemia cells and the immune microenvironment inhibit T cell-mediated immunity.
CLINICAL EVIDENCE OF DISRUPTED T CELL IMMUNITY IN AML
While the utility of allogeneic stem cell transplantation and donor lymphocyte infusions in AML demonstrates the potential for anti-leukemic T cell responses to eradicate leukemia cells, validating the importance of anti-leukemic immunity prior to transplant has been more difficult.7 There was some early clinical interest in the function of the immune system and its impact on outcome in AML. In 1971, just prior to the standardization of modern intensive induction chemotherapy (“7+3”), investigators tested a group of 25 patients with acute leukemia for skin reactivity in a battery of delayed hypersensitivity skin test antigens before and after induction chemotherapy.16 Though small, this group demonstrated a correlation between a major response to chemotherapy and appropriate skin test responses, providing evidence that cell-mediated immunity can be reduced in AML and that this reduction could have some prognostic information.16 A few years later, the same investigators performed skin testing in 55 patients with acute leukemia (34 with AML) monthly or bimonthly for at least a year.17 The vast majority of patients achieving a remission had intact skin-test responses at diagnosis (32/39), compared to only 27% (4/15) of those who with refractory disease. After the universal reduction in skin test response during chemotherapy, patients who maintained a prolonged remission more quickly recovered normal skin test responses, usually by 6 months. For those in remission, loss of recovered skin test responses seemed to predict leukemia relapse, preceding it by approximately one month. Though not definitive, these data provide some of the first correlative evidence that aspects of T cell function are important in chemotherapy response and maintenance of remission.
Reports of spontaneous remissions (SR) in AML further support the potential of immune effector cells in eradicating leukemia.18 The largest case series of SR in AML reports 46 cases, including 39 achieving spontaneous CR. Forty-four of these cases were associated with some identifiable immune-related event, including fever, infection, or blood transfusion. While mean duration of spontaneous CR was short at 5 months, 2 cases resulted in durable ongoing remissions of 8 and 10 years. Though thorough immune profiling has not been done in these cases, the available limited evidence suggests a role for both humoral and cytotoxic immunity in SR.19
ANIMAL MODELS OF ALTERED T CELL IMMUNITY IN AML
Immunocompetent animal models may offer some evidence for anti-leukemic T cell immunity. Hasegawa et al. introduced the MLL/AF9 fusion product generated by t(9;11) translocation found in human acute myelomonocytic leukemia (AMML) together with ovalbumin (OVA), a model antigen, into murine hematopoietic progenitor cells (HPCs).20 These MLL/AF9-OVA leukemia initiating cells were transplanted in wild-type and Rag2−/− mice, which lack mature B and T cells. All Rag2−/− mice succumbed to AML within 50 days, whereas six out of seven wild-type mice did not develop leukemia. Moreover, when a large number of MLL/AF9-OVA cells (3×106 cells) was introduced in the same mouse model, there was expansion of antigen-specific cytotoxic T lymphocytes (CTL) with high level expression of inhibitory markers PD-1 (programmed death 1) and LAG-3 (lymphocyte-activation gene 3) compared with non-leukemic mice.20 These data suggest that an intact adaptive immune system is necessary for leukemia eradication in a murine model. Further, it establishes the MLL/AF9-OVA model with OT-1 T cells as a viable platform to study T cells in AML.
Another murine model, described by Zhang et al, introduced the C1498 murine AML cell line to C57BL/6 mice either by subcutaneous (s.c.) or intravenous (i.v.) injection.21 Mice receiving i.v. inoculation had only minimal functional response of antigen-specific T cells compared with mice receiving s.c. inoculation suggestive that systemic presence of leukemia impaired activation of antigen-specific T cells. Moreover, tagged antigen-specific T cells had significantly decreased production of IFN-γ and TNF-α after injection with i.v. versus s.c. C1498 cells. The group hypothesized that the leukemia cells indirectly cause immune evasion through costimulatory ligand blockade. The addition of anti-CD40 antibody, a costimulatory protein on antigen presenting cells, enhanced T cell proliferation and restored IFN-γ and TNF-α production. This murine model indicates that leukemia causes immune dysfunction by blocking costimulatory signals necessary for T cell activation.
While other murine leukemia models have been used, they are limited in their ability to assess anti-leukemic immunity.22 Transplantation of oncogene-transduced HPCs requires irradiation in the recipients which can suppress the immune response. Transgenic models often have quite variable ability to develop leukemia and do not always result in the desired phenotype. Therefore, establishing and refining immunocompetent murine leukemia models will more accurately assess the immune microenvironment of AML.
