Summary
Background
Before the COVID-19 pandemic, coronaviruses caused two noteworthy outbreaks: severe acute respiratory syndrome (SARS), starting in 2002, and Middle East respiratory syndrome (MERS), starting in 2012. We aimed to assess the psychiatric and neuropsychiatric presentations of SARS, MERS, and COVID-19.
Methods
In this systematic review and meta-analysis, MEDLINE, Embase, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature databases (from their inception until March 18, 2020), and medRxiv, bioRxiv, and PsyArXiv (between Jan 1, 2020, and April 10, 2020) were searched by two independent researchers for all English-language studies or preprints reporting data on the psychiatric and neuropsychiatric presentations of individuals with suspected or laboratory-confirmed coronavirus infection (SARS coronavirus, MERS coronavirus, or SARS coronavirus 2). We excluded studies limited to neurological complications without specified neuropsychiatric presentations and those investigating the indirect effects of coronavirus infections on the mental health of people who are not infected, such as those mediated through physical distancing measures such as self-isolation or quarantine. Outcomes were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, or the Chinese Classification of Mental Disorders (third edition) or psychometric scales; quality of life; and employment. Both the systematic review and the meta-analysis stratified outcomes across illness stages (acute vs post-illness) for SARS and MERS. We used a random-effects model for the meta-analysis, and the meta-analytical effect size was prevalence for relevant outcomes, I2 statistics, and assessment of study quality.
Findings
1963 studies and 87 preprints were identified by the systematic search, of which 65 peer-reviewed studies and seven preprints met inclusion criteria. The number of coronavirus cases of the included studies was 3559, ranging from 1 to 997, and the mean age of participants in studies ranged from 12·2 years (SD 4·1) to 68·0 years (single case report). Studies were from China, Hong Kong, South Korea, Canada, Saudi Arabia, France, Japan, Singapore, the UK, and the USA. Follow-up time for the post-illness studies varied between 60 days and 12 years. The systematic review revealed that during the acute illness, common symptoms among patients admitted to hospital for SARS or MERS included confusion (36 [27·9%; 95% CI 20·5–36·0] of 129 patients), depressed mood (42 [32·6%; 24·7–40·9] of 129), anxiety (46 [35·7%; 27·6–44·2] of 129), impaired memory (44 [34·1%; 26·2–42·5] of 129), and insomnia (54 [41·9%; 22·5–50·5] of 129). Steroid-induced mania and psychosis were reported in 13 (0·7%) of 1744 patients with SARS in the acute stage in one study. In the post-illness stage, depressed mood (35 [10·5%; 95% CI 7·5–14·1] of 332 patients), insomnia (34 [12·1%; 8·6–16·3] of 280), anxiety (21 [12·3%; 7·7–17·7] of 171), irritability (28 [12·8%; 8·7–17·6] of 218), memory impairment (44 [18·9%; 14·1–24·2] of 233), fatigue (61 [19·3%; 15·1–23·9] of 316), and in one study traumatic memories (55 [30·4%; 23·9–37·3] of 181) and sleep disorder (14 [100·0%; 88·0–100·0] of 14) were frequently reported. The meta-analysis indicated that in the post-illness stage the point prevalence of post-traumatic stress disorder was 32·2% (95% CI 23·7–42·0; 121 of 402 cases from four studies), that of depression was 14·9% (12·1–18·2; 77 of 517 cases from five studies), and that of anxiety disorders was 14·8% (11·1–19·4; 42 of 284 cases from three studies). 446 (76·9%; 95% CI 68·1–84·6) of 580 patients from six studies had returned to work at a mean follow-up time of 35·3 months (SD 40·1). When data for patients with COVID-19 were examined (including preprint data), there was evidence for delirium (confusion in 26 [65%] of 40 intensive care unit patients and agitation in 40 [69%] of 58 intensive care unit patients in one study, and altered consciousness in 17 [21%] of 82 patients who subsequently died in another study). At discharge, 15 (33%) of 45 patients with COVID-19 who were assessed had a dysexecutive syndrome in one study. At the time of writing, there were two reports of hypoxic encephalopathy and one report of encephalitis. 68 (94%) of the 72 studies were of either low or medium quality.
Interpretation
If infection with SARS-CoV-2 follows a similar course to that with SARS-CoV or MERS-CoV, most patients should recover without experiencing mental illness. SARS-CoV-2 might cause delirium in a significant proportion of patients in the acute stage. Clinicians should be aware of the possibility of depression, anxiety, fatigue, post-traumatic stress disorder, and rarer neuropsychiatric syndromes in the longer term.
