Abstract
Introduction
Urinary tract infections (UTI) are a common indication for antibiotic use in the emergency department (ED). With antibiotic resistance on the rise, it is essential that antibiotics be prescribed appropriately for UTIs. Our objective was to evaluate the appropriateness of antibiotic prescriptions by ED providers for uncomplicated cystitis and pyelonephritis.
Methods
We conducted a retrospective study of females ages 2–50 years seen in an academic ED from January 2017 to April 2018 diagnosed with UTI. We assessed the appropriateness of discharge antibiotic prescriptions, as determined by adherence to clinical practice guidelines, best evidence for the particular indication (cystitis vs pyelonephritis for children and adults), and the local antibiogram.
Results
A total of 421 patients were included in this study. Of these, 60 children and 198 adults were diagnosed with cystitis, and 47 children and 116 adults were diagnosed with pyelonephritis. Treatment in the absence of true infection was common, with culture-confirmed UTI occurring in only 17/50 (34%) of children and 60/129 (47%) of adults diagnosed with cystitis, and 23/40 (58%) of children and 58/87 (67%) of adults diagnosed with pyelonephritis, among patients who had urine cultures. The type of antibiotic prescribed was appropriate in 53/60 (88%) of children and 135/198 (68%) of adults with cystitis, and 38/47 (81%) of children and 53/116 (46%) of adults with pyelonephritis. The most common inappropriate antibiotic types were beta-lactams in adults (n = 92), nitrofurantoin for pyelonephritis (n = 16), and amoxicillin (n = 15). Dosing and duration errors were also common, occurring in 122/279 (44%) of prescriptions of an appropriate antibiotic type. The frequency of errors in the type of antibiotic prescribed was similar among provider types (attending physician, resident physician, and advanced practice clinician; p = 0.926).
Conclusion
This study reveals room for improvement in antibiotic prescription practices across provider cohorts in the ED for the management of uncomplicated cystitis and pyelonephritis in females.
INTRODUCTION
Urinary tract infections (UTI) are common among females, accounting for upward of 10.5 million visits to physician offices and emergency departments (ED) per year.1 While it is important to promptly treat UTIs with antibiotics to prevent complications such as septic shock and renal scarring in children,2,3 it is also prudent to use antibiotics only when needed in an effort to reduce adverse medication effects and antibiotic resistance. With common UTI pathogens, resistance to fluoroquinolones and trimethoprim-sulfamethoxazole (TMP-SMX) has been on the rise. Additionally, there has been increasing prevalence of extended-spectrum β-lactamase and other multi-drug resistant organisms.4–6
Given the implications of inappropriate antibiotic prescription practices to individuals and populations, it is essential that providers appropriately select antibiotic treatment for the management of suspected UTIs. Guidelines from sources such as the Infectious Diseases Society of America (IDSA) and the American Academy of Pediatrics (AAP) can provide guidance.7,8 However, local factors, such as site-specific antibiotic resistance, also must be taken into account. Most organizations now publish hospital-wide antibiograms, which were present in 98% of United States hospitals in 2014.9 Although hospital antibiograms exist, it remains unknown whether providers are using this tool, in conjunction with guidelines, such as those from the IDSA, to tailor their prescription practices to their particular location. In a recent survey of pediatric providers, 70% reported access to local antibiograms and, of these, only 50% reported using the antibiogram “always” or “most of the time” when empirically prescribing antibiotics for UTIs.10 Prescribing practices for UTIs are variable and problems exist, including the use of overly broad antibiotics and treatment in the absence of true infection.11,12
To investigate antibiotic prescribing practices for suspected UTIs, we primarily sought to determine whether empiric antibiotic selection was appropriate for suspected UTIs in children and adults. Secondarily, we set out to analyze frequency of antibiotic prescription in the absence of true infection and whether variation in appropriate antibiotic selection existed between specific provider groups, including attending physicians, resident physicians, physician assistants, and nurse practitioners. We hypothesized that inappropriate antibiotics were frequently prescribed, that patients were frequently treated with antibiotics in the absence of true infection, and that variation existed between provider groups.
