Abstract
A recent outbreak of a novel Coronavirus responsible for a Severe Acute Respiratory Syndrome (SARS-CoV-2) is spreading globally. The aim of this study was to systematically review main clinical characteristics and outcomes of SARS-CoV-2 infections in pediatric age. An electronic search was conducted in PubMed database. Papers published between 1 January and 1 May 2020 including children aged 0–18 years were selected. Sixty-two studies and three reviews were included, with a total sample size of 7480 children (2428/4660 males, 52.1%; weighted mean age 7.6 years). Patients showed mainly mild (608/1432, 42.5%) and moderate (567/1432, 39.6%) signs of the infection. About 2% of children were admitted to the pediatric intensive care unit. The most commonly described symptoms were fever (51.6%) and cough (47.3%). Laboratory findings were often unremarkable. Children underwent a chest CT scan in 73.9% of all cases, and 32.7% resulted normal. Overall, the estimated mortality was 0.08%. A higher proportion of newborns was severely ill (12%) and dyspnea was the most common reported sign (40%).
Conclusion: SARS-CoV-2 affects children less severely than adults. Laboratory and radiology findings are mainly nonspecific. Larger epidemiological and clinical cohort studies are needed to better understand possible implications of COVID-19 infection in children.
|
What is Known: • A novel Coronavirus has been recently identified as responsible for a new Severe Acute Respiratory Syndrome (SARS-CoV-2) spreading globally. • There is limited evidence on SARS-CoV2 infection in children. | |
|
What is New: • Systematically reviewed available evidence showed that children with SARS-CoV-2 infection may have a less severe pattern of disease in comparison to adults. • Blood tests and radiology findings are mainly nonspecific in children but may help to identify those who are severely ill. |
Electronic supplementary material
The online version of this article (10.1007/s00431-020-03684-7) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Pediatrics, Infectious disease, Pandemic, Novel coronavirus, Neonatal
Introduction
In early January 2020, a novel type of Coronavirus (CoV) was identified in the bronchoalveolar lavage sample of a subject affected by pneumonia of unknown origin [1]. The virus was provisionally named novel coronavirus (2019-nCoV) [2] to differentiate it from the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) [3] and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) [4], responsible for two previous outbreaks, in 2002 and 2012, respectively [5]. Successively, the International Committee on Taxonomy of Viruses defined it as SARS-CoV-2 [6] and the associated disease has been called 2019 Coronavirus Disease (COVID-19). SARS-CoV-2 rapidly spread worldwide, forcing the World Health Organization (WHO) to declare the outbreak as a pandemic on 11 March [7, 8].
As of 1 May, 3,519,901 cases have been reported in 187 countries in all continents except for Antarctica, with 247,630 deaths [9]. Children seem to be less affected than adults, but data regarding epidemiologic characteristics and clinical features of COVID-19 in pediatric age are very poor and essentially based on limited case series [10, 11]. In a report of 72,314 cases from Chinese Center for Disease Control and Prevention (CDC), about 2% of all patients were aged < 19 years, but no specific clinical information was available [12].
Italy was among the first countries in the world to be hit by the COVID-19 outbreak, with 1.2% of all patients represented by children [9, 13, 14]. The estimated overall case-fatality rate in Italy resulted higher than in China (7.2% vs. 2.3%) [15], but no death in the pediatric age has been reported, confirming that the mortality remains low and no specific risk factor could be identified [16].
Neonatal SARS-CoV-2 infections are also extremely rare and, to date, there is no evidence of intrauterine infection caused by vertical transmission [17, 18]. As described in a case report and a case series, amniotic fluid, cord blood, neonatal throat swab, and colostrum samples collected from infected mothers were negative for COVID-19 [19, 20]. However, the question remains controversial, as IgM antibodies have been detected in newborns from mothers with COVID-19 [21], even though the probability of a false positivity should be taken in account. There is also growing evidence of cases of neonatal pneumonia which may be explained by SARS-CoV-2 infection [22, 23]. Currently in China, all newborns are separated from their infected mothers for at least 14 days [24], while the US CDC advise to consider a temporary separation between the infected mother and her infant on a case-by-case basis, using shared decision-making between the patient and the clinical team [25].
Despite the global interest and concern about COVID-19, clinical pattern is still unclear for the pediatric health community. The aim of our review is to provide a concise and systematic overview of the available evidence on clinical, laboratory, and radiological findings in children with SARS-CoV-2 infection.
Materials and methods
This study is in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplementary material S1) [26].
An electronic search was conducted on studies published from 1 January 2020 to 1 May 2020 in PubMed database. We used the search terms “2019 novel coronavirus OR COVID-19 OR SARS-CoV-2 AND child* OR pediatric* OR newborn OR infant” with no language restriction to include as much data as possible. However, since full texts available only in Chinese language could not be evaluated, we had to rely on English-language abstracts. For the purpose of this review, studies on children aged 0 to 18 years were included. Case reports, case series, and retrospective or observational studies were all considered eligible.
Articles were first screened by title and abstract: duplicates and those with no available English summary were excluded. Eligible full texts were then assessed for pediatric clinical, laboratory, and radiological data. Papers reporting information on both children and adults were included only if pediatric data could be retrieved. To identify missing studies, we also checked the reference list for each selected paper. Included studies were also assessed for methodological quality according to the Joanna Briggs Institute (JBI) Critical Appraisal Tools (Supplementary material S2) [27].
Three reviewers extracted data independently. A standardized table with the following information was used for data extraction: first author, date and journal of publication, study design (cohort study, case series, case report), sample size, age, mortality and morbidity rate, clinical features, laboratory and radiological results, and treatment information. All included studies were differentiated in tables for newborns and for children aged > 1 month.
During the data analysis process, clinical patterns were grouped according to the pediatric scoring for patients with COVID-19 (recommendations issued by the pediatric branch of the Chinese Medical Association [28]). In particular, cases described as “upper respiratory tract infection” (i.e., pharyngeal congestion, sore throat, and fever) with no abnormal radiographic and septic presentation were included in the “mild” symptomatic category. Children with radiological findings of “pneumonia” and no complications were categorized as “moderate.” Patients with mild or moderate clinical patterns, plus any manifestations suggesting disease progression (i.e., tachypnea, hypoxia, neurological deterioration, dehydration, myocardial injury, coagulation dysfunction, rhabdomyolysis), were considered “severe.” Critically ill children were those with a rapid disease progression in particular those who developed respiratory failure with need for mechanical ventilation (i.e., acute respiratory distress syndrome, persistent hypoxia), septic shock, or multiple organ failure (MOF) [28].
Laboratory data were presented as abnormally high or low according to the reference value reported by the paper, but normal ranges of main laboratory parameters were not always clearly defined. Similarly, radiological findings were categorized according to the available description.
A quantitative synthesis of the included studies was performed. For continuous variables, weighted mean (range) was calculated as appropriate, while categorical variables were expressed as percentages or frequencies in relation to the total or subtotal sample size, according to the number of missing data.
Results
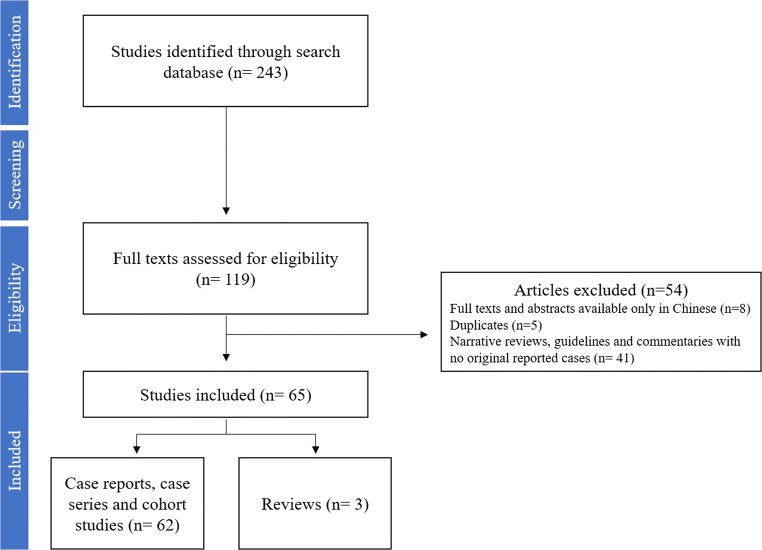
We initially identified 243 papers on SARS-CoV-2 infection in children published from 1 January 2020 to 1 May 2020. After screening the title and abstract, 119 full articles were evaluated for eligibility. At the end of the selection process (Fig. 1), 62 [10, 11, 14, 16, 22, 23, 29–84] studies and three previously published reviews [85–87] with a total sample size of 7480 children (2428/4660 males, 52.1%) were included in the systematic review (Table 1). The weighted mean age of patients was 7.6 years, ranging from 0 to 18 years. Children included were mainly Italian (3293/7479; 44.1%), from the USA (2572/7479; 34.4%), and Chinese (1358/7479; 18.2%). The most extensive retrospective study [16] described clinical characteristics of 2143 children with confirmed (n = 713) or suspected (n = 1430) SARS-CoV-2 infection. For the purpose of this review, we considered only the confirmed cases. Overall, 2926/4709 (71%) were discharged after a weighted mean hospitalization of 11.2 days (range 2–27). The estimated mortality of confirmed cases of SARS-CoV-2 infection was 0.08%, with six reported deaths: a 10-month-old baby with intussusception who developed MOF [10], a 14-year-old boy from Hubei province [16], and a preterm newborn who died from complications of sepsis [65]. Other three children died according to the report of CDC [76], but review of these cases is still ongoing to confirm COVID-19 as the likely cause of death. Significant comorbidities were reported in 129/587 (22%) patients with known underlying disease status: 58/129 (45%) were asthmatic or had chronic lung disease [61, 62, 76, 78], 30/129 (23%) had a diagnosis of congenital heart disease [32, 61, 76, 77], 15/129 (12%) were on immunosuppressive treatment [30, 76, 77], and 8/129 (6%) children had hemato-oncological diseases [31, 61, 76]. Neurological conditions, prematurity, and metabolic disease were also reported [62, 76].
Fig. 1.
PRISMA flow diagram of the included studies on children with SARS-CoV-2 infection
Table 1.