Funding
Wellcome Trust, UK National Institute for Health Research (NIHR), UK Medical Research Council, NIHR Biomedical Research Centre at University College London Hospitals NHS Foundation Trust and University College London.
Introduction
Viral infections are common and some are known to infect the CNS, causing neuropsychiatric syndromes affecting cognitive, affective, behavioural, and perceptual domains.1, 2, 3 Severe illness of diverse aetiologies is associated with subsequent psychiatric morbidity, at least some of which is attributable to its psychological impact of trauma.4, 5, 6
Coronaviruses are single-stranded RNA viruses and several subtypes affecting humans have been identified, most of which cause mild upper respiratory tract infections in immunocompetent individuals (notably, the HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 strains).7, 8 Coronaviruses have also been detected in both the brain and the cerebrospinal fluid of individuals with seizures, encephalitis, and encephalomyelitis.9 Novel strains of coronavirus caused the severe acute respiratory syndrome (SARS) outbreak, starting in 2002, and the Middle East respiratory syndrome (MERS) outbreak, starting in 2012.8
On Dec 31, 2019, WHO was made aware of several cases of atypical pneumonia in Wuhan, China, which were subsequently identified as being caused by a novel coronavirus termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2;10 panel). As the pandemic of the disease now known as COVID-19 has spread, there has been a growing recognition of the psychiatric implications of the disease.13, 14 There are several reasons why the current COVID-19 pandemic might have psychiatric consequences. Some of these reasons relate to the wider social impact of the pandemic and the governmental response, including physical distancing measures and quarantine.15, 16 Both the infected and non-infected population might be susceptible as a result of certain experiences, such as widespread anxiety,17 social isolation,16 stress in health-care workers and other essential workers,18 and unemployment and financial difficulties.19 Other experiences might be specific to individuals who are infected with the virus, such as concern about the outcome of their illness,20 stigma,21 and amnesia or traumatic memories of severe illness.22
Panel. Terminology.
Coronanavirus
A group of viruses that predominantly cause mild upper respiratory tract infections in humans.
Severe acute respiratory syndrome coronavirus (SARS-CoV)
A clade I, cluster IIb betacoronavirus that enters host cells via the angiotensin-converting enzyme 2 receptor.
Severe acute respiratory syndrome (SARS)
The clinical syndrome associated with infection with SARS-CoV that emerged in humans in 2002, affecting approximately 8096 people.11
Middle East respiratory syndrome coronavirus (MERS-CoV)
A clade II betacoronavirus that enters host cells via the dipeptidyl peptidase 4 receptor.
Middle East respiratory syndrome (MERS)
The clinical syndrome associated with infection with MERS-CoV that emerged in humans in 2012, affecting approximately 2260 people.11
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
A clade I, cluster IIa betacoronavirus with structural similarity to SARS-CoV that enters host cells via the angiotensin-converting enzyme 2 receptor.
COVID-19
The clinical syndrome—primarily a respiratory disorder—associated with infection with SARS-CoV-2.
Psychiatric
We use this term to include disorders, symptoms, and signs listed in category 06 (mental, behavioural, or neurodevelopmental disorders) of the 11th edition of the ICD.12
Neuropsychiatric
We use this term to denote psychiatric disorders, symptoms, and signs that are the result of brain damage or disease.
Neuropsychiatric consequences—ie, mental disorders that are the sequelae of brain damage or disease—can arise either through direct effects of infection of the CNS or indirectly via an immune response or medical therapy. A case series from Wuhan found that among patients admitted to hospital for infection with SARS-CoV-2, 36% had neurological features, mostly consisting of mild symptoms such as dizziness and headache, although these symptoms might be manifestations more of systemic illness than a specific neurological syndrome.23 Some patients had acute cerebrovascular disease or impaired consciousness as part of their illness.23 SARS-CoV-2 enters human host cells by the angiotensin-converting enzyme 2 receptor, which has little expression in the brain.24, 25 There has been speculation that other routes of CNS infiltration might account for the respiratory failure caused by infection with SARS-CoV-2, although there is currently no evidence.24 There is preliminary in-vitro evidence that—possibly unlike SARS coronavirus (SARS-CoV)—SARS-CoV-2 can replicate in neuronal cells, but the translation of this finding to in-vivo settings remains unclear.26 Even if severe neuropsychiatric consequences are proportionately rare, a considerable number of individuals worldwide would be affected.27, 28 Previous influenza pandemics have been associated with long-lasting neuropsychiatric consequences,29 so it is possible that other viral infections on a large scale could cause sustained mental morbidity.