METHODS
Study Design
The study was a retrospective analysis of females ages 2 to 50 years diagnosed with a UTI who were treated and discharged between January 1, 2017 – April 16, 2018, from an academic medical center ED. Females age 18 years or older were categorized as adults, while females under the age of 18 years were categorized as children. Institutional review board approval was obtained. We identified patients via an ED diagnosis of one or more of the following International Classification of Diseases (ICD-10) codes for UTI: ICD-10 CM: N39.0 (UTI, site not specified); N30.00 (acute cystitis without hematuria); N30.91 (cystitis, unspecified with hematuria); N30.80 (other cystitis without hematuria); N30.0 (acute cystitis); N30.01 (acute cystitis with hematuria); N30.90 (cystitis, unspecified without hematuria); N30 (cystitis); N30.8 (other cystitis); N30.9 (cystitis, unspecified); N30.81 (other cystitis with hematuria); N10 (acute pyelonephritis); and N12 (pyelonephritis). All data collection was conducted by PC after receiving training from KK. Approximately 5% of charts were reviewed by both authors independently to assess for congruency, including random charts and any cases in which there was uncertainty.
We excluded patients from this study if they had UTI complications (including renal abscess, septic shock, concurrent nephrolithiasis, or required hospital admission or observation); had pre-existing renal or urologic disease (including vesicoureteral reflux or other functional abnormality; obstructive uropathy, permanent or intermittent catheter, history of urinary tract surgery); were pregnant or up to six-weeks post-partum; were prisoners; had diabetes mellitus; or were immunocompromised. This included patients taking medications such as steroids, biologics, and chemotherapeutic drugs. We also excluded patients with recurrent UTI (had a UTI with or without antibiotic treatment within the prior month) and patients who were already prescribed an antibiotic for the current UTI prior to the ED visit.
Population Health Research Capsule.
What do we already know about this issue?
Previous studies have found overuse of antibiotics for urinary tract infections (UTI).
What was the research question?
To evaluate the appropriateness of antibiotic prescriptions for UTIs.
What was the major finding of the study?
Antibiotics were overused and inappropriate antibiotics were commonly prescribed for suspected UTIs.
How does this improve population health?
This study identified antibiotic misuse including overly broad antibiotics and overdiagnosis of UTIs, which can promote antimicrobial resistance.
Patients were categorized as having uncomplicated cystitis or pyelonephritis based on their ICD-10 code. In cases where the ICD-10 code CM N39.0 for “urinary tract infection, site not specified” was used, the authors categorized patients as having either cystitis or pyelonephritis based on medical chart documentation of symptoms, vital signs, physical exam, and provider impression. Patients were assigned to the pyelonephritis cohort if they had fever, flank pain, or costovertebral angle tenderness.13
Outcomes and Data Analysis
The primary outcome was the appropriateness of the antibiotic prescription for UTI, including the antibiotic type, dose, and duration. We determined a list of appropriate discharge antibiotics (Table 1, Appendix Tables 1 and 2) using guidelines from the IDSA, the AAP, UpToDate, and our hospital’s 2016 outpatient antibiogram (Appendix Figure 1).7,8,13–15 These references were selected as they represent current and reputable sources used by providers in our ED. For children, there are many potential antibiotic options with limited evidence for superiority of a particular antibiotic or regimen.16 Thus, any antibioitcs recommended by expert sources7,14,15 with favorable susceptibility at our institution (> 80% of E. coli isolates susceptible) were included. For adults, antibiotics recommended by IDSA guidelines were used.8 Based on our local susceptibilities (Appendix Figure 1), all of the antibiotic options had favorable resistance patterns to E. coli (more than 80% of isolates susceptible) and were included as appropriate.
Table 1.