Characteristics of the included studies and main outcome measures in children with documented SARS-CoV-2 infection
| Author | Cohort | Case series | Case report | Country | Language | N | Males | Age | Mortality | Still admitted at time of publication | Discharged | Days of hospitalization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu [10] | √ | – | – | China | English | 171 | 104 | Median 6.7 years (0–15 years) | 1a | 149 | 21 | NR |
| Wang [33] | √ | – | – | China | Chinese | 34 | 14 | Median 8 years | 0 | 0 | 34 | NR |
| Xia [34] | – | √ | – | China | English | 20 | 13 | Median 2.1 (0–14 years) | 0 | 0 | 20 | 12.9b |
| KSID [29] | √ | – | – | S. Korea | English | 201 | NR | 0–9 years: 16%; 9–19 years: 84% (45-day-old infant–19 years) | 0 | NR | 201 | NR |
| Dong [16] | √ | – | – | China | English | 713c | 420 | Median 7 years (2–13 years) | 1 | NR | NR | NR |
| Liu [39] | – | √ | – | China | English | 4 | 2 | 2 months −9 years | 0 | 0 | 4 | NR |
| Wang [63] | – | – | √ | China | English | 1 | 1 | Newborn (36 h) | 0 | 0 | 1 | 16 |
| Cui [64] | – | – | √ | China | English | 1 | 0 | 55 days | 0 | 0 | 1 | 11 |
| Li [57] | – | – | √ | China | English | 2 | 1 | 4 years | 0 | 1 | 1 | NR |
| Ji [41] | – | – | √ | China | English | 2 | 2 | 9–15 years | 0 | 0 | 2 | 2 |
| Liu [11] | – | √ | – | China | English | 6 | 2 | Median 3 years (1–7 years) | 0 | 0 | 6 | 7.5d |
| Zhou [42] | – | √ | – | China | Chinese | 9 | NR | 0–3 years | 0 | 0 | 9 | NR |
| Zhu [65] | – | √ | – | China | English | 10 | 8 | Newborns (7–9 days) | 1e | 4 | 5 | NR |
| Li [40] | – | √ | – | China | English | 5 | 4 | Median 3.4 years (10 months–6 years) | 0 | 2 | 3 | 12–14 |
| D’Antiga [30] | – | √ | – | Italy | English | 3f | NR | NR | 0 | 0 | 3 | NR |
| Sun [31] | – | √ | – | China | English | 8g | 6 | 2 months–15 years | 0 | 3 | 5 | NR |
| Zheng [32] | – | √ | – | China | English | 25h | 14 | Median 3 years (3 months–14 years) | 0 | 24i | 1 | NR |
| Park [43] | – | – | √ | S. Korea | English | 1 | 0 | 10 years | 0 | 0 | 1 | NR |
| Lu [66] | – | √ | – | China | English | 3 | NR | Newborns (1, 5, and 17 days) | 0 | 0 | 3 | NR |
| Liu** [44] | – | – | √ | China | English | 1 | 1 | 10 years | 0 | 0 | 1 | NR |
| Chan** [45] | – | – | √ | China | English | 1 | 1 | 10 years | 0 | 0 | 1 | NR |
| Cai** [46] | – | – | √ | China | Chinese | 1 | 1 | 7 years | 0 | 0 | 1 | NR |
| Chen** [47] | – | – | √ | China | Chinese | 1 | 1 | 13 months | 0 | 0 | 1 | NR |
| Zhang** [67] | – | – | √ | China | Chinese | 1 | 0 | 3 months | 0 | 0 | 1 | NR |
| Zeng** [68] | – | – | √ | China | Chinese | 1 | 1 | Newborn (14 days) | 0 | 0 | 1 | NR |
| Cai** [38] | – | √ | – | China | English | 10 | 4 | Median 6.2 years (3 months–11 years) | 0 | 0 | 10 | NR |
| Kam** [48] | – | – | √ | China | English | 1 | 1 | 6 months | 0 | 0 | 1 | 18 |
| Feng** [35] | – | √ | – | China | Chinese | 15 | 5 | 4–14 years | 0 | 0 | 15 | NR |
| Wang** [36] | √ | – | – | China | Chinese | 31 | NR | 7 years (6 months–17 years) | 0 | 7 | 24 | NR |
| Zhang** [49] | – | √ | – | China | Chinese | 2 | 0 | 14 months | 0 | 0 | 2 | NR |
| Zhao** [50] | – | – | √ | China | Chinese | 1 | 1 | 13 years | 0 | 0 | 2 | NR |
| Wei [51] | – | √ | – | China | English | 9 | 2 | 1–11 months | 0 | 0 | 9 | NR |
| Shen [52] | – | √ | – | China | English | 28 | NR | 1 months–17 years | 0 | 0 | 28 | NR |
| Lou [53] | – | √ | – | China | English | 3 | 1 | 6 months and 6–8 years | 0 | 0 | 3 | 10 |
| Qian [54] | – | – | √ | China | English | 1 | 0 | 13 months | 0 | 0 | 1 | NR |
| Wang [70] | – | – | √ | China | Chinese | 1 | NR | Newborn (19 days) | 0 | 0 | 1 | 14 |
| Su [55] | – | √ | – | China | English | 9 | 3 | Median 3.5 years (11 months–9 years) | 0 | 0 | 9l | NR |
| Zeng [23] | – | √ | – | China | English | 3 | 3 | Newborns (48 h) | 0 | 0 | 3 | 2–11 |
| Le [69] | – | – | √ | Vietnam | English | 1 | 0 | 3 months | 0 | 0 | 1 | 14 |
| Tang [56] | – | – | √ | China | English | 1 | 1 | 10 years | 0 | 0 | 1 | NR |
| Xu [37] | – | √ | – | China | English | 10 | 6 | Median 6.6 years (2 months–15 years) | 0 | 6 | 4 | 10.5 |
| ISS [14]*** | √ | – | – | Italy | English | 3293 | 1683 | <17 yearsm | 0 | 134 | 2277 | NR |
| Pan [58] | – | – | √ | China | English | 1 | 1 | 3 years | 0 | 0 | 1 | NR |
| Chen [62] | √ | – | – | China | English | 31 | 13 | 1.5–17 years | 0 | 8 | 23 | NR |
| Xing [59] | – | √ | – | China | English | 3 | 2 | Median 4.2 years | 0 | 0 | 3 | 23 |
| Qiu [60] | √ | – | – | China | English | 36 | 23 | Mean 8.3 years (1–16 years) | 0 | 0 | 36 | 14 |
| Zhang [61] | √ | – | – | China | English | 34 | 14 | Median 33 months (10–94 months) | 0 | 0 | 34 | NR |
| Dong [22] | – | – | √ | China | English | 1 | 0 | Newborn (from birth) | 0 | 0 | 1 | NR |
| Shen [71] | – | √ | – | China | English | 9 | 3 | Median 8 yrs. (1–12 years) | 0 | 3 | 6 | 15.3 |
| Han [72] | – | √ | – | China | English | 7 | 4 | Mean 1.3 years (2 months–13 years) | 0 | 0 | 7 | 10 |
| Kamli-Aghdam [73] | – | – | √ | Iran | English | 1 | 1 | Newborn (15 days) | 0 | 0 | 1 | 6 |
| Canarutto [74] | – | – | √ | Italy | English | 1 | 1 | Newborn (32 days) | 0 | 0 | 1 | 5 |
| Li [75] | √ | – | – | China | English | 40 | 23 | Mean 5.09 ± 4.71 years | 0 | 0 | 40 | NR |
| CDC [76] | √ | – | – | USA | English | 2572 | NR | Median 11 years (0–17 years) | 3 | 147/745 | NR | NR |
| Parri [77] | √ | – | – | Italy | English | 100■ | 57 | Median 3.3 years (0–17.5 years) | 0 | 67 | 33 | NR |
| Tagarro [78] | √ | – | – | Spain | English | 41 | 18 | Median 1 year (0.35–8.5 years) | 0 | 25 | NR | NR |
| See [79] | – | √ | – | Malaysia | English | 4 | 3 | Median 6.5 years (20 months–11 years) | 0 | 4 | 4 | NR |
| Tan [80] | – | √ | – | China | English | 10 | 3 | Median 7.5 years (13 months–12 years) | 0 | 10 | 10 | 17.2 |
| Du [81] | – | √ | – | China | English | 14 | 6 | Median 6.2 years (0–16 years) | 0 | NR | NR | NR |
| Zhu [84] | – | √ | – | China | English | 10 | 5 | Median 9 years (19 months–14 years) | 0 | 10 | 5 | NR |
| Buonsenso [82] | – | – | √ | Italy | English | 2 | 2 | Newborn (14 days) | 0 | 0 | 2 | NR |
| Han [83] | – | – | √ | S. Korea | English | 1 | 0 | Newborn (27 days) | 0 | 1 | 1 | 18 |
| Total | 13 | 26 | 23 | 7480 | 2428/4660■■ | WMn 7.6 years | 6 | 605 | 2926 | WMo 11.2 days | ||
| 52.1% | (range 0–18 years) | 0.08%p | 12.9%q | 71%q | (range 2–27) |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported
a10-month-old baby with intussusception and MOF; bmedian; c713 confirmed; dmedian; edied because of sepsis, MOF, and DIC (preterm); f3/200 screened children on immunosuppressive treatment; gcomplications: 2/8 MOF, 3/8 still in PICU; h2 CHD; i24 still admitted but recovering; l5 discharged children were admitted again because their stool resulted + for SARS-CoV-2; m(25 0–1 year; 9 2–6; 25 > 7), no child in PICU; nweighted mean (N = 1396); oweighted mean (N = 130); pcalculated on the total of the confirmed case (N = 7480); qcalculated on the total of children with reported status on hospitalization (N = 4709)
**Studies included in the review by Henry et al. [67]
***Last update, 1 May 2020
■Since these 100 children have been included in the ISS National Registry [14], we excluded them from the analysis reported in this table
■■The percentage has been calculated on the total of patients with reported information on gender
Clinical features
Clinical findings were available in 1780 children aged 1 month to 18 years (49 studies) [10, 11, 16, 30–62, 64, 67, 69, 71, 72, 76–81, 84] (Table 2). Severe and critically ill children accounted for 2% (30/1475) and 0.6% (10/1475) of the total sample size, respectively (see also Table 5). The most commonly described symptoms in pediatric age were fever (51.6%), cough (47.3%), and sore throat (17.9%). Rarely children were also dyspneic (7.7%) and required oxygen supplementation for SpO2 below 92% (3.3%) [10, 31]. Extrarespiratory symptoms were mainly represented by diarrhea (9.7%), vomiting (7.2%), and fatigue (10.6%). A familial history of positive contact could be identified in 73.3% of the cases.