Research in context.
Evidence before this study
Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) cause severe respiratory illness, and a few studies have examined the acute and post-illness psychiatric and neuropsychiatric outcomes of these diseases. The COVID-19 pandemic has affected a large proportion of the world's population, but relatively little is known about its potential direct effects on mental health. MEDLINE, Embase, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature databases were searched from inception to March 18, 2020, for terms relating to coronavirus infection and psychiatric presentations. medRxiv, bioRxiv, and PsyArXiv were searched for relevant preprints published between Jan 1, 2020, and April 10, 2020. Studies were included if they provided numerical or formal qualitative data on psychiatric presentations of coronavirus outbreaks. The majority of studies were of low or moderate quality.
Added value of this study
This systematic review and meta-analysis suggests that among patients admitted to hospital for severe SARS or MERS coronavirus infections, delirium is common acutely, whereas post-traumatic stress disorder, depression, anxiety, and fatigue are common in the following months. Preliminary data suggest patients with COVID-19 might experience delirium, confusion, agitation, and altered consciousness, as well as symptoms of depression, anxiety, and insomnia.
Implications of all the available evidence
Previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages. In the current COVID-19 pandemic, there is already evidence of delirium acutely and clinicians should be alert to the possibility of high rates of common mental disorders in the longer term. High-quality, peer-reviewed research into psychiatric symptoms of patients infected with SARS-CoV-2 as well as into potential mitigating factors and interventions is needed.
We are not aware of a systematic review of the psychiatric consequences of all forms of coronavirus infection, including the recent data on COVID-19, to inform clinicians of the possible longer-term consequences of this pandemic. We aimed to examine the two previous coronavirus epidemics, SARS and MERS, to identify the possible psychiatric and neuropsychiatric implications for the current pandemic. We also examined the early data from the COVID-19 outbreak.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched MEDLINE, Embase, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature databases for studies or abstracts published between database inception and March 18, 2020. We used a combined set of keywords (appendix pp 6–8) to identify human studies reporting on a broad range of psychiatric presentations, symptom severity, diagnoses, employment, and quality of life in association with coronavirus exposure. Neuropsychiatric concepts such as confusion and cognition were included, but we did not include neurological disorders such as stroke, seizure, and encephalomyelitis because these disorders do not necessarily have psychiatric presentations and any psychiatric presentations would be captured by the psychiatric search terms. Our definitions are included in the panel.
For MEDLINE, the terms were: (((coronavir* OR alphacoronavirus OR betacoronavirus OR COVID OR COVID-19 OR “severe acute respiratory syndrome” OR SARS OR “Middle East respiratory syndrome” OR MERS OR “infectious bronchitis vir*” OR “infectious bronchitis”).ti,ab OR (exp coronaviridae/ OR exp “severe acute respiratory syndrome”/)) AND ((deliri* OR sleep OR insomnia OR somnolence OR hypersomnolence OR parasomnia OR “movement disorder” OR neuropsych* OR dement* OR cogniti* OR irritability OR hallucinat* OR delusion* OR apath* OR indifference OR agitat* OR euphori* OR elation OR elated OR disinhibit* OR aggressi* OR amnes* OR catatoni* OR personality OR psycho* OR mental OR mood OR affective OR depress* OR anxi* OR “obsessive compulsive” OR OCD OR “panic disorder” OR post-trauma* OR posttrauma* OR PTSD OR neurosis OR neurotic OR bipolar OR mania OR manic OR schizophreni* OR “intelligence quotient” OR IQ OR “mental retardation” OR “intellectual disability” OR “learning disability” OR autis* OR asperger* OR “attention deficit” OR ADHD OR hyperactivity OR hyperkinetic OR suicid* OR emotion* OR appetite OR fatigu* OR tired* OR confus* OR “quality of life” OR QoL OR employment OR unemployment).ti,ab OR (exp delirium/ OR exp sleep/ OR exp wakefulness/ OR exp sleep/ OR exp “disorders of excessive somnolence”/ OR exp parasomnias/ OR exp “psychomotor disorders”/ OR exp dementia/ OR exp “neurocognitive disorders”/ OR exp hallucinations/ OR exp delusions/ OR exp apathy/ OR exp “psychomotor agitation”/ OR exp euphoria/ OR exp aggression/ OR exp amnesia/ OR exp catatonia/ OR exp “personality disorders”/ OR exp “schizophrenia spectrum and other psychotic disorders”/ OR exp “mental disorders”/ OR exp “mood disorders”/ OR exp depression/ OR exp anxiety/ OR exp “anxiety disorders”/ OR exp “obsessive-compulsive disorder”/ OR exp “panic disorder”/ OR exp “stress disorders, post-traumatic”/ OR exp “bipolar and related disorders”/ OR exp schizophrenia/ OR exp “intellectual disability”/ OR exp “autism spectrum disorder”/ OR exp “asperger syndrome”/ OR exp “attention deficit and disruptive behavior disorders”/ OR exp “attention deficit disorder with hyperactivity”/ OR exp “motor activity”/ OR exp suicide/ OR exp emotions/ OR exp appetite/ OR exp “FEEDING AND EATING DISORDERS”/ OR exp FATIGUE/ OR exp CONFUSION/ OR exp “quality of life”/ OR exp employment/ OR exp unemployment/))) [Humans].