List of appropriate antibiotic types for the management of uncomplicated cystitis and pyelonephritis.
| Age group | Infection type | Appropriate medications |
|---|---|---|
| Children14,15 | Uncomplicated cystitis | Amoxicillin / clavulanic acid (immediate release formulations) 1st–3rd generation cephalosporin Nitrofurantoin Trimethoprim-sulfamethoxazole |
| Pyelonephritis | Amoxicillin / clavulanic acid (immediate release formulations) 1st–3rd generation cephalosporin Trimethoprim-sulfamethoxazole |
|
| Adults8,13 | Uncomplicated cystitis | Fosfomycin Nitrofurantoin Trimethoprim-sulfamethoxazole |
| Pyelonephritis | Ciprofloxacin Levofloxacin Trimethoprim-sulfamethoxazole |
We did not include beta-lactams as appropriate options for adults in accordance with the IDSA guidelines, which recommend against routine use for UTI due to inferior efficacy, as there are numerous other antibiotic options for adults with favorable local susceptibilities. Allergies were reviewed during chart review, and no patients were allergic to all appropriate first-line options. There are no standardardized antibiotic recommendations in place in our ED. Our hospital antibiogram is available on the hospital infonet, and pocket cards are distributed periodically. It provides susceptibilities for various organisms for inpatients and outpatients, but does not provide empiric treatment recommendations for infection types, such as UTI.
The secondary outcomes were the frequency of positive urine culture (when obtained) and the appropriateness of discharge antibiotic prescriptions between provider types (resident physician, advanced practice clinician, and attending physician). UTI was defined as growth of greater than or equal to 100,000 colonies of a single uropathogen via clean catch, growth of greater than or equal to 50,000 colonies of a single uropathogen via direct catheterization, or growth of greater than or equal to 50,000 colonies of a single uropathogen via clean catch in the presence of a positive urinalysis.13,14 We performed data analysis using descriptive statistics and chi-squared tests where applicable.
RESULTS
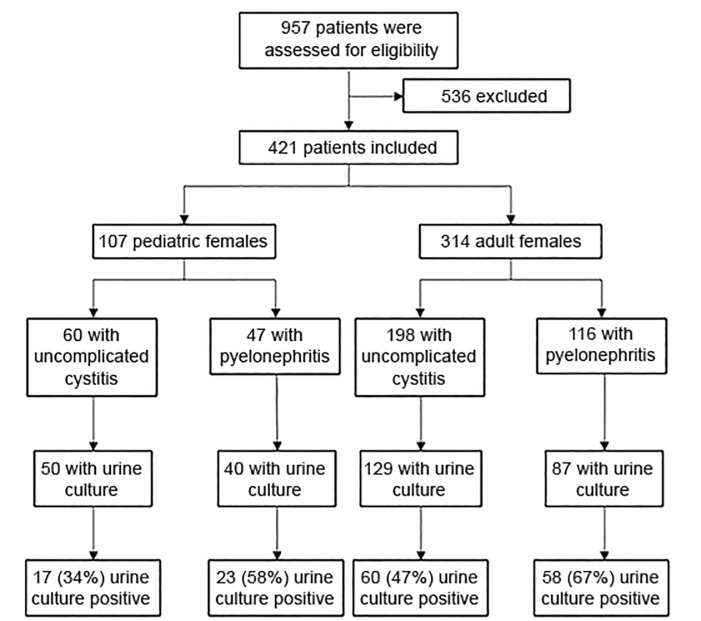
During the study period, 421 patients met inclusion criteria (Figure 1). Common reasons for exclusion in adults included diabetes mellitus, presence of nephrolithiasis, and current treatment with antibiotics or immunosuppressing medications. Common reasons for exclusion in children included vesicoureteral reflux and current antibiotic treatment. Of the total 421 patients, 258 (61.3%) were classified as having cystitis and 163 were classified as having pyelonephritis (38.7%) (Figure 1). The median age for children was nine years and the median age for adults was 27 years.
Figure 1.
Flow diagram of study participants diagnosed with urinary tract infections.
Urine cultures were performed in 73% of patients (306/421), with higher utilization of urine culture in patients with pyelonephritis (127/163, 78%) than in patients with cystitis (179/258, 69%) (Figure 1). The presence of UTI was not confirmed for a substantial proportion of patients diagnosed with cystitis, whereas more patients diagnosed with pyelonephritis had a culture-proven UTI (Figure 1). A vast majority of the true positive urine cultures grew E. coli (80%).