Table 2.
Clinical features in children with documented SARS-CoV-2 infection
| Author | N | Clinical features | Common symptoms | Extrarespiratory Symptoms | Contact | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Mild | Moderate | Severe | Critical | Fever | Cough | Sore throat | Runny nose | Dyspneic | Diarrhea | Vomiting | Fatigue | Family | Unknown | ||
| Lu [10] | 171 | 27 (16%) | 33 (19%) | 111 (65%) | 0 | 0 | 71 (41%) | 89 (52%) | 79 (46%) | 0 | 4 (2%) | 15 (9%) | 11 (6%) | 13 (8%) | 154 (90%)a | 15 (9%) |
| Wang [33] | 34 | 3 (9%) | 9 (26%) | 22 (65%) | 0 | 0 | 17 (50%) | 13 (38%) | 0 | 0 | 0 | 0 | 0 | 0 | 28 (82%) | 6 (18%) |
| Xia [34] | 20 | 2 (10%) | 6 (30%) | 12 (60%) | 0 | 0 | 12 (60%) | 13 (65%) | 1 (5%) | 0 | 2 (10%) | 3 (15%) | 1 (5%) | 1 (5%) | 13 (65%) | 7 (35%) |
| Dong [16] | 731 | 94 (4%) | 1091 (51%) | 831 (39%) | 112 (5%) | 13 (0.6%) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Liu [39] | 4 | 1 (25%) | 3 (75%) | 0 | 0 | 0 | 3 (75%) | 3 (75%) | 0 | 0 | 0 | 0 | 0 | 1 (25%) | NR | NR |
| Li [57] | 2 | 0 | 2 (100%) | 0 | 0 | 0 | 0 | 2 (100%) | 0 | 2 (100%) | 0 | 0 | 0 | 0 | 2 (100%) | 0 |
| Ji [41] | 2 | 0 | 2 (100%) | 0 | 0 | 0 | 1 (50%) | 0 | 1 (50%) | 0 | 0 | 1 (50%) | 0 | 0 | 2 (100%) | 0 |
| Liu [11] | 6 | 0 | 2 (33%) | 4 (67%) | 0 | 0 | 6 (100%) | 6 (100%) | 0 | 0 | 0 | 0 | 4 (47%) | 0 | NR | NR |
| Zhou [42] | 9 | 5 (56%) | 4 (44%) | 0 | 0 | 0 | 4 (44%) | 2 (22%) | 0 | 1 (11%) | 0 | 0 | 0 | 0 | 9 (100%) | 0 |
| Li [40] | 5 | 4 (80%) | 1 (20%) | 0 | 0 | 0 | 1 (20%) | 0 | 1 (20%) | 1 (20%) | 0 | 0 | 0 | 0 | 4 (80%) | 1 (20%) |
| D’Antiga [30] | 3 | 3 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR |
| Sun [31] | 8 | 0 | 0 | 0 | 5 (62%) | 3 (38%) | 6 (75%) | 6 (75%) | 0 | 0 | 8 (100%) | 3 (38%) | 4 (50%) | 1 (12%)b | 5 (62%) | 2 (25%)c |
| Zheng [32] | 25 | 0 | 8 (32%) | 15 (60%) | 0 | 2 (8%) | 13 (52%) | 11 (44%) | 0 | 2 (8%) | 2 (8%) | 3 (12%) | 2 (8%) | 0d | 21 (84%) | 4 (16%) |
| Park [43] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Liu** [44] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Chan** [45] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Cai** [46] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Chen** [47] | 1 | 0 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0e | NR | NR |
| Cai** [38] | 10 | 0 | 6 (60%) | 4 (40%) | 0 | 0 | 8 (80%) | 6 (60%) | 4 (40%) | 5 (50%) | 0 | 0 | 0 | 0 | 6 (60%) | 4 (40%) |
| Kam** [48] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Feng** [35] | 15 | 10 (67%) | 5 (33%) | 0 | 0 | 0 | 5 (33%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR |
| Wang** [36] | 31 | 4 (13%) | 13 (42%) | 14 (45%) | 0 | 0 | 20 (64%) | 14 (45%) | 0 | 0 | 0 | 3 (10%) | 0 | 3 (10%) | 28 (90%) | 3 (10%) |
| Zhang** [49] | 2 | 0 | 2 (100%) | 0 | 0 | 0 | 2 (100%) | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (100%) | 0 |
| Zhao** [50] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Wei [51] | 9 | 1 (11%) | 6 (67%) | 0 | 0 | 0 | 4 (44%) | 2 (22%) | 0 | 2 (22%) | 0 | 0 | 0 | 0 | 9 (100%) | 0 |
| Shen [52] | 28 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 28 (100%) | 0 |
| Lou [53] | 3 | 0 | 3 (100%) | 0 | 0 | 0 | 3 (100%) | 1 (33%) | 0 | 2 (67%) | 0 | 2 (67%) | 0 | 2 (67%) | 3 (100%) | 0 |
| Qian [54] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Su [55] | 9 | 6 (67%) | 3 (33%) | 0 | 0 | 0 | 2 (22%) | 1 (11%) | 0 | 0 | 0 | 0 | 0 | 0 | 9 (100%) | 0 |
| Xu [37] | 10 | 1 (10%) | 9 (90%) | 0 | 0 | 0 | 7 (70%) | 5 (0%) | 4 (40%) | 2 (20%) | 0 | 3 (30%) | 0 | 0 | 7 (70%) | 3 (30%) |
| Tang [56] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Pan [58] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Chen [62] | 31 | 12 (39%) | 19 (61%) | 0 | 0 | 0 | 14 (45%) | 13 (42%) | 2 (6%) | 3 (10%) | 0 | 0 | 0 | 2 (6%) | 29 (94%) | 2 (6%) |
| Xing [59] | 3 | 0 | 3 (100%) | 0 | 0 | 0 | 3 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR |
| Qiu [60] | 36 | 10 (28%) | 7 (19%) | 19 (53%) | 0 | 0 | 13 (36%) | 7 (19%) | 2 (5%) | 1 (3%) | 1 (3%) | 2 (5%) | 2 (5%) | 0 | 32 (89%) | 2 (5%) |
| Zhang [61] | 34 | 0 | 34 (100%) | 0 | 0 | 0 | 26 (76%) | 20 (83%) | 0 | 7 (21%) | 3 (9%) | 4 (12%) | 4 (12%) | 0 | 13 (38%) | 0 |
| Shen [71] | 9 | 2 (22%) | 7 (78%) | 0 | 0 | 0 | 3 (33%) | 1 (11%) | 1 (11%) | 0 | 0 | 2 (22%) | 0 | 0 | 9 (100%) | 0 |
| Han [72] | 7 | 0 | 4 (57%) | 3 (43%) | 0 | 0 | 5 (71%) | 5 (71%) | 1 (14%) | 0 | 3 (43%) | 4 (57%) | 0 | 0 | 7 (100%) | 0 |
| Le [69] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | NR | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Cui [64] | 1 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | NR | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) | 0 |
| Zhang** [67] | 1 | 0 | 1 (100%) | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Li [75] | 40 | 0 | 0 | 39 (97%) | 1 (3%) | 0 | 21 (52%) | 25 (62%) | 2 (5%) | 2 (5%) | 1 (3%) | 2 (5%) | NR | 4 (10%) | NR | NR |
| CDC [76] | 291 | NR | NR | NR | NR | NR | 163 (56%) | 158 (54%) | 71 (24%) | 21 (7%) | 39 (13%) | 37 (13%) | 31 (11%) | 66 (23%) | 168 (58%) | 16 (5%) |
| Parri [77] | 100 | 21 (21%) | 58 (58%) | 19 (19%) | 1 (1%) | 1 (1%) | 54 (54%) | 44 (44%) | 4 (4%) | 22 (22%) | 11 (11%) | 9 (9%) | 10 (10%) | 9 (9%) | 45 (45%) | 48 (48%) |
| Tagarro [78] | 41 | 0 | 33 (80%) | 4 (10%) | 4 (10%) | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 16 (39%) | 25 (61%) |
| See [79] | 4 | 1 (25%) | 3 (75%) | 0 | 0 | 0 | 2 (50%) | 2 (50%) | 0 | 1 (25%) | 0 | 1 (25%) | 0 | 0 | 2 (50%) | 1 (25%) |
| Tan [80] | 10 | 2 (20%) | 7 (70%) | 1 (10%) | 0 | 0 | 4 (40%) | 3 (30%) | 0 | 0 | 0 | 0 | 1 (10%) | 0 | 9 (90%) | 1 (1%) |
| Du [81] | 14 | 8 (57%) | 6 (43%) | 0 | 0 | 0 | 5 (36%) | 3 (21%) | 1 (7%) | NR | 0 | 0 | 0 | 1 (7%) | 14 (5%) | 0 |
| Zhu [84] | 10 | NR | NR | NR | NR | NR | 4 (40%) | 3 (30%) | 0 | 0 | 0 | 0 | 0 | 0 | 7 (70%) | 0 |
| Total | 1780 | 223 | 618 | 568 | 30 | 10 | 503 | 461 | 174 | 75 | 75 | 94 | 70 | 103 | 690 | 127 |
| % | 15.1 a | 41.9 a | 38.5 a | 2 a | 0.7 a | 51.6b | 47.3b | 17.9b | 7.7b | 7.7b | 9.7b | 7.2b | 10.6b | 73.3c | 13.5 c | |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported
aCalculated on the total of reported symptoms (N = 1475); bcalculated on the total of reported symptoms (N = 1016); ccalculated on the total of reported symptoms (N = 941)
**Studies included in the review by Henry et al. [67]
Table 5.