Given that this field is developing rapidly, we also searched the preprint servers medRxiv, PsyArXiv, and bioRxiv for studies published between Jan 1, 2020, and April 10, 2020, with the terms “coronavirus” or “COVID-19” in the title or abstract. In addition, relevant experts in the field were individually contacted and the references of other review articles were examined.
Duplicate references were removed electronically and manually. Titles, abstracts, and full texts of articles were independently screened by two reviewers (JPR and EC). Where there was disagreement on the inclusion of a title or abstract, it was retained for the next stage of screening. Disagreement on the inclusion of a full-text article was discussed with an independent arbiter (DO). Reasons for exclusion of full texts were collected.
We included English-language studies that reported the psychiatric and neuropsychiatric features of suspected or confirmed cases of three types of coronavirus infection (SARS-CoV, MERS coronavirus [MERS-CoV], and SARS-CoV-2). Randomised controlled trials, cohort studies, case-control studies, cross-sectional studies, case series, case reports, and qualitative studies were included. Preprints and letters were included if they described original research that contained data on patients with suspected or laboratory-confirmed coronavirus infection, if data on individuals infected with coronavirus were distinguishable from data on any individuals not infected, and if specific neuropsychiatric features were listed, but conference abstracts were excluded because they lacked sufficient information for quality assessment and data extraction.
We excluded studies limited to neurological complications without specified neuropsychiatric presentations, but we included neuropsychiatric presentations (eg, cognitive impairment, apathy, insomnia, altered consciousness, and delirium). We excluded studies investigating the indirect effects of coronavirus infections on the mental health of people not infected mediated through physical distancing measures such as self-isolation or quarantine, because these have been recently appraised.16
This systematic review followed PRISMA guidelines (appendix pp 2–5), although the study protocol was not registered.
Data extraction
Data were extracted by two of three independent reviewers (EC, DO, and JPR). Where relevant data were missing from a report, the author was contacted. Descriptive variables extracted were setting (ie, country), population type (eg, pregnant women and children), study design (eg, cohort and case-control), virus subtype (SARS-CoV, MERS-CoV, and SARS-CoV-2), diagnostic criteria for viral infection (eg, WHO guidelines), timing (acute vs post-illness), follow-up time, nature of the control group, number of cases, number of controls, age, and gender. Randomised controlled trials, for the purposes of this review, were treated as cohort studies. For example, if a trial investigated the effects of an antiviral medication versus placebo, data from all participants regardless of treatment group would be extracted together.
Outcomes
Outcomes were divided into number of signs or symptoms; symptom severity (ie, anxiety, depression, or trauma); proportion of diagnoses (ie, anxiety, depression, and post-traumatic stress disorder); quality of life scores; and proportion of individuals employed. If more than one dataset was reported for the same group of patients, the outcomes that were assessed after the longest follow-up were used, and point prevalence values were used if available. Studies were categorised as examining the acute versus post-illness psychiatric consequences of infection on the basis of whether they collected information during the patient's illness or the period after the illness. Factors associated with the development of adverse outcomes were extracted and reported if odds ratios were reported or could be robustly calculated.