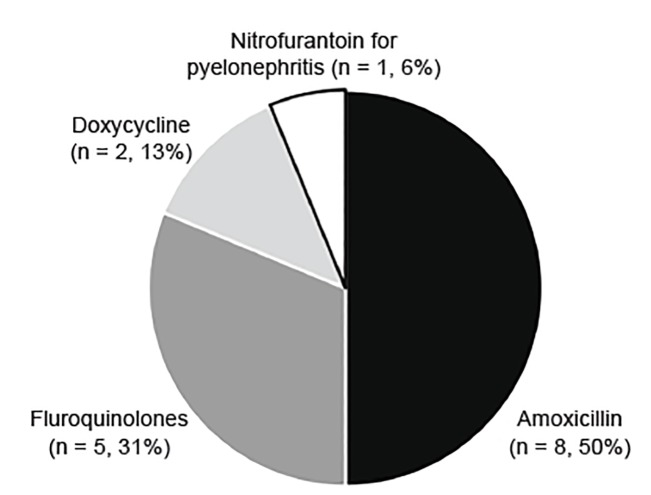
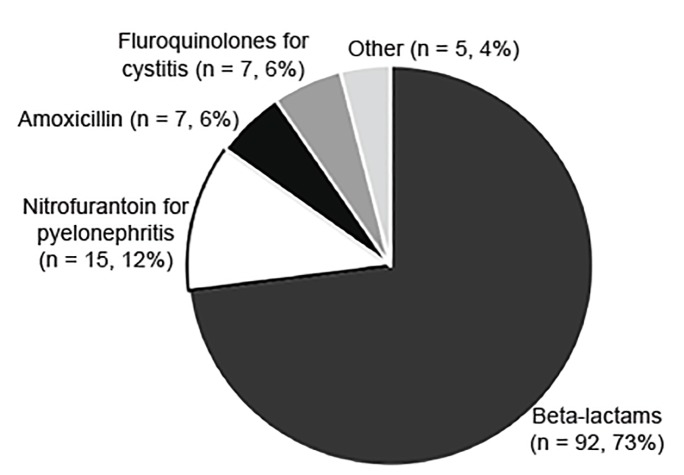
The appropriateness of discharge antibiotic prescriptions (Appendix Figure 2) is shown in Table 2. The majority of patients received an appropriate antibiotic type, except for adults with pyelonephritis (46%). Figures 2 and 3 detail the reasons for lack of accordance with clinical guidelines for antibiotic type in children and adults, respectively. Errors in dosing and duration were common, with only 157 (56%) of those with appropriate antibiotic type having correct dosing and duration when compared to our established criteria (Appendix Tables 1 and 2). Duration was more frequently incorrect than dosing. Antibiotics were commonly prescribed for a longer, rather than shorter, duration than is specified in the Appendix Tables 1 and 2. Of the 104 patients with incorrect duration, 73 patients (70%) received antibiotics for a longer duration and 31 patients (30%) received antibiotics for a shorter duration.
Table 2.
Appropriateness of antibiotic prescriptions for urinary tract infection.
| Age group | Children, n (%) | Adult, n (%) | ||
|---|---|---|---|---|
| Diagnosis | Cystitis | Pyelonephritis | Cystitis | Pyelonephritis |
| Total | 60 (14%) | 47 (11%) | 198 (47%) | 116 (28%) |
| Appropriate discharge antibiotic type | 53/60 (88%) | 38/47 (81%) | 135/198 (68%) | 53/116 (46%) |
| + Appropriate dosea | 48/60 (80%) | 36/47 (77%) | 128/198 (65%) | 49/116 (42%) |
| + Appropriate dose and durationb | 41/60 (68%) | 22/47 (47%) | 68/198 (34%) | 26/116 (22%) |
Appropriate antibiotic type and correct dose.
Appropriate antibiotic type and correct dose and duration.
Figure 2.
Inappropriate antibiotic types prescribed for children with urinary tract infections.
Figure 3.
Inappropriate antibiotic types prescribed for adult women with urinary tract infections.