T CELL ALTERATIONS IN AML
Augmented T Regulatory Cell Number and Impact
Regulatory T cells (Treg) are critical for maintaining immune tolerance to self-antigen, among other roles, but they may also impair the anti-leukemic immune response. Multiple studies have identified a higher frequency of Tregs in newly diagnosed and relapsed/refractory AML patients compared with healthy controls (HC) in peripheral blood (PB) and bone marrow (BM).23–29 Several groups have also reported increased expansion of PB Tregs during lymphocyte recovery after induction and cytotoxic maintenance chemotherapy.30–32
Shenghui et al. investigated Treg frequency using the phenotype CD4+CD25+CD127lo in 182 newly diagnosed patients with AML. Tregs were increased in both the BM and PB (mean 11.8% versus 9.2%, respectively) compared with HC (mean 5.4%).33 In the presence of these leukemia-associated BM and PB Tregs, in vitro proliferation of CD4+CD25- T cells was reduced significantly relative to HC Tregs. Interestingly, BM Tregs possessed greater immunosuppression compared with PB Tregs (undivided cells: HC 52.0% versus PB 58.9% versus BM 65.3%). Follow up of 58 patients from the original cohort showed that those patients with persistently elevated PB Treg frequency after achieving CR had increased risk of relapse, consistent with other studies.26, 27, 34 Wang et al. found higher numbers of CD4+CD25hi T cells in PB of 36 patients at AML diagnosis, which was correlated with increased KI67 expression in AML patient-derived CD4+CD25hi Tregs compared with control Tregs (17.5% versus 1.9%).25 Activated CD4+CD25- T cells underwent less proliferation and also had decreased cytokine production of IL-2 and IFN-γ in the presence of these Tregs. 25
Despite several studies correlating Treg presence and number in AML and some data suggesting outcomes may be affected by Tregs, it remains unclear how Tregs should be understood in AML pathogenesis, prognosis, and treatment. Animal studies do provide some evidence that Treg targeting could be beneficial. A model of murine AML confirmed increased presence of Tregs in AML, and Treg depletion improved long-term survival.35 Emerging data indicates that the absolute number of Tregs may play a role in high risk MDS progression to AML. In a study of 42 MDS patients, those categorized as high risk had increased absolute number of Tregs with concomitant expansion of myeloid-derived suppressor cells (MDSC), leading to immune suppression and progression to AML.36 In contrast, a retrospective study found that increased numbers of Tregs during lymphocyte recovery after induction therapy correlated with to improved response to therapy and overall survival.37 The conflicting data makes it difficult to make specific conclusions about the role of Tregs in human AML. Further research should focus on ways to determine the role of Tregs in AML and whether inhibition of Treg effects would augment anti-leukemic immune response and improve outcomes.
Diminished Function of T Helper Cells
Helper T cell function may be compromised in AML. Studies in the 1970s and 1980s discussed above showed that loss of delayed hypersensitivity response (absence of T helper populations) correlated with response to chemotherapy and risk of relapse.16, 17 There are several observational studies which have found T helper 1 (Th1) populations have a decreased frequency and lower expression of interferon gamma (IFN-γ) in both PB and BM of AML patients compared with healthy donors.26, 38–41 Moreover, a correlation in increased frequency of Th17 cells, which secrete IL-17 and can inhibit Th1 IFN-γ production, accompanied such a decrease in Th1 cells in diagnostic BM and PB samples in several studies (Figure 1).42, 43 These data are not complete and a thorough understanding of Th subsets in AML remains elusive.
Figure 1.
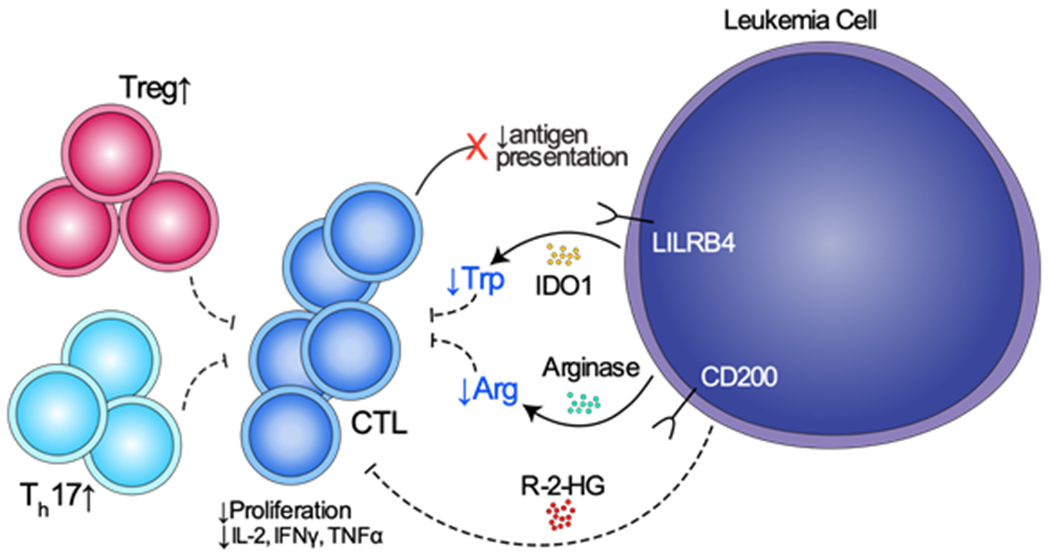
Proposed mechanisms of T cell suppression and promotion of T cell dysfunction by AML. LILRB4 is an inhibitory receptor found in monocytic AML that directly suppresses T cell proliferation. CD200 is a surface receptor found on leukemic blasts that is associated with increase in Tregs and reduce cytotoxic T cell function. IDO1 and arginase II are enzymes that reduce the presence of amino acids tryptophan and arginine, respectively, inhibiting T cell proliferation. 2-HG is an oncometabolite produced by IDH mutated AML that inhibits T cell proliferation and function.