Data analysis
The meta-analysis was planned for the proportion of psychiatric diagnoses; severity of anxiety, depression, and post-traumatic symptoms; quality of life; and proportion of individuals employed. We used a random-effects model because high heterogeneity was expected. The effect size measures were prevalence with 95% CIs (for number of signs or symptoms, quality of life scores, and employment) and mean difference with 95% CIs (for symptom severity and proportion of diagnoses). We defined point prevalences for number of psychiatric symptoms, proportion of diagnoses (defined by ICD-10, DSM-IV, or Chinese Classification of Mental Disorders [third edition] criteria or by validated psychometric scales with established cutoffs), and proportion of patients in employment as the proportion of cases over the sample size.30 For studies using cutoff scores on symptom rating scales, this percentage represents the presence of clinically significant symptoms reflected by the number of patients scoring above the defined cutoff. We also synthesised prevalences for individuals admitted to an intensive care unit (ICU) and undergoing mechanical ventilation for each coronavirus subtype for comparability. We used mean difference for symptom severity and quality-of-life outcomes, with negative values indexing lower symptom severity and higher quality of life, and positive values indexing higher symptom severity and lower quality of life, in patients with coronavirus infection than among healthy controls. Where continuous data (ie, symptom scores and quality of life) did not have a sufficient number of studies reporting suitable control group data to produce mean differences, we calculated sample size-weighted mean scores for all the studies reporting data alongside 95% CIs in addition to any potential meta-analytical summary effect. We calculated I2 as a measure of between-study heterogeneity. We did not assess funnel plot asymmetry because of an insufficient number of studies.31 Sensitivity analyses were done to assess the contribution of individual studies for the meta-analyses of diagnoses. Data were analysed using R (version 3.3.2) and the meta package (version 4.11) for prevalence data, and RevMan Web (version 5.3) for continuous outcomes. The threshold for significance was set to p values of less than 0·05.
To assess study quality, we adapted the Newcastle Ottawa Scale to enhance its relevance to the specific requirements of this review, such as including laboratory verification, as described in full in the appendix (pp 9–10).32
Role of the funding source
The funders of the individuals working on the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. JPR, EC, and DO had access to the raw data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
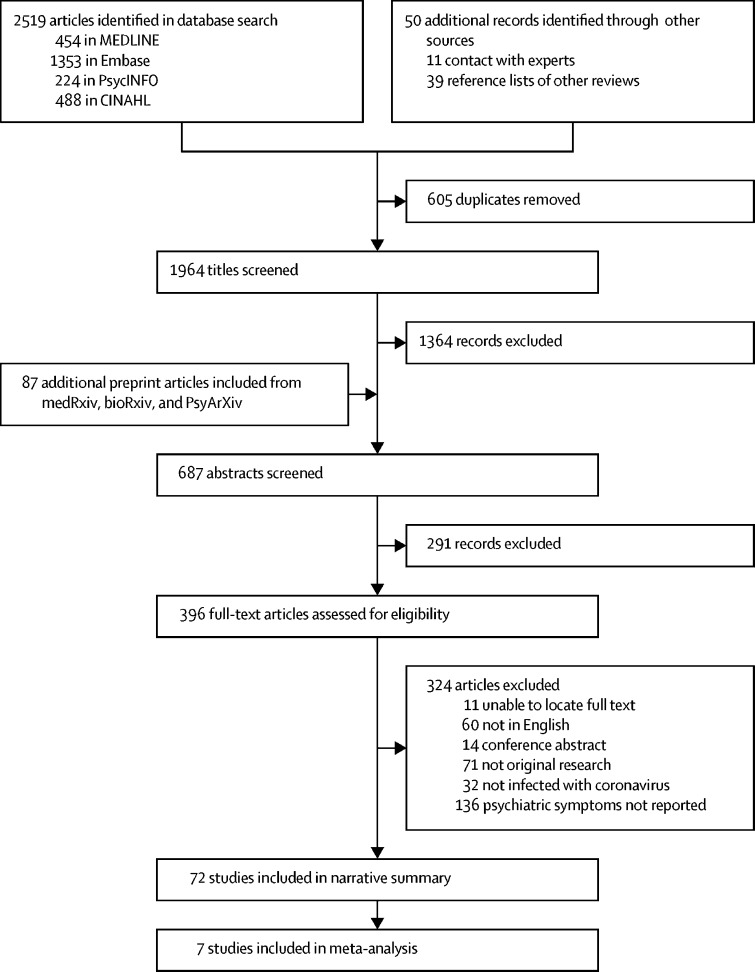
The systematic search identified 1963 studies and 87 preprints, of which 65 independent studies21, 23, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95 and seven medRxiv preprints96, 97, 98, 99, 100, 101, 102 were included in the analyses (figure 1). The number of cases in the included studies ranged from 1 to 997, and the mean age of samples ranged from 12·2 years (SD 4·1) to 68·0 years (single case report). Studies covered China, Canada, France, Hong Kong, Saudi Arabia, South Korea, Japan, Singapore, the UK, and the USA. Several studies had overlapping samples, which made it difficult to estimate the exact number of unique cases identified, although a minimum estimate of total cases was 3559. 47 studies involved SARS-CoV (2068 cases), 13 studies were of MERS-CoV (515 cases), and 12 studies (including seven preprints) described SARS-CoV-2 (976 cases). There were 6390 controls, 2410 of whom were from general population samples used to compare quality-of-life outcomes.