Treatments used in children with documented SARS-CoV-2 infection
| Author | N | PICU | MV | Noninvasive Ox | Symptomatic alone | Antiviral | Antibiotic | IVIg | CCS | IFN | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu [10] | 171 | 3 (2%)a | 3 (2%)a | NR | NR | NR | NR | NR | NR | NR | NR |
| Wang [33] | 34 | 0 | 0 | 0 | 0 | 20 (59%)b | 0 | 0 | 0 | 0 | 0 |
| Li [57] | 2 | 0 | 0 | 2 (100%) | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Ji [41] | 2 | 0 | 0 | 0 | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Liu [11] | 6 | 1 (17%) | 1 (17%) | 1 (17%) | 0 | 6 (100%)c | 0 | 1 (17%) | 4 (67%) | 0 | 0 |
| Li [40] | 5 | 0 | 0 | 0 | 0 | 2 (40%) | 2 (40%) | 5 (100%) | 0 | 2 (40%) | 3 (60%)d |
| Sun [31] | 8 | 2 (25%) | 2 (25%) | 6 (75%) | 0 | 8 (100%) | 5 (62%) | 4 (50%) | 5 (62%) | 0 | 1 (12%)e |
| Zheng [32] | 25 | 2 (8%) | 2 (8%) | 0 | 0 | 12 (48%) | 13 (52%) | 2 (8%) | 0 | 12 (48%) | 1 (4%)f |
| Park [43] | 1 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Liu** [44] | 1 | 0 | 0 | 0 | 0 | 1 (100%)g | 0 | 0 | 1 (100%) | 0 | 0 |
| Cai** [46] | 10 | 0 | 0 | 0 | 5 (50%) | 0 | 5 (50%) | 0 | 0 | 0 | 0 |
| Wang** [36] | 31 | 0 | 0 | 0 | 31 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Zhang** [49] | 2 | 0 | 0 | 0 | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Wei [51] | 9 | 0 | 0 | 0 | 9 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Lou [53] | 3 | 0 | 0 | 0 | 1 (33%) | 0 | 0 | 0 | 0 | 2 (67%) | 0 |
| Xu [37] | 10 | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 1 (10%) | 0 | 10 (100%) | 0 |
| Su [55] | 9 | 0 | 0 | 0 | 0 | 1 (11%)h | 0 | 0 | 0 | 9 (100%) | 0 |
| Pan [58] | 1 | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR |
| Chen [62] | 31 | 0 | 0 | 0 | 0 | 3 (10%) | 1 (3%) | 0 | 0 | 30 (97%) | 0 |
| Xing [59] | 3 | 0 | 0 | 0 | 0 | 3 (100%) | 0 | 0 | 0 | 3 (100%) | 3 (100%) |
| Qiu [60] | 36 | 0 | 0 | 6 (17%) | 0 | 14 (39%) | 0 | 0 | 0 | 36 (100%) | 0 |
| Zhang [61] | 34 | 0 | 0 | 3 (10%) | 0 | 28 (82%) | 30 (88%) | 0 | 5 (15%) | 28 (82%) | 0 |
| Shen [71] | 9 | 0 | 0 | 9 (100%) | 0 | 9 (100%) | 5 (56%) | 1 (11%) | 1 (11%) | 0 | 0 |
| Han [72] | 7 | 0 | 0 | 2 (29%) | 4 (57%) | 0 | 0 | 0 | 1 (14%) | 0 | 0 |
| Cui [64] | 1 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 1 (100%) | 1 (100%) |
| Le [69] | 1 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 |
| Li [75] | 40 | 1 (2%) | 1 (2%) | NR | 0 | 20 (50%) | 13 (31%) | 4 (10%) | 3 (7.5%) | 40 (100%) | 0 |
| CDC [76] | 745 | 15 (2%) | 15 (2%) | NR | NR | NR | NR | NR | NR | NR | NR |
| Parri [77] | 100 | 1 (1%) | 1 (1%) | 8 (8%) | NR | NR | NR | NR | NR | NR | NR |
| Tagarro [78] | 41 | 4 (10%) | 1 (3%) | 3 (7%) | NR | NR | NR | NR | NR | NR | NR |
| See [79] | 4 | 0 | 0 | 0 | 3 (75%) | 0 | 1 (25%) | 0 | 0 | 0 | 0 |
| Tan [80] | 10 | 0 | 0 | 0 | 9 (90%) | 0 | 1 (10%) | 0 | 0 | 0 | 0 |
| Zhu [84] | 10 | 0 | 0 | 1 (10%) | 4 (40%) | 5 (50%) | 1 (10%) | 0 | 0 | 4 (40%) | 0 |
| Total | 1402 | 29 | 26 | 41 | 73 | 132 | 80 | 18 | 20 | 177 | 9 |
| % | 2.1 | 1.9 | 9.2i | 21.2j | 38.4 j | 23.3 j | 5.2 j | 5.8 j | 51.5 j | 2.6j |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported, PICU pediatric intensive care unit, MV mechanical ventilation, Noninvasive Ox noninvasive oxygen, IVIg intravenous immunoglobulin, CCS corticosteroids, IFN interferon
aAll with coexisting conditions (hydronephrosis, leukemia, and intussusception); blopinavir and ritonavir; cribavirin 2/6; oseltamivir 6/6; d3/5 montelukast; e1/8 plasmapheresis; f1 also kidney replacement; gribavirin; hribavirin; icalculated on the total of reported treatments (N = 957); jcalculated on the total of reported treatments (N = 1058)
**Studies included in the review by Henry et al. [67]
Laboratory investigations
Table 3 summarizes the main laboratory investigations reported in 655 children (38 studies) [10, 11, 31–34, 36–50, 55, 58–62, 64, 67, 69, 71, 72, 75, 77, 79–81, 84]. The full blood cell count was unremarkable in most patients, with less than one fifth of them (17.1%) showing low white blood cell (WBC) and lympho- or neutropenia (13.3%). Elevated inflammatory indexes such as C-reactive protein (CRP) and procalcitonin (PCT) were shown by 31.1% of children. Creatine kinase (CPK) and liver enzymes were also altered, as shown by 14.5% and 12.4% of all patients, respectively.
Table 3.
Lab investigations in children with documented SARS-CoV-2 infection
| Author | N | Low WBC* | High WBC* | Lymphopenia/neutropenia* | Low Plt* | High Plt* | High CRP-PCT* | High CPK* | High transaminase* |
|---|---|---|---|---|---|---|---|---|---|
| Lu [10] | 171 | 45 (26%) | 0 | 6 (3%) | NR | NR | 105 (61%) | NR | 25 (15%) |
| Wang [33] | 34 | 1 (3%) | 5 (16%) | 1 (3%) | NR | NR | 1 (3%) | NR | NR |
| Xia [34] | 20 | 4 (20%) | 2 (10%) | 7 (35%) | NR | NR | 16 (80%) | 5 (25%) | 5 (25%) |
| Liu [39] | 4 | 1 (25%) | 0 | 0 | NR | NR | 1 (25%) | NR | NR |
| Ji [41] | 2 | 0 | 1 (50%) | 0 | NR | NR | 1 (50%) | NR | NR |
| Liu [11] | 6 | 4 (67%) | 0 | 6 (100%) | NR | NR | NR | NR | NR |
| Zhou [42] | 9 | 0 | 2 (22%) | 0 | NR | NR | NR | NR | NR |
| Li [40] | 5 | 0 | 2 (40%) | 0 | NR | NR | 1 | NR | NR |
| Sun [31] | 8 | 1 (12%) | 6 (75%) | 1 (12%) | 2 (25%) | 1 (12%) | 4 (50%) | 0 | 2 (25%) |
| Zheng [32] | 25 | NR | NR | 10 (40%) | NR | NR | NR | 0 | 0 |
| Park [43] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR |
| Liu** [44] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Chan** [45] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | NR | 0 |
| Cai** [46] | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 0 |
| Chen** [47] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) | 1 (100%) | NR |
| Cai** [38] | 10 | 1 (10%) | 3 (30%) | 3 (30%) | 1 (10%) | 2 (20%) | 3 (30%) | 5 (50%) | 2 (20%) |
| Kam** [48] | 1 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 0 | NR | NR | NR |
| Wang** [36] | 31 | 2 (6%) | 3 (10%) | 2 (6%) | 0 | 2 (6%) | 4 (13%) | 4 (13%) | 6 (19%) |
| Zhang** [49] | 2 | 0 | 2 (100%) | NR | 0 | 2 (100%) | 1 (50%) | NR | 2 (100%) |
| Zhao** [50] | 1 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 |
| Xu [37] | 10 | 3 (30%) | 0 | 5 (50%) | 0 | 0 | 6 (60%) | 0 | 1 (10%) |
| Su [55] | 9 | 3 (33%) | 1 (11%) | 3 (33%) | 1 (11%) | 0 | 0 | 6 (66%) | 0 |
| Pan [58] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chen [62] | 31 | 12 (39%) | 17 (55%) | 0 | 1 (3%) | 0 | 4 (13%) | 2 (6%) | 2 (6%) |
| Xing [59] | 3 | 0 | 0 | 1 (33%) | 0 | 2 (67%) | 1 (33%) | 0 | 0 |
| Qiu [60] | 36 | 7 (19%) | 0 | 11 (31%) | NR | NR | 6 (17%) | 1 (3%) | 3 (8%) |
| Zhang [61] | 34 | 0 | 17 (50%) | 0 | NR | NR | 17 (50%) | 0 | NR |
| Shen [71] | 9 | 0 | 1 (11%) | 0 | NR | NR | 1 (11%) | NR | 2 (22%) |
| Han [72] | 7 | 0 | 2 (29%) | 0 | 0 | 1 (14%) | 3 (43%) | 4 (57%) | 3 (43%) |
| Cui [64] | 1 | 0 | 1 (100%) | 0 | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) |
| Zhang** [67] | 1 | 0 | 0 | NR | 0 | 1 (100%) | 1 (100%) | NR | NR |
| Le [69] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 |
| Li [75] | 40 | NR | NR | NR | NR | NR | 1 (2%) | NR | 0 |
| Parri [77] | 100 | 11 (11%) | 11 (11%) | 14 (14%) | NR | NR | 4 (4%) | NR | 10 (10%) |
| See [79] | 4 | NR | NR | NR | NR | NR | NR | NR | 0 |
| Tan [80] | 10 | 0 | 1 (10%) | 0 | NR | NR | 1 (10%) | 1 (10%) | 2 (20%) |
| Du [81] | 14 | 4 | 0 | 9 | 0 | 2 | 5 | 4 | 1 |
| Zhu [84] | 10 | 0 | 0 | 0 | NR | NR | 0 | NR | 2 (20%) |
| Total | 655 | 100 | 80 | 81 | 7 | 14 | 190 | 37 | 69 |
| % | 17.1a | 13.7a | 13.3b | 5.1c | 10.3c | 31.1d | 14.5e | 12.4f |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported, WBC white blood cell, Plt platelet, CRP C-reactive protein, PCT procalcitonin, CPK creatine kinase
aCalculated on the total of reported labs (N = 586); bcalculated on the total of reported labs (N = 608); ccalculated on the total of reported labs (N = 136); dcalculated on the total of reported laboratory investigations (N = 610); ecalculated on the total of reported labs (N = 255); fcalculated on the total of reported labs (N = 557)
*Normal ranges of main laboratory parameters were not always clearly defined. However, most of the studies defined values above 5 mg/L as high CRP and above 0.5 ng/mL as high PCT
**Studies included in the review by Henry et al. [67]
Radiology findings
Of the 674 children who had radiological examinations (40 studies) [10, 11, 31–34, 36–50, 53, 55, 57–62, 64, 67, 69, 71, 72, 75, 77, 79–81, 84], up to 49.1% of them showed abnormalities, even if asymptomatic (17/113, 15%) (Table 4). Most patients underwent a chest CT scan (73.9%) that resulted normal in 198 out of 605 patients (32.7%), whereas typical ground-glass opacities (GGO), nonspecific unilateral and bilateral lesions were identified in 29.4%, 26.6%, and 23.2% of patients, respectively.