A total of 130 unique prescribers treated the 421 patients included in the study. We assessed the frequency of appropriate discharge medication prescription type, independent of dosage and duration, for cystitis and pyelonephritis combined across the following three cohorts: attending physician (18/27, 67%); resident physician (168/249, 67%); and advanced practice clinician (93/145, 64%). No difference was found between the groups (p = 0.926).
DISCUSSION
In this study we found a number of areas for improvement in the initial management of female UTIs in an academic ED. Furthermore, we demonstrated that opportunity for improvement exists across all provider cohorts studied. These findings can guide improvement efforts for judicious use of antibiotics. Overtreatment of UTI was common, in particular for cystitis. As many as two-thirds of children who were treated for cystitis had a negative urine culture. Strategies to improve diagnostic accuracy and/or decrease unnecessary antibiotic treatment include the use of a decision aid, such as that by McIsaac et al for females with suspected cystitis.17 This simple decision aid using three variables (dysuria, leukocytes greater than a trace amount, and positive nitrites) was statistically associated with a positive urine culture result.
Strategies to reduce antibiotic overuse include delaying antibiotic prescription if infection is uncertain and discontinuing antibiotics if the urine culture results as negative. In a study by Knottnerus et al, many women were willing to delay antibiotic initiation for cystitis while awaiting urine culture result. More than half of these women experienced resolution of symptoms within a week without antibiotic therapy.18 In the pediatric and adult settings, nurse, clinician, and pharmacist follow-up after urine culture result has led to decreased total antibiotic days.19,20 A common theme is the need for improved, ED-based antimicrobial stewardship strategies, as the ED is unique and may require its own specific interventions. 21
Overtreatment of UTIs was also found in the form of excessive antibiotic duration (Table 2), particularly for cystitis. The variation in length of antibiotic courses could be due in part to limited evidence for superiority of particular regimens, especially for children.16,22 However, longer than recommended antibiotic courses can contribute to antibiotic resistance.23 Potential strategies for improving dosing and duration include interventions to the electronic health record, which have been demonstrated to improve appropriate antibiotic delivery.24,25
Finally, we investigated the utilization of appropriate antibiotic types given the local antibiogram and evidence-based guidelines. Adherence to the IDSA recommendations can increase narrow spectrum antibiotic use and decrease unnecessary antibiotic days.26,27 In our study, we found overuse of fluoroquinolones for cystitis (albeit at lower rates than in other reported studies).26,27 This antibiotic class can cause serious advserse events, promote antibiotic resistance, and predispose to Clostridum difficile infections.28–31 Other deviations from the guidelines included the use of potentially ineffective antimicrobials, such as amoxicillin and nitrofurantoin for pyelonephritis. While clinical outcomes were not tracked, the liklihood of treatment failure is high with these agents, with E. coli resistance to amoxicillin being widespread.8
The most common deviation from IDSA guidelines in adults was treatment with beta-lactams (Figure 3). The significance of this is unclear despite how common this practice is at our institution. These antibiotics are likely prescribed because local susceptibilities are favorable for 1st- and 2nd-generation cephalosporins. Furthermore, additional studies have been published since the IDSA guidelines in 2011 supporting efficacy of beta-lactams for the treatment of UTIs in adults.32–35 The use of beta-lactams could be studied further to provide more potential treatment options, especially given that antibiotic resistance to many other antibiotic classes is on the rise.
LIMITATIONS
This study analyzed adherence to clinical guidelines and expert recommendations, assuming that these promote optimal care. Additional limitations of this study are associated with its retrospective design. The determination of antibiotic appropriateness relied on diagnosis of either cystitis or pyelonephritis; however, some patients had a non-specific diagnosis of “urinary tract infection, site not specified.” In this case, they were assigned to either the cystitis or pyelonephritis group based on the study team’s interpretation of the patient’s signs and symptoms. Errors in this designation by the study team would have affected the assessment of the appropriateness of antibiotics received. In an effort to check cohort assignment, we performed statistical analysis and found no significant difference in the appropriateness of discharge antibiotic type between patients whom the study team assigned to cystitis vs pyelonephritis and patients who had an ED diagnosis code for cystitis or pyelonephritis specifically (p > 0.05). Additionally, we did not assess why providers chose antibiotics that deviated from the recommendations, such as resistance in past urine cultures or lack of awareness of the guidelines. Furthermore, urine culture was used to assess presence of true infection; however, not all patients received cultures, which could affect our conclusions about the prevalence of true UTI in our study.