Figure 1.
Study selection
CINAHL=Cumulative Index to Nursing and Allied Health Literature.
25 studies (table 1) investigated the features of acute SARS (1991 cases) and MERS (489 cases). They include six qualitative studies, two case reports, three case series, one cross-sectional study, one randomised controlled trial, and 12 cohort studies. Two studies43, 73 systematically assessed signs and symptoms in a representative cohort using a tailored Neuropsychiatric Symptom Checklist, the combined results of which are shown in table 2. During the acute illness, common symptoms among patients admitted to hospital for SARS or MERS included depressed mood (42 [32·6%; 95% CI 24·7–40·9] of 129 patients), anxiety (46 [35·7%; 27·6–44·2] of 129), impaired memory (44 [34·1%; 26·2–42·5] of 129), impaired concentration or attention (39 [38·2%; 29·0–47·9] of 102; in one study), and insomnia (54 [41·9%; 22·5–50·5] of 129). Notably, confusion was reported by 36 (27·9%; 95% CI 20·5–36·0) of 129 patients despite mean ages in the included studies of 37·6 years (SD 12·4) and 41·2 years (18·6). In one study,33 13 (0·7%) of 1744 patients with SARS in Hong Kong were diagnosed with steroid-induced psychotic disorders. In addition, two studies in which disorders were not systematically assessed reported cases of depression (two cases73), anxiety disorder (two cases73), acute stress reaction (two cases73), psychotic depression (one case33), psychotic disorder not specified (one case33), and deterioration of dementia (one case73). Five qualitative studies investigated the experiences of individuals infected with SARS-CoV and MERS-CoV.34, 35, 36, 37, 38 Loneliness, boredom, and frustration resulting from isolation were prominent.35, 36, 37, 38, 39 Individuals were often concerned about family members who were already infected, spreading the virus to other acquaintances, and death.34, 36, 37, 38 However, two studies noted the enormous gratitude felt by patients for the support they received.37, 38
Table 1.
Studies reporting acute psychiatric and neuropsychiatric outcomes of SARS and MERS infections
| Setting | Virus subtype | Study design | Special population | Sample size | Age, years | Male cases (%) | Female cases (%) | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al (2017)66 | South Korea | MERS-CoV | Case report | .. | 1 case | 68·0 | 1 (100%) | 0 | Symptoms: confusion and drowsiness |
| Schneider et al (2004)67 | USA | SARS-CoV | Case report | Pregnant | 1 case | NR | 0 | 1 (100%) | Symptoms: anxiety |
| Guery et al (2013)68 | France | MERS-CoV | Case series | .. | 2 cases | 64·0, 51·0 | 2 (100%) | 0 | Symptoms: confusion and disorientation |
| Cheng et al (2004)94 | Hong Kong | SARS-CoV | Case series | .. | 10 cases | Mean 34·8 (SD 15·6) | 4 (40%) | 6 (60%) | Diagnoses: adjustment disorder, organic hallucinosis, organic manic disorder, and mental disorder not otherwise specified; symptoms: depressed mood, suicidal ideas, anxiety, visual and auditory hallucinations, suspiciousness, persecutory beliefs, delusions of grandeur, elated mood, increased energy, increased activity, and mood swings |