T Cell Exhaustion in AML
After prolonged antigen exposure, T cells enter a state of exhaustion, characterized by sustained expression of immune checkpoint receptors, loss of effector functions, and loss of differentiation potential.44 Murine myeloid leukemia models and in vitro co-cultures have demonstrated increased inhibitory immune checkpoint receptors on T cells can promote AML progression, but this connection is less clear in human data.45–47
Williams et al performed a study immunophenotyping 107 patients with AML (39 newly diagnosed and 68 relapsed) based on surface phenotype and immune checkpoint expression.27 Though not significant, there was a trend toward increased co-expression of PD-1 with T-cell immunoglobulin and mucin-domain containing-3 (Tim3) and LAG-3 in CD4+ and CD8+ effector subsets in diagnostic and relapsed samples versus HCs. Additionally, expression of OX40 (co-stimulatory marker) was increased in CD4+ and CD8+ T cells consistent with activation of antigen experienced T cells. However, the study was limited as the T cell profiling occurred at one timepoint for these patients who received multiple modalities of therapy. No correlations were clear with respect to the T cell subsets studied and prognosis or response to therapy. In contrast, Knaus et al. assessed not only the immune checkpoint receptor expression but also T cell differentiation state and functional studies of T cells from patients with AML at diagnosis and after induction chemotherapy.48 The group found that there was significantly increased frequency of terminally differentiated effector cells (CCR7-CD45RA+ and CD27-CD45RA+) with decreased frequency of naïve (CCR7+CD45RA+) and naïve-like cells (CD27+CD45RA+) at diagnosis. There was a higher percentage CD57 (a marker of senescence), 2B4, and PD-1 on AML CD8+ T cells compared with HCs. Transcriptional profiling of the AML CD8+ T cells showed upregulation of inhibitory receptor genes including CD244 (encoding 2B4), CD160, LILRB1, LAG3, and TIGIT. There was also downregulation of costimulatory receptor genes CD40L and CD28. However, in vitro stimulation of CD8+ T cells from newly diagnosed AML patients versus HCs did not show a significant difference in cytokine production. Thus, while T cells are more antigen experienced at diagnosis, they are not losing their functional cytokine capacity.
Beyond diagnostic patient samples showing an increase in antigen experienced T cells, studies have evaluated immune checkpoints on T cells based on response to therapy. A study of 59 patients with de novo AML demonstrated that CD8+ T cells with increased co-expression of PD-1 and TIGIT (T cell immunoreceptor with Ig and ITIM domains) were associated with failure to achieve remission after induction chemotherapy.49 Knaus et al determined that the percentage of cells expressing PD-1 and Tim3 were increased in the BM compared to pretreatment levels in patients who failed to achieve remission.48 Moreover, transcriptional profiling of PB AML CD8+ T cells showed gene expression upregulation of costimulatory markers (ICOS, CD28) and downregulation of coinhibitory genes (CD244, CD160) in patients achieving complete remission (CR) versus no response. Moreover, in a phase I dose escalation trial combining selinexor, a selective inhibitor of nuclear export, with high dose cytarabine and mitoxantrone, multi-parameter flow cytometry was performed on PB and BM samples at diagnosis and after induction therapy in 26 patients.50 There was increased Tim3 expression on BM T cells in treatment failure patients. The frequency of PD-1+CD4+ and PD-1+CD8+ T cells was higher at the time of diagnosis in treatment failure patients compared with those that achieved CR. Thus, the data implicates co-expression of immune checkpoint receptors as possible markers for therapy response but which markers are potential targets for immunotherapy are unknown.
Emerging data in AML also reveals the use of novel markers to characterize T cell state. Two members of the cell fate and differentiation-related T-box family of transcription factors, T-bet and Eomesodermin (Eomes), have been implicated in T cell exhaustion. Within chronic viral infections involving exhausted T cells, T-bet maintains the presence of nonterminal T progenitor cells and Eomes regulates terminally exhausted CD8+ T cells.51 Eomes expressing CD8+ T cells had higher co-expression of PD-1 and TIGIT consistent with an exhausted T cell phenotype and functional impairment.51, 52 In a study of 59 newly diagnosed AML patients, the presence of Eomes+T-betlo CD8+ T cells was associated with poor response to induction chemotherapy and shorter overall survival.53 In comparison to diagnosis, patients achieving a CR had statistically significant decrease in Eomes+T-betlo CD8+ T cells. Patients with primary refractory disease had a much higher percentage of Eomes+T-betlo CD8+ cells which, in turn, correlated significantly with lower overall survival. Using in vitro stimulation, Eomes+T-betlo CD8+ cells produced significantly less IFN-γ and TNF-α consistent with decreased functional capability. Further supporting this idea, a single arm phase II clinical trial for relapsed/refractory AML patients treated with azacytidine and nivolumab used mass cytometry to profile bone marrow aspirate T cells before and during treatment.54 There was a small increase in a cluster of CD8+ T cells with phenotype CD45RA+PD1loTbethiEomeslo at diagnosis and post treatment in patients whom responded compared to no response. Differential expression of T-box transcription factors may be involved in regulating T cell exhaustion in AML and contribute to therapy response and relapse, though further study is warranted.
The upregulation of inhibitory immune checkpoint co-receptors possibly controlled by T-box transcription factors leads to a T cell profile that favors leukemic evasion and progression. However, presence of antigen experienced T cells does not alone indicate the T cells are dysfunctional as in vitro studies have showed normal proliferation and cytokine expression of effector T cells.48 Additionally, clinical trials using checkpoint inhibitors as monotherapy have not demonstrated a dramatic response in patients with AML.55, 56 Therefore, given the co-expression of immune checkpoint receptors present on T cells, immunotherapy involving more targets may be necessary to achieve robust anti-leukemic response.
Epigenetic Changes of T Cells
Dysregulated epigenetic mechanisms have been well established in pathogenesis of AML.57 There is now emerging pre-clinical data that unresponsiveness and/or resistance to immunotherapy may be the result of epigenetic modifications leading to a dysfunctional T cell state.58, 59 In a 30 AML patient cohort from Switzerland, transcriptomic analysis of BM CD8+ T cells revealed downregulation of genes involved in signaling pathways for T cell activation, differentiation, and function including NF-kB, Wnt, FoxO, T cell receptor, and cytokine/chemokine signaling.60 The small number of upregulated genes were involved in control of histone deacetylation and epigenetic regulation. Thus, epigenetic modifications of T cells can affect T cell receptor activation and function in AML. However, more evidence is needed to verify the importance of epigenetic changes in T cell activation and function in AML.