CONCLUSION
This study reveals gaps in the management of children and adults with urinary tract infections, including administration of antibiotics in the absence of true infection and inappropriate choice of discharge antibiotic type, dose, or duration. These shortcomings occurred across all three provider types studied and represent areas for further education and quality improvement to promote antimicrobial stewardship.
Supplementary Information
Footnotes
Section Editor: Stephen Y. Liang, MD, MPHS
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. No author has professional or financial relationships with any companies that are relevant to this study. There are no conflicts of interest or sources of funding to declare.
REFERENCES
- 1.Schappert S, Rechtsteiner E. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13. 2007;(169):1–38. [PubMed] [Google Scholar]
- 2.Johnson J, Russo T. Acute pyelonephritis in adults. N Engl J Med. 2018;378(1):48–59. doi: 10.1056/nejmcp1702758. [DOI] [PubMed] [Google Scholar]
- 3.Karavanaki KA, Soldatou A, Koufadaki AM, et al. Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr. 2017;106(1):149–54. doi: 10.1111/apa.13636. [DOI] [PubMed] [Google Scholar]
- 4.Dancer SJ, Kirkpatrick P, Corcoran DS, et al. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum beta-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2013;41(2):137–42. doi: 10.1016/j.ijantimicag.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez GV, Babiker A, Master RN, et al. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob Agents Chemother. 2016;60(5):2680–3. doi: 10.1128/AAC.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidell MR, Palchak M, Mohr J, et al. Fluoroquinolone and third-generation-cephalosporin resistance among hospitalized patients with urinary tract infections due to Escherichia coli: Do rates vary by hospital characteristics and geographic region? Antimicrob Agents Chemother. 2016;60(5):3170–3. doi: 10.1128/AAC.02505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinl infect dis. 2011;52(5):103–120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 9.Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200. [PMC free article] [PubMed] [Google Scholar]
- 10.Selekman RE, Allen IE, Copp HL. Determinants of practice patterns in pediatric UTI management. J Pediatr Urol. 2016;12(5):308. doi: 10.1016/j.jpurol.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Sayyed B, Le J, Al-Tabbaa MM, et al. Uncomplicated urinary tract infection in ambulatory primary care pediatrics: Are we using antibiotics appropriately? J Pediatr Pharmacol Ther. 2019;24(1):39–44. doi: 10.5863/1551-6776-24.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien K, Hillier S, Simpson S, et al. An observational study of empirical antibiotics for adult women with uncomplicated UTI in general practice. J Antimicrob Chemother. 2007;59(6):1200–3. doi: 10.1093/jac/dkm108. [DOI] [PubMed] [Google Scholar]
- 13.Hooton TM, Gupta K. Acute simple cystitis in women. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate Inc.; [Accessed April 14, 2018]. Available at: https://www.uptodate.com. [Google Scholar]
- 14.Shaikh N, Hoberman A. Urinary tract infections in infants older than one month and young children: acute management, imaging, and prognosis. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate Inc.; [Accessed on April 14, 2018]. Available at: https://www.uptodate.com. [Google Scholar]
- 15.Palazzi DL, Campbell JR. Acute infectious cystitis: management and prognosis in children older than two years and adolescents. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate Inc; [Accessed on Jan 2, 2020]. Available at: https://www.uptodate.com. [Google Scholar]
- 16.Fitzgerald A, Mori R, Lakhanpaul M, et al. Antibiotics for treating lower urinary tract infection in children. Cochrane Database Syst Rev. 2012;(8):CD006857. doi: 10.1002/14651858.CD006857.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIsaac WJ, Moineddin R, Ross S. Validation of a decision aid to assist physicians in reducing unnecessary antibiotic drug use for acute cystitis. Arch Intern Med. 2007;167(20):2201–6. doi: 10.1001/archinte.167.20.2201. [DOI] [PubMed] [Google Scholar]