| Arabi et al (2015)70 | Saudi Arabia | MERS-CoV | Case series | .. | 3 cases | Mean 58·7 (SD 6·9) | 3 (100%) | 0 | Symptoms: confusion |
| Avendano et al (2003)71 | Toronto, ON, Canada | SARS-CoV | Cohort | Health-care workers | 14 cases | Mean 43·9 (SD 10·2) | 3 (21%) | 11 (79%) | Symptoms: anxiety |
| Hong et al (2018)72 | South Korea | MERS-CoV | Cohort | .. | 30 cases | Mean 49·0 (SD 13·0) | 19 (63%) | 11 (37%) | Symptoms: altered mental status |
| Kim et al (2018)73 | South Korea | MERS-CoV | Cohort | .. | 27 cases | Mean 41·2 (SD 18·6) | 10 (37%) | 17 (63%) | Diagnoses: adjustment disorders, depressive disorders, acute stress disorders, delirium, and anxiety disorders; DSM-IV criteria; symptoms: insomnia, depressive mood, tension, disorientation, impaired memory, auditory hallucinations, and aggressive outbursts; scales: PHQ-9, IES-R, PTD-PTNB-PTSS, and KNHANES-short form |
| Alhumaid et al (2018)74 | Saudi Arabia | MERS-CoV | Cohort | .. | 107 cases | Median 54·5 (range 21·0–97·0) | 74 (69%) | 33 (31%) | Symptoms: confusion |
| Noorwali et al (2015)75 | Saudi Arabia | MERS-CoV | Cohort | .. | 261 cases | Median 47·5 (range 8·0–90·0) | 171 (66%) | 90 (34%) | Symptoms: altered consciousness |
| Saad et al (2014)76 | Saudi Arabia | MERS-CoV | Cohort | .. | 70 cases | Median 62·0 (range 1·0–90·0) | 46 (66%) | 24 (34%) | Symptoms: confusion |
| Mackay et al (2005)77 | Toronto, ON, Canada | SARS-CoV | Cohort | .. | 246 cases | NR | 95 (39%) | 151 (61%) | Symptoms: agitation, confusion, and hallucinations |
| Sheng et al (2005)43 | Hong Kong | SARS-CoV | Cohort | .. | 102 cases | Mean 37·6 (SD 12·4) | 35 (34%) | 67 (66%) | Scales: NPSC (reporting a broad range of neuropsychiatric symptoms) and GHQ-28 |
| Leung et al (2004)65 | Hong Kong | SARS-CoV | Cohort | Children | 44 cases | Mean 12·2 (SD 4·1) | 20 (45%) | 24 (55%) | Symptoms: visual hallucinations, auditory hallucinations, impaired attention span, forgetfulness, emotional lability, and depressed mood |
| Lee et al (2004)33 | Hong Kong | SARS-CoV | Cohort | .. | 1744 cases | Mean 32·8 (SD 14·1) | 18 (40%) | 27 (60%) | Diagnoses: steroid-induced manic episode, steroid-induced psychotic disorder, major depressive episode with psychotic features, and psychotic disorder not otherwise specified |
| Lau et al (2004)78 | Hong Kong | SARS-CoV | Cohort | .. | 88 cases | Mean 42·1 (SD 14·0) | 33 (38%) | 55 (63%) | Symptoms: confusion, anxiety, and depression |
| Chua et al (2004)79 | Hong Kong | SARS-CoV | Cohort | .. | 79 cases; 145 controls | 34·0 (estimated) | 27 (34%) | 52 (66%) | Scales: PSS-10 |
| Jeong et al (2016)80 | South Korea | MERS-CoV | Cross-sectional | .. | 36 cases; 1656 controls | Mean 52·3 (SD 15·0) | 18 (50%) | 18 (50%) | Scales: STAXI and GAD-7 |
| Koller et al (2006)35 | Toronto, ON, Canada | SARS-CoV | Qualitative | Children | 5 cases | NR | NR | NR | Qualitative: sadness, worry, and fear |
| Almutairi et al (2018)34 | Saudi Arabia | MERS-CoV | Qualitative | Health-care workers | 7 cases | Mean 47·0 (SD 15·9) | 3 (43%) | 4 (57%) | Qualitative: anxiety, fear, and despair |
| Mok et al (2005)37 | Hong Kong | SARS-CoV | Qualitative | Nurses | 10 cases | Range 20·0–47·0 | 2 (20%) | 8 (80%) | Qualitative: uncertainty, guilt, fear of death, isolation, and loneliness |