ACUTE MYELOID LEUKEMIA HETEROGENEITY
Immunosuppressive Soluble Factors
AML cells are capable of manufacturing soluble factors, enzymes, and oncometabolites in the immune environment to suppress T cells and T cell function. IDO1 (indoleamine 2,3-dioxygenase 1) catabolizes the degradation of tryptophan to N-formylkynurenine and is one of the most studied enzymes produced by AML.61 Various studies have shown that IDO is constitutively expressed by BM and PB AML blasts. By comparison, this is not the case in normal CD34+ hematopoietic progenitor cells and may portend a worse prognosis in AML.62–64 Mechanistically, the reduction in local tryptophan concentration and accumulation of toxic tryptophan metabolites cooperate to arrest T cells in the G1 phase of the cell cycle and halting their proliferation (Figure 1).65 Further, tryptophan-derived metabolites like L-kynurenine inhibit antigen-specific T cell proliferation and induce T cell apoptosis.61 In addition, there is an association between IDO+ AML and frequency of Tregs compared with IDO- AML and HCs.66, 67 Similarly, arginine catabolism is another immunosuppressive feature of AML. In a study by Mussai et al, 15 newly diagnosed AML samples were examined for enzymatic arginase II activity and T cell proliferation.68 When T cells were cultured in vitro with these AML patients’ plasma, there was decreased T cell proliferation that was partially restored with inhibitors of arginase activity indicating that arginase II is partially responsible for T cell suppression in AML (Figure 1).69 Moreover, in both in vitro and murine models, arginase II polarized healthy monocytes to an M2-like macrophage phenotype which produce the immunosuppressive cytokine IL-10 and inhibit T cell function.68, 70
AML can also produce oncometabolites that can interfere with T cell function. Approximately 15% of AML has a mutation in the gene encoding for the protein isocitrate dehydrogenase 1 or 2 (IDH1/2), which introduces neomorphic function in this metabolic enzyme.2, 71 The result is the accumulation of the oncometabolite (R)-2-hydroxyglutarate (2-HG) leading to epigenetic changes, aberrant gene expression, cell proliferation, and leukemia progression.72, 73 Bunse et al studied T cell activity in the presence of 2-HG, proposing that 2-HG is directly toxic to T cells (Figure 1). 74 These investigators showed that 2-HG could inhibit ATP synthase, resulting in AMPK activation. This activation resulted in decreased T cell proliferation and function via inhibition of nuclear factor of activated T cells (NFAT) transcription using in vitro studies.74 Corroborative data from Zhang et al showed decreased proliferation, cytokine production, and migration of T cells in the presence of 2-HG with a murine model.75 2-HG also is implicated in T cell metabolism as accumulation of physiologic levels of 2-HG leads to Th17 cell differentiation via glutamate-dependent metabolic changes in an autoimmune murine model.76 While this data is in the context of glioma and autoimmune models, 2-HG likely has similar roles in the setting of mIDH AML, though this connection has not been studied in depth.
Surface Receptor Heterogeneity
Leukemia cells have fluctuating capability of many direct and indirect interactions with T cells. More differentiated subtypes of AML, such as AMML, may retain some functions unique to their normal counterparts. Such heterogeneity results in variability in the production of receptors and soluble factors that can interact with T cells and alter their function. One such receptor, leukocyte immunoglobulin-like receptor B4 (LILRB4), is a novel inhibitory immune checkpoint receptor that can be expressed on both healthy monocytes, as well as AMML cells, and directly suppresses T cell proliferation with in vitro co-culture assays (Figure 1).46 This marker may also be associated with decreased survival in the subset of AML patients that express this marker.77, 78 Another group of investigators recently demonstrated using single cell RNA sequencing that CD14+ AML cells (monocyte-like) displayed in vitro inhibition of T cell activation, supplying further evidence that differentiated monocytic AML cells possess unique immunomodulatory functions that contribute to leukemia progression.28
In several studies of AML patient samples, high expression of CD200, a type I membrane glycoprotein within the immunoglobulin superfamily, on leukemic blasts correlates with poor prognosis in core binding factor and cytogenetically normal AML.79–81 More recent studies have also found that CD200 expression on AML is both associated with increases in Tregs and reduced memory T cell function in human studies (Figure 1).82, 83 Similarly, in multiple solid tumors as well as multiple myeloma and B cell lymphomas, high expression of CD200 is associated with lower overall survival.84, 85 When CD200 binds its receptor, CD200R, found on myeloid cells and lymphocytes, it can inhibit macrophage function, induce Treg expansion, and influence a switch from Th1 phenotype to inhibitory Th2 cells; thus, CD200 may suppress anti-leukemic immunity and promote progression of disease.85–87
AML cell expression of major histocompatibility complex II (MHC II) molecules allows for antigen presentation and targeting by T cells for elimination. However, in the post allogeneic SCT setting, AML has been shown to downregulate MHC class II genes to evade immune surveillance.28, 88 In a study comparing diagnostic and post allogeneic SCT relapse samples, 50% of patients (17/34) were found to have MHC class II gene downregulation at relapse using RNA sequencing and flow cytometry.88 Further, genes involved in antigen presentation and T cell co-stimulatory molecules, including CD86, were downregulated in six out of seven patients tested. Using IFN-γ stimulation, which is known to upregulate MHC class II expression, this reduction of MHC class II expression was reversed in three samples tested. This phenomenon of MHC II downregulation was not shared by post chemotherapy relapses included in this study. However, using transcriptional profiling of AML leukemic stem cells and progenitor cells, there was a significant downregulation of genes involved in signaling pathways mediating antigen-presentation and interactions with immune cells from diagnostic samples (Figure 1).60 Consequently, the impact of leukemic manipulation of antigen presentation on T cell function warrants further investigation.