- 18.Knottnerus BJ, Geerlings SE, Mol van Charante EP, et al. Women with symptoms of uncomplicated urinary tract infection are often willing to delay antibiotic treatment: a prospective cohort study. BMC Fam Pract. 2013;14:71. doi: 10.1186/1471-2296-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Rowan N, Pflugeisen BM, et al. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594–8. doi: 10.1016/j.ajem.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Saha D, Patel J, Buckingham D, et al. Urine culture follow-up and antimicrobial stewardship in a pediatric urgent care network. Pediatrics. 2017;139(4):e20162103. doi: 10.1542/peds.2016-2103. (2017) [DOI] [PubMed] [Google Scholar]
- 21.May L, Cosgrove S, L’Archeveque M, et al. A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med. 2013;62(1):69–77. doi: 10.1016/j.annemergmed.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriakidou KG, Rafailidis P, Matthaiou DK, et al. Short- versus long-course antibiotic therapy for acute pyelonephritis in adolescents and adults: a meta-analysis of randomized controlled trials. Clin Ther. 2008;30(10):1859–68. doi: 10.1016/j.clinthera.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Spellberg B. The new antibiotic mantra: “Shorter is better”. JAMA Intern Med. 2016;176(9):1254–5. doi: 10.1001/jamainternmed.2016.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hincker A, Ben Abdallah A, Avidan M, et al. Electronic medical record interventions and recurrent perioperative antibiotic administration: a before-and-after study. Can J Anaesth. 2017;64(7):716–23. doi: 10.1007/s12630-017-0885-1. [DOI] [PubMed] [Google Scholar]
- 25.Kaushal R, Barker KN, Bates DW. How can information technology improve patient safety and reduce medication errors in children’s health care? JAMA Pediatrics. 2001;155(9):1002–7. doi: 10.1001/archpedi.155.9.1002. [DOI] [PubMed] [Google Scholar]
- 26.Percival KM, Valenti KM, Schmittling SE, et al. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129–33. doi: 10.1016/j.ajem.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 27.Hecker MT, Fox CJ, Son AH, et al. Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS One. 2014;9(2):e87899. doi: 10.1371/journal.pone.0087899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber SG, Gold HS, Hooper DC, et al. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9(11):1415–22. doi: 10.3201/eid0911.030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCusker ME, Harris AD, Perencevich E, et al. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg Infect Dis. 2003;9(6):730–3. doi: 10.3201/eid0906.020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu DI, Okamoto MP, Murthy R, et al. Fluoroquinolone-resistant Pseudomonas aeruginosa: risk factors for acquisition and impact on outcomes. J Antimicrob Chemother. 2005;55(4):535–41. doi: 10.1093/jac/dki026. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. [Accessed January 14, 2020]. Available at: https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass.htm.
- 32.Vogler S, Pavich E. Pyelonephritis treatment in the community emergency department: cephalosporins vs. first-line agents. Am J Emerg Med. 2018;36(11):2054–7. doi: 10.1016/j.ajem.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez M, Collvinent B, Miro O, et al. Short-term effectiveness of ceftriaxone single dose in the initial treatment of acute uncomplicated pyelonephritis in women. A randomised controlled trial. Emerg Med J. 2002;19(1):19–22. doi: 10.1136/emj.19.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monmaturapoj T, Montakantikul P, Mootsikapun P, et al. A prospective, randomized, double dummy, placebo-controlled trial of oral cefditoren pivoxil 400mg once daily as switch therapy after intravenous ceftriaxone in the treatment of acute pyelonephritis. Int J Infect Dis. 2012;16(12):843–9. doi: 10.1016/j.ijid.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Jernelius H, Zbornik J, Bauer CA. One or three weeks’ treatment of acute pyelonephritis? A double-blind comparison, using a fixed combination of pivampicillin plus pivmecillinam. Acta Med Scand. 1988;223(5):469–77. doi: 10.1111/j.0954-6820.1988.tb15899.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.