Table 4.
Radiological findings in children with documented SARS-CoV-2 infection
| Author | N | Abnormal radiological findings | Chest X-ray | CT scan | GGO | Local patchy | Bilateral patchy | Normal |
|---|---|---|---|---|---|---|---|---|
| Lu [10] | 171 | 51 (30%) | 0 | 111 (65%) | 56 | 32 (33%) | 21 (12%) | 60 (35%) |
| Wang [33] | 34 | 34 (100%) | 0 | 34 (100%) | NR | NR | NR | NR |
| Xia [34] | 20 | 16 (80%) | 0 | 20 (100%) | 12 (60%) | 6 (20%) | 6 (20%) | 4 (20%) |
| Liu [39] | 4 | 3 (75%) | 0 | 4 (100%) | 1 (25%) | 1 (25%) | 1 (25%) | 1 (25%) |
| Li [57] | 2 | 2 (100%) | 0 | 2 (100%) | 0 | 1 (50%) | 1 (50%) | 0 |
| Ji [41] | 2 | 0 | 0 | 2 (100%) | 0 | 0 | 0 | 2 (100%) |
| Liu [11] | 6 | 4 (67%) | 0 | 6 (100%) | 1 (17%) | 3 (50%) | 0 | 2 (33%) |
| Zhou [42] | 9 | 9 (100%) | 0 | 9 (100%) | 6 (67%) | 7 (78%) | 0 | 0 |
| Li [40] | 5 | 3 (60%) | 0 | 5 (100%) | 3 (60%) | 0 | 0 | 2 (40%) |
| Sun [31] | 8 | 8 (100%) | 0 | 8 (100%) | 6 (75%) | 0 | 8 (100%) | 0 |
| Zheng [32] | 25 | 17 (68%) | 0 | 25 (100%) | 0 | 5 (20%) | 12 (48%) | 8 (32%) |
| Park [43] | 1 | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 1 (100%) |
| Liu** [44] | 1 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 |
| Chan** [45] | 1 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 |
| Cai** [46] | 1 | 1 (100%) | NR | NR | NR | NR | NR | NR |
| Chen** [47] | 1 | 1 (100%) | NR | NR | NR | NR | NR | NR |
| Cai** [38] | 10 | 4 (40%) | 10 (100%) | 0 | 0 | 4 (40%) | 0 | 6 (60%) |
| Feng** [35] | 15 | 9 (60%) | 0 | 15 (100%) | 9 (60%) | 7 (47%) | 2 (13%) | 6 (40%) |
| Wang** [36] | 31 | 14 (45%) | 0 | 31 (100%) | 9 (29%) | 0 | 0 | 0 |
| Zhang** [49] | 2 | 1 (50%) | 0 | 2 (100%) | NR | NR | NR | 1 (50%) |
| Zhao** [50] | 1 | 1 (100%) | NR | NR | NR | NR | NR | NR |
| Lou [53] | 3 | 3 (100%) | 0 | 3 (100%) | 3 (100%) | 0 | 0 | 0 |
| Xu [37] | 10 | 5 (50%) | 10 (100%) | 10 (100%) | 5 (50%) | 0 | 0 | 5 (50%) |
| Su [55] | 9 | 1 (11%) | 0 | 9 (100%) | 1 (11%) | 1 (11%) | 0 | 8 (89%) |
| Pan [58] | 1 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) |
| Chen [62] | 31 | 11 (35%) | 0 | 31 (100%) | 11 (35%) | 8 (26%) | 3 (10%) | 20 (65%) |
| Xing [59] | 3 | 2 (67%) | 0 | 3 (100%) | 2 (67%) | 2 (67%) | 0 | 1 (33%) |
| Qiu [60] | 36 | 19 (53%) | 0 | 36 (100%) | 19 (53%) | 0 | 0 | 17 (47%) |
| Zhang [61] | 34 | 28 (82%) | 0 | 34 (100%) | 0 | 14 (41%) | 14 (41%) | 6 (18%) |
| Shen [71] | 9 | 2 (22%) | 0 | 9 (100%) | 2 (22%) | 2 (22%) | 0 | 7/(78%) |
| Han [72] | 7 | 2 (29%) | 0 | 7 (100%) | NR | NR | NR | 5 (71%) |
| Li [75] | 40 | 39 (97%) | 0 | 40 (100%) | NR | 13 (32.5%) | 26 (65%) | 1 (2%) |
| Parri [77] | 100 | 15 (15%) | 35 (35%) | 0 | 14 (14%) | NR | NR | 15 (15%) |
| See [79] | 4 | 2 (50%) | 2 (50%) | 0 | NR | 1 (25%) | 1 (25%) | 0 |
| Tan [80] | 10 | 5 (50%) | 0 | 10 (100%) | 5 (50%) | NR | NR | 5 (50%) |
| Du [81] | 14 | 6 (43%) | 0 | 14 (100%) | NR | 6 (43%) | 5 (36%) | 8 (57%) |
| Zhu [84] | 10 | 5 (50%) | 0 | 10 (100%) | NR | 3 (30%) | 2 (20%) | 5 (50%) |
| Zhang** [67] | 1 | 1 (100%) | NR | NR | NR | NR | NR | NR |
| Cui [64] | 1 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 |
| Le [69] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) |
| Total | 674 | 331 | 59 | 495 | 169 | 117 | 102 | 198 |
| % | 49.1 | 8.8a | 73.9a | 29.4b | 26.6c | 23.2c | 32.7d |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported, GGO ground-glass opacities
aCalculated on the total of reported imaging (N = 670); bcalculated on the total of reported imaging (N = 574); ccalculated on the total of reported imaging (N = 440); dcalculated on the total of reported imaging (N = 605)
**Studies included in the review by Henry et al. [67]
Treatment
Of all patients, about 2% were admitted in the pediatric intensive care unit (PICU) and required mechanical ventilation (MV) (Table 5) [10, 11, 31–33, 36–38, 40, 41, 43, 44, 49, 51, 53, 55, 57–62, 71, 72, 75–80, 84]. Most authors described the use of nebulized Interferon (IFN) (51.5%), and of other antiviral agents (38.4%) or antibiotics (23.3%). The use of intravenous immunoglobulin (IVIg) and corticosteroids (CCS) was less frequently described (5.2% and 5.8%, respectively).
Neonatal cases
A few case reports [22, 49, 63, 68, 70, 73, 74, 82, 83] and case series [23, 65, 66] including a total of 25 newborns (17 males, 74%) with SARS-CoV-2 were identified (Table 6). Neonates were usually screened because of a history of primary maternal infection (84%). Similarly to older children, most of them were asymptomatic (20%) or had mild (48%) and moderate (20%) signs of clinical infection. However, a slightly higher proportion of them was severely ill (12%). Dyspnea was the most common reported sign in neonatal age (40%). Fever (32%) and feeding intolerance (24%) were also described. Blood tests showed high WBC (20%), CRP and/or PCT (12%), CPK (20%), and liver enzymes (16%).
Table 6.