In addition to altering expression of MHC II molecules, AML cells appear to alter actin polymerization and immune synapse formation with T cells. Le Dieu et al used gene expression profiling of T cells to demonstrate differential expression of actin polymerization and cell polarization genes between T cells from AML samples and age-matched HCs.11 Using confocal imaging, they found dysfunctional actin organization and impaired immunological synapse formation between T cells and AML blasts. Knaus et al. also showed downregulation of genes involved in T cell adhesion and migration by transcriptional profiling of AML CD8+ T cells, consistent with the findings from Le Dieu et al.48 This evidence suggests that T cells are ineffectively activated and unable to form functional immune synapses with leukemia cells. Further investigation of alterations in actin polymerization and cell-cell interactions, especially with imaging techniques, may help elucidate the specific mechanisms causing impaired immunological synapse formation.
DISCUSSION
The ability to boost effector T cell function or restore function of exhausted T cells has become a key therapeutic intervention in solid tumors and some hematologic malignancies but remains elusive in AML. The data presented in this review reveals that comprehensive understanding of the immune microenvironment of AML is lacking. Moreover, the translational studies presented are generally correlative from patient data without significant mechanistic insight. While there is promising evidence of T cell suppression due to leukemic manipulation of the immune microenvironment, there is much work to be done. To overcome the multifaceted immune suppression observed in AML, a number of questions remain only partially or completely unanswered: 1) Do specific subtypes of AML utilize distinctive combinations of immune evasion mechanisms? 2) Are there additional mechanisms of immune escape? 3) Is there an optimal time to consider immunotherapy for AML? 4) What are the best targets for immunotherapy in AML?
Given the dynamic and heterogeneous nature of the AML microenvironment, an understanding of the leukemic microenvironment at the single cell level will be essential.89 Single cell advances have the potential to pave the way to a comprehensive and detailed understanding of the leukemia-associated immune response. Discovering T cell phenotypes that are present at specific disease time points, dissecting the functional heterogeneity of T cell populations, and identifying barriers and targets through emerging single cell technologies will be invaluable in overcoming T cell suppression and immune evasion by AML.
Acknowledgements
This study was supported by 1K23HL138291-01 K23 NHLBI (P.B.F.) and 1T32CA217834-01A1 T32 (Z.L.).
Footnotes
Competing Interests
Dr. Ferrell receives funding support from Incyte, Forma Therapeutics, and Astex Pharmaceuticals and is a consultant for Agios Pharmaceuticals. Dr. Li and Dr. Philip have no competing interests to declare.
References
- 1.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2016. In. Bethesda, MD. [Google Scholar]
- 2.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. The New England journal of medicine 2016; 374(23): 2209–2221. e-pub ahead of print 2016/06/09; doi: 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollyea DA. New drugs for acute myeloid leukemia inspired by genomics and when to use them. Hematology. American Society of Hematology. Education Program 2018; 2018(1): 45–50. e-pub ahead of print 2018/12/07; doi: 10.1182/asheducation-2018.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerrano M, Itzykson R. New Treatment Options for Acute Myeloid Leukemia in 2019. Current oncology reports 2019; 21(2): 16 e-pub ahead of print 2019/02/05; doi: 10.1007/s11912-019-0764-8 [DOI] [PubMed] [Google Scholar]
- 5.Lamble AJ, Lind EF. Targeting the Immune Microenvironment in Acute Myeloid Leukemia: A Focus on T Cell Immunity. Frontiers in oncology 2018; 8: 213 e-pub ahead of print 2018/06/29; doi: 10.3389/fonc.2018.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Critical reviews in oncology/hematology 2016; 103: 62–77. e-pub ahead of print 2016/06/02; doi: 10.1016/j.critrevonc.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 7.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75(3): 555–562. e-pub ahead of print 1990/02/01; [PubMed] [Google Scholar]
- 8.Boddu P, Kantarjian H, Garcia-Manero G, Allison J, Sharma P, Daver N. The emerging role of immune checkpoint based approaches in AML and MDS. Leukemia & lymphoma 2018; 59(4): 790–802. e-pub ahead of print 2017/07/07; doi: 10.1080/10428194.2017.1344905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Bewersdorf JP, Stahl M, Zeidan AM. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: The dawn of a new era? Blood reviews 2019; 34: 67–83. e-pub ahead of print 2018/12/17; doi: 10.1016/j.blre.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. Journal of immunology (Baltimore, Md. : 1950) 2001; 167(10): 6021–6030. e-pub ahead of print 2001/11/08; [DOI] [PubMed] [Google Scholar]
- 11.Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood 2009; 114(18): 3909–3916. e-pub ahead of print 2009/08/28; doi: 10.1182/blood-2009-02-206946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barosi G An immune dysregulation in MPN. Current hematologic malignancy reports 2014; 9(4): 331–339. e-pub ahead of print 2014/08/21; doi: 10.1007/s11899-014-0227-0 [DOI] [PubMed] [Google Scholar]
- 13.Fozza C, Crobu V, Isoni MA, Dore F. The immune landscape of myelodysplastic syndromes. Critical reviews in oncology/hematology 2016; 107: 90–99. e-pub ahead of print 2016/11/09; doi: 10.1016/j.critrevonc.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 14.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9(1): 34 e-pub ahead of print 2017/04/20; doi: 10.1186/s13073-017-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klco JM, Spencer DH, Miller CA, Griffith M, Lamprecht TL, O’Laughlin M et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer cell 2014; 25(3): 379–392. doi: 10.1016/j.ccr.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh EM, Whitecar JP, Jr., McCredie KB, Bodey GP, Sr., Freireich EJ. Chemotherapy, immunocompetence, immunosuppression and prognosis in acute leukemia. The New England journal of medicine 1971; 285(22): 1211–1216. e-pub ahead of print 1971/11/01; doi: 10.1056/nejm197111252852201 [DOI] [PubMed] [Google Scholar]
- 17.Hersh EM, Gutterman JU, Mavligit GM, McCredie KB, Burgess MA, Matthews A et al. Serial studies of immunocompetence of patients undergoing chemotherapy for acute leukemia. The Journal of clinical investigation 1974; 54(2): 401–408. e-pub ahead of print 1974/08/01; doi: 10.1172/jci107775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashidi A, Fisher SI. Spontaneous remission of acute myeloid leukemia. Leukemia & lymphoma 2015; 56(6): 1727–1734. e-pub ahead of print 2014/10/07; doi: 10.3109/10428194.2014.970545 [DOI] [PubMed] [Google Scholar]
- 19.Muller-Schmah C, Solari L, Weis R, Pfeifer D, Scheibenbogen C, Trepel M et al. Immune response as a possible mechanism of long-lasting disease control in spontaneous remission of MLL/AF9-positive acute myeloid leukemia. Annals of hematology 2012; 91(1): 27–32. e-pub ahead of print 2011/10/01; doi: 10.1007/s00277-011-1332-y [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa K, Tanaka S, Fujiki F, Morimoto S, Nakajima H, Tatsumi N et al. An Immunocompetent Mouse Model for MLL/AF9 Leukemia Reveals the Potential of Spontaneous Cytotoxic T-Cell Response to an Antigen Expressed in Leukemia Cells. PLoS ONE 2015; 10(12): e0144594 e-pub ahead of print 2015/12/15; doi: 10.1371/journal.pone.0144594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. The Journal of clinical investigation 2013; 123(5): 1999–2010. e-pub ahead of print 2013/04/27; doi: 10.1172/jci63980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almosailleakh M, Schwaller J. Murine Models of Acute Myeloid Leukaemia. International journal of molecular sciences 2019; 20(2). e-pub ahead of print 2019/01/24; doi: 10.3390/ijms20020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou W Regulatory T cells, tumour immunity and immunotherapy. Nature reviews. Immunology 2006; 6(4): 295–307. e-pub ahead of print 2006/03/25; doi: 10.1038/nri1806 [DOI] [PubMed] [Google Scholar]
- 24.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. Journal for immunotherapy of cancer 2013; 1(13). e-pub ahead of print 2013/12/20; doi: 10.1186/2051-1426-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zheng J, Liu J, Yao J, He Y, Li X et al. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. European journal of haematology 2005; 75(6): 468–476. e-pub ahead of print 2005/11/30; doi: 10.1111/j.1600-0609.2005.00537.x [DOI] [PubMed] [Google Scholar]
- 26.Tian T, Yu S, Liu L, Xue F, Yuan C, Wang M et al. The Profile of T Helper Subsets in Bone Marrow Microenvironment Is Distinct for Different Stages of Acute Myeloid Leukemia Patients and Chemotherapy Partly Ameliorates These Variations. PLoS ONE 2015; 10(7). doi: 10.1371/journal.pone.0131761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2018. e-pub ahead of print 2018/12/01; doi: 10.1002/cncr.31896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Galen P, Hovestadt V, Wadsworth Ii MH, Hughes TK, Griffin GK, Battaglia S et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019; 176(6): 1265–1281.e1224. e-pub ahead of print 2019/03/05; doi: 10.1016/j.cell.2019.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood 2011; 118(19): 5084–5095. e-pub ahead of print 2011/09/02; doi: 10.1182/blood-2011-07-365817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol 2010; 11: 38 e-pub ahead of print 2010/07/14; doi: 10.1186/1471-2172-11-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood 2011; 117(2): 608–617. doi: 10.1182/blood-2010-04-277939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtenegger FS, Lorenz R, Gellhaus K, Hiddemann W, Beck B, Subklewe M. Impaired NK cells and increased T regulatory cell numbers during cytotoxic maintenance therapy in AML. Leukemia research 2014; 38(8): 964–969. doi: 10.1016/j.leukres.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 33.Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z et al. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. International journal of cancer 2011; 129(6): 1373–1381. e-pub ahead of print 2010/11/26; doi: 10.1002/ijc.25791 [DOI] [PubMed] [Google Scholar]
- 34.Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research 2009; 15(10): 3325–3332. e-pub ahead of print 2009/05/07; doi: 10.1158/1078-0432.Ccr-08-3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood 2009; 114(18): 3793–3802. e-pub ahead of print 2009/09/03; doi: 10.1182/blood-2009-03-208181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology 2016; 5(2): e1062208 e-pub ahead of print 2016/04/09; doi: 10.1080/2162402x.2015.1062208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menter T, Kuzmanic B, Bucher C, Medinger M, Halter J, Dirnhofer S et al. Beneficial role of increased FOXP3(+) regulatory T-cells in acute myeloid leukaemia therapy response. British journal of haematology 2018; 182(4): 581–583. e-pub ahead of print 2017/06/28; doi: 10.1111/bjh.14819 [DOI] [PubMed] [Google Scholar]