Clinical features and laboratory results in newborns and infants ≤ 3 months of age with documented SARS-CoV-2 infection
| Author | N | Clinical features | Symptoms | Family contact | Labs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Mild | Moderate | Severe | Fever | Cough | Dyspneic | Vomiting | Feeding intolerance | High WBC | Low L | Low Plt | High Plt | High CRP-PCT | High CPK | High transaminase | |||
| Wang [63] | 1 (1 m) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | NR | 1 (100%) | NR | NR | 0 | 1 (100%) | 1 (100%) |
| Zhu [65] | 10 (8 m) | 0 | 8 (80%) | 0 | 2 (20%) | 2 (20%) | 0 | 6 (60%) | 0 | 4 (40%) | 10 (100%) | 3 (30%) | 0 | 2 (20%) | 0 | 2 (20%) | 2 (20%) | 2 (20%) |
| Lu [66] | 3 | 1 (33%) | 1 (33%) | 0 | 1 (3%) | 1 (33%) | 0 | 1 (33%) | 1 (33%) | 0 | 3 (100%) | NR | NR | NR | NR | NR | NR | NR |
| Zeng** [68] | 1 (1 m) | 0 | 1 (100%) | 0 | 0 | NR | NR | NR | NR | 0 | NR | 0 | NR | 0 | 1 (100%) | 1 (100%) | NR | NR |
| Wang [70] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | NR | NR | NR | NR | NR | NR | NR | NR |
| Zeng [23] | 3 (3 m) | 0 | 0 | 3 (100%) | 0 | 2 (67%) | 0 | 1 (33%) | 0 | 1 (33%) | 3 (100%) | 2 (67%) | 2 (67%) | NR | NR | 0 | 2 (67%) | 1 (33%) |
| Kamli-Aghdam [73] | 1 (1 m) | 0 | 0 | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) | 0 | 0 | NR | 0 | 0 | 0 | 0 | 0 | NR | NR |
| Canarutto [74] | 1 (1 m) | 0 | 1 (100%) | 0 | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | NR | 0 | 1 (100%) | 0 | 0 | 0 | NR | NR |
| Dong [22] | 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Buonsenso [82] | 2 (2 m) | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (100%) | NR | NR | NR | NR | NR | NR | NR |
| Han [83] | 1 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 25 (17 m) | 5 | 12 | 5 | 3 | 0 | 8 | 2 | 3 | 6 | 21 | 0 | 5 | 4 | 2 | 1 | 3 | 5 |
| % | 20.0 | 48.0 | 20.0 | 12.0 | 0.0 | 32,0 | 8.0 | 12 | 24 | 84 | 0 | 20 | 16 | 8 | 4 | 12 | 20 | |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported, WBC white blood cell, L lymphocyte, Plt platelet, CRP C-reactive protein, PCT procalcitonin, CPK creatine kinase
**Studies included in the review by Henry et al. [67]
Unlike older children, newborns underwent a chest X-ray in most cases (64%) (Table 7). Abnormal radiological findings could be recognized in less than half of them (48%), but specific lesions were not so frequently described: 4% had GGO, 20% unilateral patchy area, and 12% bilaterally. Poor information on treatment options was obtainable from the included reports, and most patients (48%) received only symptomatic therapies.
Table 7.
Radiological findings and treatments used in newborns and infants ≤ 3 months of age with documented SARS-CoV-2 infection
| Author | N | Radiology findings | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abnormal | Chest X-ray | CT-scan | GGO | Local patchy | Bilateral patchy | Normal | PICU | MV | Symptomatic alone | Antibiotic | IVIg | IFN | ||
| Wang [63] | 1 (1 m) | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) | 0 | 0 | ||||||
| Zhu [65] | 10 (8 m) | 7 (70%) | 10 (100%) | 0 | 1 (10%) | 1 (10%) | 3 (30%) | 3 (30%) | 0 | 0 | 9 (90%) | 0 | 1 (10%) | 0 |
| Lu [66] | 3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Zeng** [68] | 1 (1 m) | 1 (100%) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Wang [70] | 1 | NR | NR | NR | NR | NR | NR | NR | 0 | 0 | 0 | 0 | 0 | 1 (100%) |
| Zeng [23] | 3 (3 m) | 3 (100%) | 3 (100%) | 0 | 0 | 3 (100%) | 0 | 0 | 1 (33%) | 1 (33%) | 2 (67%) | 1 (33%) | 0 | 0 |
| Kamli-Aghdam [73] | 1 (1 m) | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 1 (100%) | 0 | 0 |
| Canarutto [74] | 1 (1 m) | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 1 (100%) | 0 | 0 | 0 |
| Dong [22] | 1 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Buonsenso [82] | 2 (2 m) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Han [83] | 1 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 1 (100%) | 0 | NR | NR |
| Total | 25 (15 m) | 12 | 16 | 2 | 1 | 5 | 3 | 7 | 2 | 1 | 2 | 12 | 1 | 2 |
| % | 48 | 64 | 8 | 4 | 20 | 12 | 28 | 8 | 4 | 8 | 48 | 4 | 8 | |
SARS-CoV-2 severe acute respiratory syndrome–Coronavirus-2, NR not reported, GGO ground-glass opacities, PICU pediatric intensive care unit, MV mechanical ventilation, IVIg intravenous immunoglobulin, CCS corticosteroids, IFN interferon
**Studies included in the review by Henry et al. [67]
Discussion
The knowledge about epidemiological and clinical features of novel Coronavirus disease needs to be continuously updated. However, current data have been reported mainly from adult patients, while many aspects of the infection remain unclear in children. This systematic review fully assesses epidemiological trends and clinical characteristics of SARS-CoV-2 infections in pediatric age, giving an analytical summary of the available heterogeneous evidence.
Children are less likely to develop severe symptoms of COVID-19 than adults, with 95% of all cases ranging from asymptomatic to mild-moderate clinical patterns, as described by different case series [10, 11, 30, 33–62, 71, 72, 76, 77, 81]. Moreover, only 2% of patients were admitted to PICU or required MV [10, 31, 32, 75–78]. Overall, six deaths were reported (mortality rate 0.08%): most patients developed complications [10, 16], including a preterm newborn who died from sepsis [65]. In adulthood, over two thirds of those who died from COVID-19 had a comorbidity [88], while about 20% of children with underlying disease could be identified, and none of them showed worse clinical course of the infection in comparison to previously healthy patients [10, 30, 32, 61, 62]. The explanation for the lower mortality rate in children might rely not only on epidemiological reasons. Some authors hypothesized a molecular mechanism making children less susceptible to SARS-CoV-19 infection. The pathogenesis of human coronavirus mainly depends on the interactions between its transmembrane spike glycoprotein (S-protein) and specific cell receptors of angiotensin-converting enzyme II (ACE2) [89]. It has been shown that the expression of this enzyme starts to increase in later childhood [90], and this may protect children from the most aggressive pattern of the infection.
However, children might not be tested for SARS-CoV-2 as frequently as adults. Most countries choose to test and centralize only symptomatic patients. We may speculate that a small number of children have been tested because they are mostly asymptomatic and therefore just locked down at home. Moreover, the current gold standard for the diagnosis of SARS-CoV-2 infection is real time-polymerase chain reaction (RT-PCR) on respiratory tract specimen. The diagnostic accuracy of RT-PCR highly depends on the virus-specific diagnostic window (e.g., the viral load may decrease from the second week of the disease), and the analytical sensitivity of this assay is potentially plagued by false SARS-CoV-2 negativity attributable to the low viral loads, especially in asymptomatic or mild symptomatic patients that might transmit the disease as well [91]. There are very recent data from a German study indicating that viral loads in the very young (age group 0–6 years) do not significantly differ from those of adults. This means that even if children are less often symptomatic, they might be as infectious as adults [92].
The majority of pediatric patients infected with SARS-CoV-2 have been part of a family cluster outbreak or had a household positive contact (85% of all reported cases) [10, 32–34, 36, 58, 60–62]. Several other studies documented how COVID-19 spreads within families [93]. Preliminary findings suggested differences in the onset and duration of viral shedding between and within family clusters, with adult subjects remaining positive for a prolonged time [93]. Further investigation with the inclusion of the virus genome may be worthy.
Clinical features
Infected children usually show typical symptoms of acute respiratory infections including fever (49.6%) and cough (45.5%) [10, 11, 16, 30–62, 64, 67, 69, 71, 72, 76–81, 84]. In particular, some authors reported that up to one third of symptomatic children may have high fever [10, 77], but generally below 39 °C [38, 77]. Differently from adults, children are more likely to present with extra-respiratory symptoms [94]: diarrhea (9.4%) and vomiting (7.3%) are the most frequently reported ones. It has been showed that, when present, gastrointestinal symptoms usually anticipate the typical respiratory pattern [95]. Previous studies on SARS-CoV cases demonstrated the viral detection in gut biopsy specimens and stool of recovered patients, indicating a possible gastrointestinal tract tropism that may partially provide explanations for extra-respiratory symptoms and persistent viral shedding through fecal–oral route [96]. There is growing evidence that this mechanism of excretion may be typical also for SARS-CoV-2 [2, 37]. As described in a case series of ten infected children, SARS-CoV-2 remained detectable in rectal swabs after nasopharyngeal swabs turned negative [37]. However, the extra-pulmonary detection of viral RNA does not mean infectious virus is present, but two independent laboratories from China declared that they have successfully isolated live 2019-nCoV from the stool of patients (unpublished) [95]. Moreover, recent analysis revealed that ACE2 was expressed also in upper esophagus and absorptive enterocytes from ileum and colon [61].
According to evaluated studies, clinical presentation in newborns could be slightly different than in older children, with a higher proportion of them (12%) presenting with a severe pattern [65, 66]. Even if the vertical transmission for SARS-CoV-2 has not been demonstrated [19, 97], in 84% of neonatal cases the mother was infected [23, 63, 65, 66, 82, 83]. Moreover, nosocomial infection may also occur, and strict measures to reduce this risk should be always observed [24]. However, some authors hypothesized that 2019-nCoV infection and morbidity in newborns may be related to possible hypoxemia in the infected mother that increases the risk of perinatal adverse events such as birth asphyxia and premature birth [65, 98]. Evidence is still too limited.
Laboratory findings
Overall, no significant abnormalities were observed and this was consistent with the results of a previously published review including a total sample size of 66 children with confirmed SARS-CoV-2 infection [85]. In particular, full blood cell count was normal in most patients, with only two case series reporting high rates of lymphopenia (10/25, 40% [32] and 11/36, 30.1% [60], respectively). However, this finding seems to be in contrast with adult data, as low lymphocyte count has been noted in up to 80% of infected critically ill subjects [91, 99]. The limited number of severe COVID-19 infection may in part explain the lack of significant lymphopenia in children.
Our results suggested that inflammatory indexes may be abnormal in one third of children with SARS-CoV-2 infection (30.9%), while Henry et al. described only 10–13% of cases with high CRP and/or PCT [85]. This controversial finding could be explained by the high heterogeneity in defining a cut-off of abnormal values across all the included studies. However, in adults a PCT value of ≥ 0.5 ng/mL was observed to be associated with a near fivefold increase in risk of severe clinical course of COVID-19 infection [100].