- 38.Schnorfeil FM, Lichtenegger FS, Emmerig K, Schlueter M, Neitz JS, Draenert R et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. Journal of hematology & oncology 2015; 8: 93 e-pub ahead of print 2015/07/30; doi: 10.1186/s13045-015-0189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun YX, Kong HL, Liu CF, Yu S, Tian T, Ma DX et al. The imbalanced profile and clinical significance of T helper associated cytokines in bone marrow microenvironment of the patients with acute myeloid leukemia. Human immunology 2014; 75(2): 113–118. e-pub ahead of print 2013/11/26; doi: 10.1016/j.humimm.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 40.Musuraca G, De Matteis S, Napolitano R, Papayannidis C, Guadagnuolo V, Fabbri F et al. IL-17/IL-10 double-producing T cells: new link between infections, immunosuppression and acute myeloid leukemia. Journal of translational medicine 2015; 13: 229 e-pub ahead of print 2015/07/16; doi: 10.1186/s12967-015-0590-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian T, Yu S, Wang M, Yuan C, Zhang H, Ji C et al. Aberrant T helper 17 cells and related cytokines in bone marrow microenvironment of patients with acute myeloid leukemia. Clinical & developmental immunology 2013; 2013: 915873 e-pub ahead of print 2013/09/12; doi: 10.1155/2013/915873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and interleukin-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer science 2014; 105(8): 933–942. e-pub ahead of print 2014/06/04; doi: 10.1111/cas.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y et al. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clinical and experimental immunology 2009; 158(2): 199–204. e-pub ahead of print 2009/09/10; doi: 10.1111/j.1365-2249.2009.04011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry EJ. T cell exhaustion. Nature immunology 2011; 12(6): 492–499. e-pub ahead of print 2011/07/09; [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011; 117(17): 4501–4510. e-pub ahead of print 2011/03/10; doi: 10.1182/blood-2010-10-310425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng M, Gui X, Kim J, Xie L, Chen W, Li Z et al. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature 2018; 562(7728): 605–609. e-pub ahead of print 2018/10/20; doi: 10.1038/s41586-018-0615-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaBelle JL, Hanke CA, Blazar BR, Truitt RL. Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7–1+, but not B7–2+, murine myelogenous leukemia. Blood 2002; 99(6): 2146–2153. e-pub ahead of print 2002/03/06; [DOI] [PubMed] [Google Scholar]
- 48.Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI insight 2018; 3(21). e-pub ahead of print 2018/11/06; doi: 10.1172/jci.insight.120974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M, Bu J, Zhou M, Sido J, Lin Y, Liu G et al. CD8(+)T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clinical immunology (Orlando, Fla.) 2018; 190: 64–73. e-pub ahead of print 2017/09/13; doi: 10.1016/j.clim.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 50.Dama P, Tang M, Fulton N, Kline J, Liu H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. Journal for immunotherapy of cancer 2019; 7(1): 175 e-pub ahead of print 2019/07/12; doi: 10.1186/s40425-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science (New York, N.Y.) 2012; 338(6111): 1220–1225. e-pub ahead of print 2012/12/01; doi: 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia B, Wang L, Claxton DF, Ehmann WC, Rybka WB, Mineishi S et al. Bone marrow CD8 T cells express high frequency of PD-1 and exhibit reduced anti-leukemia response in newly diagnosed AML patients. Blood cancer journal 2018; 8(3): 34 e-pub ahead of print 2018/03/23; doi: 10.1038/s41408-018-0069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia B, Zhao C, Rakszawski KL, Claxton DF, Ehmann WC, Rybka WB et al. Eomes+T-betlow CD8+ T cells are functionally impaired and are associated with poor clinical outcome in patients with acute myeloid leukemia (AML). Cancer research 2019. e-pub ahead of print 2019/02/03; doi: 10.1158/0008-5472.Can-18-3107 [DOI] [PubMed] [Google Scholar]
- 54.Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer discovery 2019; 9(3): 370–383. e-pub ahead of print 2018/11/10; doi: 10.1158/2159-8290.Cd-18-0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masarova L, Kantarjian H, Ravandi F, Sharma P, Garcia-Manero G, Daver N. Update on Immunotherapy in AML and MDS: Monoclonal Antibodies and Checkpoint Inhibitors Paving the Road for Clinical Practice In: Naing A, Hajjar J (eds). Immunotherapy. Springer International Publishing: Cham, 2018, pp 97–116. [DOI] [PubMed] [Google Scholar]
- 56.Lee JB, Chen B, Vasic D, Law AD, Zhang L. Cellular immunotherapy for acute myeloid leukemia: How specific should it be? Blood reviews 2019; 35: 18–31. e-pub ahead of print 2019/03/04; doi: 10.1016/j.blre.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 57.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood 2016; 127(1): 42–52. e-pub ahead of print 2015/12/15; doi: 10.1182/blood-2015-07-604512 [DOI] [PubMed] [Google Scholar]
- 58.Kelderman S, Schumacher TN, Haanen JB. Acquired and intrinsic resistance in cancer immunotherapy. Molecular oncology 2014; 8(6): 1132–1139. e-pub ahead of print 2014/08/12; doi: 10.1016/j.molonc.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017; 545(7655): 452–456. e-pub ahead of print 2017/05/18; doi: 10.1038/nature22367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radpour R, Riether C, Simillion C, Hopner S, Bruggmann R, Ochsenbein AF. CD8(+) T cells expand stem and progenitor cells in favorable but not adverse risk acute myeloid leukemia. Leukemia 2019. e-pub ahead of print 2019/03/17; doi: 10.1038/s41375-019-0441-9 [DOI] [PubMed] [Google Scholar]
- 61.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The Journal of experimental medicine 2002; 196(4): 459–468. e-pub ahead of print 2002/08/21; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia 2007; 21(2): 353–355. e-pub ahead of print 2006/12/16; doi: 10.1038/sj.leu.2404485 [DOI] [PubMed] [Google Scholar]
- 63.Fukuno K, Hara T, Tsurumi H, Shibata Y, Mabuchi R, Nakamura N et al. Expression of indoleamine 2,3-dioxygenase in leukemic cells indicates an unfavorable prognosis in acute myeloid leukemia patients with intermediate-risk cytogenetics. Leukemia & lymphoma 2015; 56(5): 1398–1405. e-pub ahead of print 2014/09/25; doi: 10.3109/10428194.2014.953150 [DOI] [PubMed] [Google Scholar]
- 64.Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica 2008; 93(12): 1894–1898. e-pub ahead of print 2008/12/04; doi: 10.3324/haematol.13113 [DOI] [PubMed] [Google Scholar]
- 65.Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leukemia research 2009; 33(3): 490–494. e-pub ahead of print 2008/07/22; doi: 10.1016/j.leukres.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 66.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood 2007; 109(7): 2871–2877. e-pub ahead of print 2006/12/14; doi: 10.1182/blood-2006-07-036863 [DOI] [PubMed] [Google Scholar]
- 67.Arandi N, Ramzi M, Safaei F, Monabati A. Overexpression of indoleamine 2,3-dioxygenase correlates with regulatory T cell phenotype in acute myeloid leukemia patients with normal karyotype. Blood research 2018; 53(4): 294–298. e-pub ahead of print 2018/12/28; doi: 10.5045/br.2018.53.4.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 2013; 122(5): 749–758. e-pub ahead of print 2013/06/05; doi: 10.1182/blood-2013-01-480129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mussai F, Wheat R, Sarrou E, Booth S, Stavrou V, Fultang L et al. Targeting the arginine metabolic brake enhances immunotherapy for leukaemia. International journal of cancer 2018; 0(0). doi: 10.1002/ijc.32028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orleans-Lindsay JK, Barber LD, Prentice HG, Lowdell MW. Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function--implications for the adoptive immunotherapy of leukaemia. Clinical and experimental immunology 2001; 126(3): 403–411. e-pub ahead of print 2001/12/12; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 2013; 339(6127): 1621–1625. doi: 10.1126/science.1231677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia 2017; 31(2): 272–281. e-pub ahead of print 2016/10/11; doi: 10.1038/leu.2016.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DiNardo CD, Propert KJ, Loren AW, Paietta E, Sun Z, Levine RL et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood 2013; 121(24): 4917–4924. e-pub ahead of print 2013/05/04; doi: 10.1182/blood-2013-03-493197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nature medicine 2018; 24(8): 1192–1203. e-pub ahead of print 2018/07/11; doi: 10.1038/s41591-018-0095-6 [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Sorensen MD, Kristensen BW, Reifenberger G, McIntyre TM, Lin F. D-2-Hydroxyglutarate Is an Intercellular Mediator in IDH-Mutant Gliomas Inhibiting Complement and T Cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2018; 24(21): 5381–5391. e-pub ahead of print 2018/07/15; doi: 10.1158/1078-0432.Ccr-17-3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu T, Stewart KM, Wang X, Liu K, Xie M, Ryu JK et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 2017; 548(7666): 228–233. e-pub ahead of print 2017/08/08; doi: 10.1038/nature23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang X, Kim J, Deng M, John S, Chen H, Wu G et al. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell cycle (Georgetown, Tex.) 2016; 15(1): 25–40. e-pub ahead of print 2015/12/05; doi: 10.1080/15384101.2015.1121324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dobrowolska H, Gill KZ, Serban G, Ivan E, Li Q, Qiao P et al. Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytometry. Part B, Clinical cytometry 2013; 84(1): 21–29. e-pub ahead of print 2012/10/03; doi: 10.1002/cyto.b.21050 [DOI] [PubMed] [Google Scholar]
- 79.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK et al. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia 2007; 21(3): 566–568. e-pub ahead of print 2007/01/26; doi: 10.1038/sj.leu.2404559 [DOI] [PubMed] [Google Scholar]
- 80.Damiani D, Tiribelli M, Raspadori D, Sirianni S, Meneghel A, Cavalllin M et al. Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget 2015; 6(30): 30212–30221. e-pub ahead of print 2015/09/05; doi: 10.18632/oncotarget.4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zahran AM, Mohammed Saleh MF, Sayed MM, Rayan A, Ali AM, Hetta HF. Up-regulation of regulatory T cells, CD200 and TIM3 expression in cytogenetically normal acute myeloid leukemia. Cancer Biomark 2018; 22(3): 587–595. e-pub ahead of print 2018/05/31; doi: 10.3233/cbm-181368 [DOI] [PubMed] [Google Scholar]
- 82.Coles SJ, Hills RK, Wang EC, Burnett AK, Man S, Darley RL et al. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia 2012; 26(9): 2146–2148. doi: 10.1038/leu.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coles SJ, Hills RK, Wang EC, Burnett AK, Man S, Darley RL et al. Expression of CD200 on AML blasts directly suppresses memory T-cell function. Leukemia 2012; 26(9): 2148–2151. doi: 10.1038/leu.2012.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E et al. CD200 is a new prognostic factor in multiple myeloma. Blood 2006; 108(13): 4194–4197. e-pub ahead of print 2006/09/02; doi: 10.1182/blood-2006-06-029355 [DOI] [PubMed] [Google Scholar]
- 85.Moreaux J, Veyrune JL, Reme T, De Vos J, Klein B. CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun 2008; 366(1): 117–122. e-pub ahead of print 2007/12/07; doi: 10.1016/j.bbrc.2007.11.103 [DOI] [PubMed] [Google Scholar]
- 86.Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. Journal of immunology (Baltimore, Md. : 1950) 2007; 178(9): 5595–5605. e-pub ahead of print 2007/04/20; doi: 10.4049/jimmunol.178.9.5595 [DOI] [PubMed] [Google Scholar]
- 87.Kawasaki BT, Farrar WL. Cancer stem cells, CD200 and immunoevasion. Trends in immunology 2008; 29(10): 464–468. e-pub ahead of print 2008/09/09; doi: 10.1016/j.it.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 88.Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. The New England journal of medicine 2018; 379(24): 2330–2341. e-pub ahead of print 2018/11/01; doi: 10.1056/NEJMoa1808777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á et al. The bone marrow microenvironment at single-cell resolution. Nature 2019. doi: 10.1038/s41586-019-1104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