Other significant reported laboratory investigations were high CPK values (13.6%) and liver enzymes (12.3%). These enzymes could be often altered during viral infections [101]. In particular, high CPK levels or aspartate aminotransferase activity correlated with more severe clinical patterns in adult patients with SARS-CoV-2 infection [102]. Abnormal transaminase levels may also express a sign of direct liver damage. Recently published data demonstrated enrichment of ACE2 expression in cholangiocytes (59.7% of cells) suggesting that SARS-CoV-2 might lead to direct damage of intrahepatic bile ducts [103].
Radiology findings
Half of the children who had radiological examinations showed abnormalities. The sensitivity of chest X-ray is supposed to be inferior to that of CT-scan: in adults, nonspecific patchy peripheral and peri-bronchial opacities may be shown in all lung zones, according to the severity of the infection [104]. As children usually develop milder patterns of the disease, chest X-ray may fail to identify typical lesions, and it is mainly adopted in newborns and younger infants [23, 65, 69]. We identified 15% of asymptomatic children with abnormal radiological findings across all studies; however, both clinical and radiological patterns were specifically described only in a few case series, therefore this proportion of patients may be even higher. CT scan is frequently performed in children with suspected or confirmed SARS-CoV-2 infection (up to 74% of all cases reported). The most frequently recognizable lesions are represented by GGO, with unilateral or bilateral distribution [33–35]. However, CT scan resulted completely normal in more than one third of asymptomatic patients who underwent this examination [10, 33–36, 44, 45, 55, 63]. The use of CT scan as a diagnostic screening tool in confirmed or suspected COVID-19 patients is supported by recent evidence showing that its sensitivity could be greater than that of real-time PCR in detecting the virus (98% vs. 71%, respectively) [105]. However, routine use of CT scan has several obvious implications, in particular in a pediatric setting where concern about unnecessary exposure to radiation source should be raised. Therefore, other possible diagnostic imaging tools may be used. Lung ultrasound (LUS) has been successfully adopted in adult subjects with SARS-CoV-2 infection [106]. A recently published Italian case series reported LUS findings in eight children with COVID-19 infection: subpleural consolidations and confluent B-lines were identified in all patients and results were confirmed by a chest radiography [107].
Treatment
Currently, a few registered drug trials for COVID-19 experimental treatment included children [108]. Symptomatic treatment alone was used in most cases, in particular in newborns [65].
The only therapeutic recommendation in pediatric age is to use nebulized IFN and oral antiviral agents (i.e., lopinavir/ritonavir), with CCS for complications (acute respiratory distress syndrome, encephalitis, hemophagocytic syndrome, or septic shock) and IVIg for severe cases [28]. However, none of these therapies have shown a clear benefit in the treatment of SARS-CoV-2, and neither the WHO nor the US CDC recommends any specific treatment in children [25, 109].
The main limitation of our review is the difficulty to retrieve the full text of some Chinese studies; thus, we had to rely on English-language summaries when data were easily retrievable or publications that referenced papers published in Chinese. Given the large amount of currently published papers on COVID-19, we have decided to base our research on a single but robust database. However, this strategy might implicate possible selection bias of the included studies. Moreover, our findings are essentially based on limited case series and case reports, therefore laboratory parameters of interest were not consistently reported and reference ranges were not always clearly defined. Similarly, radiological patterns were difficult to compare, as the description of the included cases was not standardized.
However, despite the scarce and extremely heterogeneous evidence on pediatric patients with COVID-19, the use of systematic databases allowed us to review at a glance and interpret the majority of published studies up to 1 May 2020.
Conclusion
SARS-CoV-2 affects children less commonly and severely in comparison to adults, with an estimated very low mortality rate, but there is growing evidence showing that they are as susceptible to become infected as adults. This could be due either to the fact that children are less frequently exposed to the main sources of transmission (in particular, nosocomial), and that they tend to show milder symptoms, therefore may be less often tested. In symptomatic patients, laboratory and radiology findings are mainly nonspecific, but could help identifying those who are severely ill. Larger epidemiological and clinical cohort studies are needed to better understand possible implications of COVID-19 infection in children.
Electronic supplementary material
(DOC 64 kb)
(DOCX 45 kb)
Acknowledgments
The authors are grateful to all the residents in Pediatrics at the University of Udine for their valuable contribution.
Authors’ Contributions
I.L. conceptualized the systematic review, performed the electronic search, evaluated articles for eligibility, extracted relevant data, interpreted the results, and drafted sections of the manuscript.
C.P. contributed to study design, screened search results, reviewed all included studies, and reviewed and revised the manuscript.
M.B. and M.F. contributed to study design, developed search terms and performed the electronic search, and reviewed and revised the manuscript.
A.N. contributed to study design, reviewed and revised the manuscript, and provided mentorship.
A.P., E.V., and P.C. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Compliance with ethical statement
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
The original online version of this article was revised: The bibliographic information of Zhang et al. 2020 (reference [61]), listed incorrectly and is now correctly presented in the reference list of the of the above article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/1/2021
A correction to this paper has been published: <ExternalRef><RefSource>https://doi.org/10.1007/s00431-021-03961-z</RefSource><RefTarget Address="10.1007/s00431-021-03961-z" TargetType="DOI"/></ExternalRef>
Contributor Information
Ilaria Liguoro, Email: ilarialiguoro@gmail.com.
Chiara Pilotto, Email: chiara.pilotto@asufc.sanita.fvg.it.
Margherita Bonanni, Email: marghe.bonanni@gmail.com.
Maria Elena Ferrari, Email: mary6ferrari@gmail.com.
Anna Pusiol, Email: anna.pusiol@asufc.sanita.fvg.it.
Agostino Nocerino, Email: agostino.nocerino@asufc.sanita.fvg.it.
Enrico Vidal, Email: enrico.vidal@asufc.sanita.fvg.it.
Paola Cogo, Email: paola.cogo@uniud.it.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RAM, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus ADME, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RAM, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LLM, Snijder EJ, Stephens GM, Woo PCY, Zaki AM, Zambon M, Ziebuhr J. Middle East respiratory syndrome Coronavirus (MERS-CoV): announcement of the Coronavirus study group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA (2020) Insights into the recent 2019 novel Coronavirus (SARS-CoV-2) in light of past human Coronavirus outbreaks. Pathogens 9. 10.3390/pathogens9030186 [DOI] [PMC free article] [PubMed]
- 6.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020.
- 8.Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
- 9.Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases 0: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed]
- 10.Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Zhang Q, Chen J et al (2020) Detection of Covid-19 in children in early January 2020 in Wuhan, China. New England Journal of Medicine [DOI] [PMC free article] [PubMed]
- 12.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 13.Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 14.COVID-19 Integrated Surveillance GB—ISS. https://www.iss.it/covid-19-integrated-surveillance. Accessed 30 Mar 2020
- 15.Onder G, Rezza G, Brusaferro S (2020) Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 10.1001/jama.2020.4683 [DOI] [PubMed]
- 16.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S (2020) Epidemiological characteristics of 2143 pediatric patients with 2019 Coronavirus disease in China. Pediatrics.:e20200702. 10.1542/peds.2020-0702
- 17.Schwartz DA (2020) An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal Coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 10.5858/arpa.2020-0901-SA [DOI] [PubMed]
- 18.Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. 2020;55:435–437. doi: 10.1002/uog.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Z, Wang J, Mo Y, Duan W, Xiang G, Yi M, Bao L, Shi Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13:818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X (2020) Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 10.1001/jama.2020.4861 [DOI] [PMC free article] [PubMed]
- 22.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J (2020) Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed]
- 23.Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W (2020) Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan. China JAMA Pediatr. 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed]
- 24.Wang L, Shi Y, Xiao T, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (first edition) Ann Transl Med. 2020;8:47. doi: 10.21037/atm.2020.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC (2020) Coronavirus Disease 2019 (COVID-19). In: Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html.
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z-M, Fu J-F, Shu Q, Chen YH, Hua CZ, Li FB, Lin R, Tang LF, Wang TL, Wang W, Wang YS, Xu WZ, Yang ZH, Ye S, Yuan TM, Zhang CM, Zhang YY (2020) Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 10.1007/s12519-020-00345-5 [DOI] [PMC free article] [PubMed]
- 29.Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology et al. Report on the epidemiological features of Coronavirus Disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Antiga L (2020) Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 10.1002/lt.25756 [DOI] [PubMed]
- 31.Sun D, Li H, Lu X-X, Xiao H, Ren J, Zhang FR, Liu ZS (2020) Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed]
- 32.Zheng F, Liao C, Fan Q-H, Chen HB, Zhao XG, Xie ZG, Li XL, Chen CX, Lu XX, Liu ZS, Lu W, Chen CB, Jiao R, Zhang AM, Wang JT, Ding XW, Zeng YG, Cheng LP, Huang QF, Wu J, Luo XC, Wang ZJ, Zhong YY, Bai Y, Wu XY, Jin RM. Clinical characteristics of children with Coronavirus Disease 2019 in Hubei. China Curr Med Sci. 2020;40:275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XF, Yuan J, Zheng YJ et al (2020) [retracted: Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen]. Zhonghua Er Ke Za Zhi 58:E008. 10.3760/cma.j.issn.0578-1310.2020.0008 [DOI] [PubMed]
- 34.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58:E011. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:1–4. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai J, Xu J, Lin D, Yang, Xu L, Qu Z, Zhang Y, Zhang H, Jia R, Liu, Wang X, Ge Y, Xia A, Tian H, Chang H, Wang C, Li J, Wang J, Zeng M (2020) A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed]
- 39.Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Inf Secur. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Cui H, Li K, et al (2020) Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol 1–4. 10.1007/s00247-020-04656-7 [DOI] [PMC free article] [PubMed]
- 41.Ji L-N, Chao S, Wang Y-J, Li XJ, Mu XD, Lin MG, Jiang RM (2020) Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J Pediatr. 10.1007/s12519-020-00356-2 [DOI] [PMC free article] [PubMed]
- 42.Zhou Y, Yang G-D, Feng K, Huang H, Yun YX, Mou XY, Wang LF. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:215–220. doi: 10.7499/j.issn.1008-8830.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of Coronavirus Disease 2019 in Korea. J Korean Med Sci. 2020;35:e124. doi: 10.3346/jkms.2020.35.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan JF-W, Yuan S, Kok K-H, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, Tsoi HW, Lo SKF, Chan KH, Poon VKM, Chan WM, Ip JD, Cai JP, Cheng VCC, Chen H, Hui CKM, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai JH, Wang XS, Ge YL, Xia AM, Chang HL, Tian H, Zhu YX, Wang QR, Zeng JS. First case of 2019 novel coronavirus infection in children in Shanghai. Zhonghua Er Ke Za Zhi. 2020;58:86–87. doi: 10.3760/cma.j.issn.0578-1310.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Chen F, Liu ZS, Zhang FR, Xiong RH, Chen Y, Cheng XF, Wang WY, Ren J. First case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:179–182. doi: 10.3760/cma.j.issn.0578-1310.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Kam K, Yung CF, Cui L, et al A well infant with Coronavirus Disease 2019 with high viral load. Clin Infect Dis 10.1093/cid/ciaa201 [DOI] [PMC free article] [PubMed]
- 49.Zhang G-X, Zhang A-M, Huang L, Cheng LY, Liu ZX, Peng XL, Wang HW. Twin girls infected with SARS-CoV-2. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:221–225. doi: 10.7499/j.issn.1008-8830.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao R, Shen X, Yu K, Sheng (2020) [A case of children with 2019 novel Coronavirus infection]. Zhejiang Med J
- 51.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen K-L, Yang Y-H (2020) Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr. 10.1007/s12519-020-00344-6 [DOI] [PMC free article] [PubMed]
- 53.Lou XX, Shi CX, Zhou CC, Tian YS. Three children who recovered from novel coronavirus 2019 pneumonia. J Paediatr Child Health. 2020;56:650–651. doi: 10.1111/jpc.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian G, Yang N, Ma AHY, Wang L, Li G, Chen X, Chen X (2020) A COVID-19 transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis. 10.1093/cid/ciaa316 [DOI] [PMC free article] [PubMed]
- 55.Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, Sun J, Liu Y, Yang C, Geng J, Zhang Z, Gai Z. The different clinical characteristics of corona virus disease cases between children and their families in China—the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang A, Tong Z-D, Wang H-L, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB (2020) Detection of novel Coronavirus by RT-PCR in stool specimen from asymptomatic child. China Emerging Infect Dis 26. 10.3201/eid2606.200301 [DOI] [PMC free article] [PubMed]
- 57.Li Y, Guo F, Cao Y, Li LF, Guo YJ. Insight into COVID-2019 for pediatricians. Pediatr Pulmonol. 2020;55:E1–E4. doi: 10.1002/ppul.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan X, Chen D, Xia Y, Wu X, Li T, Ou X, Zhou L, Liu J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing Y, Ni W, Wu Q, et al (2020) Prolonged presence of SARS-CoV-2 in feces of pediatric patients during the convalescent phase. medRxiv 2020.03.11.20033159. 10.1101/2020.03.11.20033159
- 60.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D (2020) Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed]
- 61.Zhang C, Gu J, Chen Q, Deng NA et al. (2020) Clinical Characteristics of 34 Children with Coronavirus Disease-2019 in the West of China: a Multiple-center Case Series. medRxiv
- 62.Chen C, Cao M, Peng L, et al (2020) Coronavirus Disease-19 among children outside Wuhan, China. Social Science Research Network, Rochester, NY
- 63.Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L (2020) A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 10.1093/cid/ciaa225 [DOI] [PMC free article] [PubMed]
- 64.Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, Wang L, Chen Y, Liu W, Zhang K, Wu Y, Yang Z, Tao J, Feng J, Liu K, Ye X, Wang R, Zhang X, Zha Y. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221:1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Q, Shi Y. Coronavirus disease (COVID-19) and neonate: what neonatologist need to know. J Med Virol. 2020;92:564–567. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang YH, Lin DJ, Xiao MF, et al. 2019 novel coronavirus infection in a three-month-old baby. Zhonghua Er Ke Za Zhi. 2020;58:182–184. doi: 10.3760/cma.j.issn.0578-1310.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Zeng LK, Tao XW, Yuan WH, et al. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:E009. doi: 10.3760/cma.j.issn.0578-1310.2020.0009. [DOI] [PubMed] [Google Scholar]
- 69.Le HT, Nguyen LV, Tran DM, et al. The first infant case of COVID-19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Health. 2020;4:405–406. doi: 10.1016/S2352-4642(20)30091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Wang D, Chen G-C, Tao XW, Zeng LK. SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:211–214. doi: 10.7499/j.issn.1008-8830.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Q, Guo W, Guo T, Li J, He W, Ni S, Ouyang X, Liu J, Xie Y, Tan X, Zhou Z, Peng H. Novel coronavirus infection in children outside of Wuhan. China Pediatr Pulmonol. 2020;55:1424–1429. doi: 10.1002/ppul.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Y-N, Feng Z-W, Sun L-N, Ren XX, Wang H, Xue YM, Wang Y, Fang Y (2020) A comparative-descriptive analysis of clinical characteristics in 2019-Coronavirus-infected children and adults. J Med Virol. 10.1002/jmv.25835 [DOI] [PubMed]
- 73.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020;52:1–3. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canarutto D, Priolo A, Russo G, Pitea M, Vigone MC, Barera G. COVID-19 infection in a paucisymptomatic infant: raising the index of suspicion in epidemic settings. Pediatr Pulmonol. 2020;55:E4–E5. doi: 10.1002/ppul.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H, Chen K, Liu M, Xu H, Xu Q (2020) The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Inf Secur. 10.1016/j.jinf.2020.04.001 [DOI] [PMC free article] [PubMed]
- 76.CDC COVID-19 Response Team Coronavirus Disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parri N, Lenge M, Buonsenso D, Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group (2020) Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 10.1056/NEJMc2007617 [DOI] [PMC free article] [PubMed]
- 78.Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, Calvo C (2020) Screening and severity of Coronavirus Disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed]
- 79.See KC, Liew SM, Ng DCE, Chew EL, Khoo EM, Sam CH, Sheena D, Zahilah Filzah Z, Chin SY, Lee PY, Tan LP, Farah Najwa Z, Sabrina S, Them WW, Saipriya T, Muhammad Zamakhshari ZA, Cheah WK, Peariasamy K, Goh PP, Ibrahim H. COVID-19: four paediatric cases in Malaysia. Int J Infect Dis. 2020;94:125–127. doi: 10.1016/j.ijid.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan Y-P, Tan B-Y, Pan J, Wu J, Zeng SZ, Wei HY. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. doi: 10.1016/j.jcv.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du W, Yu J, Wang H et al (2020) Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection. 10.1007/s15010-020-01427-2 [DOI] [PMC free article] [PubMed]
- 82.Buonsenso D, Costa S, Sanguinetti M, Cattani P, Posteraro B, Marchetti S, Carducci B, Lanzone A, Tamburrini E, Vento G, Valentini P (2020) Neonatal late onset infection with severe acute respiratory syndrome Coronavirus 2. Am J Perinatol. 10.1055/s-0040-1710541 [DOI] [PMC free article] [PubMed]
- 83.Han MS, Seong M-W, Heo EY, Park JH, Kim N, Shin S, Cho SI, Park SS, Choi EH (2020) Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin Infect Dis. 10.1093/cid/ciaa447 [DOI] [PMC free article] [PubMed]
- 84.Zhu L, Wang J, Huang R, Liu L, Zhao H, Wu C, Zhu C. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55:1430–1432. doi: 10.1002/ppul.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henry BM, Lippi G, Plebani M (2020) Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med 0. 10.1515/cclm-2020-0272 [DOI] [PubMed]
- 86.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL (2020) Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed]
- 88.Guan W-J, Liang W-H, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX (2020) Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J:2000547. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed]
- 89.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muus C, Luecken MD, Eraslan G, et al (2020) Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv 2020.04.19.049254. 10.1101/2020.04.19.049254
- 91.Guan W-J, Ni Z-Y, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones TC, Mühlemann B, Veith T, et al An analysis of SARS-CoV-2 viral load by patient age. 19
- 93.Song R, Han B, Song M, Wang L, Conlon CP, Dong T, Tian D, Zhang W, Chen Z, Zhang F, Shi M, Li X (2020) Clinical and epidemiological features of COVID-19 family clusters in Beijing. China J Infect. 10.1016/j.jinf.2020.04.018 [DOI] [PMC free article] [PubMed]
- 94.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis. Treatment and Prevention Options in Children The Pediatric Infectious Disease Journal. 2020;1:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leung WK, To K-F. Chan PKS, et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/s0016-5085(03)01215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, et al (2020) Vertical transmission of Coronavirus Disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol 1–5. 10.1080/15513815.2020.1747120 [DOI] [PMC free article] [PubMed]
- 98.Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case–control study | medRxiv. 10.1101/2020.03.10.20033605v1. Accessed 2 Apr 2020
- 99.Yang X, Yu Y, Xu J, Shu H, Xia J', Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sellers SA, Hagan RS, Hayden FG, Fischer WA. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11:372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chai X, Hu L, Zhang Y, et al (2020) Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020.02.03.931766. 10.1101/2020.02.03.931766
- 104.Silverstein WK, Stroud L, Cleghorn GE, Leis JA. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;395:734. doi: 10.1016/S0140-6736(20)30370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;200432:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buonsenso D, Piano A, Raffaelli F, et al Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. 5 [DOI] [PubMed]
- 107.Denina M, Scolfaro C, Silvestro E, Pruccoli G, Mignone F, Zoppo M, Ramenghi U, Garazzino S (2020) Lung ultrasound in children with COVID-19. Pediatrics.:e20201157. 10.1542/peds.2020-1157 [DOI] [PubMed]
- 108.Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A, Abzug MJ, MacBrayne CE, Soma VL, Dulek DE, Vora SB, Waghmare A, Wolf J, Olivero R, Grapentine S, Wattier RL, Bio L, Cross SJ, Dillman NO, Downes KJ, Timberlake K, Young J, Orscheln RC, Tamma PD, Schwenk HT, Zachariah P, Aldrich M, Goldman DL, Groves HE, Lamb GS, Tribble AC, Hersh AL, Thorell EA, Denison MR, Ratner AJ, Newland JG, Nakamura MM (2020) Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc. 10.1093/jpids/piaa045
- 109.Organization WH . Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 64 kb)
(DOCX 45 kb)