| Tiwari et al (2003)38 | Hong Kong | SARS-CoV | Qualitative | .. | 12 cases | NR | NR | NR | Qualitative: fear and frustration |
| Li et al (2004)39 | Hong Kong | SARS-CoV | Qualitative | Children | 4 cases | Range 7·0–13·0 | 2 (50%) | 2 (50%) | Qualitative: social isolation; symptoms: psychological distress |
| Maunder et al (2003)36 | Toronto, ON, Canada | SARS-CoV | Qualitative | .. | 19 cases | NR | NR | NR | Symptoms: insomnia, anxiety, and exacerbation of a panic disorder |
| Loutfy et al (2003)81 | Toronto, ON, Canada | SARS-CoV | Randomised controlled trial treated as a cohort study | .. | 22 cases | Median 48·0 (range 27·0–56·0) | 6 (27%) | 16 (73%) | No depression with interferon alfacon-1 treatment |
Proportions might not sum to 100% as a result of rounding. GAD-7=Generalised Anxiety Disorder-7. GHQ-28=General Health Questionnaire-28. IES-R=Impact of Event Scale Revised. KNHANES=Korea National Health and Nutrition Examination Survey. MERS-CoV=Middle East respiratory syndrome coronavirus. NPSC=Neuropsychiatric Symptom Checklist. NR=not reported. PHQ-9=Patient Health Questionnaire-9. PSS-10=Perceived Stress Scale-10. PTD-PTNB-PTSS=Peri-Traumatic Dissociation–Post-Traumatic Negative Beliefs–Post-Traumatic Social Support scale. SARS-CoV=severe acute respiratory syndrome coronavirus. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. STAXI=State-Trait Anger Expression Inventory.
Table 2.
Prevalence of psychiatric and neuropsychiatric signs and symptoms reported by acute and post-illness studies that used systematic assessments39, 43, 46, 48, 54, 73, 83, 86, 92, 93
|
Acute |
Post-illness |
|||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Cases | Sample size | Prevalence (95% CI) | Studies | Cases | Sample size | Prevalence (95% CI) | |
| Any | 1 | 17 | 27 | 63·0% (43·8–80·4) | 1 | 0 | 4 | 0 (0·0–39·1) |
| Insomnia | 2 | 54 | 129 | 41·9% (22·5–50·5) | 4 | 34 | 280 | 12·1% (8·6–16·3) |
| Anxiety | 2 | 46 | 129 | 35·7% (27·6–44·2) | 2 | 21 | 171 | 12·3% (7·7–17·7) |
| Impaired concentration or attention | 1 | 39 | 102 | 38·2% (29·0–47·9) | 2 | 34 | 171 | 19·9% (14·2–26·2) |
| Impaired memory | 2 | 44 | 129 | 34·1% (26·2–42·5) | 3 | 44 | 233 | 18·9% (14·1–24·2) |
| Depressed mood | 2 | 42 | 129 | 32·6% (24·7–40·9) | 5 | 35 | 332 | 10·5% (7·5–14·1) |
| Confusion | 2 | 36 | 129 | 27·9% (20·5–36·0) | 1 | 1 | 621 | 0·2% (0·0–0·7) |
| Emotional lability | 1 | 30 | 102 | 29·4% (0·4–7·3) | 1 | 24 | 102 | 23·5% (15·8–32·3) |
| Altered consciousness | 1 | 17 | 82 | 20·7% (12·6–30·3) | NA | NA | NA | NA |
| Pressured speech | 1 | 21 | 102 | 20·6% (13·3–29·0) | 1 | 12 | 102 | 11·8% (6·1–18·8) |
| Euphoria | 1 | 8 | 102 | 7·8% (3·3–14·0) | 1 | 11 | 102 | 10·8% (5·4–17·6) |
| Aggression | 1 | 2 | 27 | 7·4% (0·2–21·1) | 1 | 1 | 102 | 1·0% (0·0–4·2) |
| Irritability | 1 | 5 | 102 | 4·9% (1·4–10·1) | 3 | 28 | 218 | 12·8% (8·7–17·6) |
| Auditory hallucinations | 2 | 6 | 129 | 4·7% (1·6–9·1) | 1 | 1 | 102 | 1·0% (0·0–4·2) |
| Persecutory ideas | 1 | 4 | 102 | 3·9% (0·9–8·7) | 1 | 2 | 102 | 2·0% (0·0–5·8) |
| Visual hallucinations | 1 | 2 | 102 | 2·0% (0·0–5·8) | NA | NA | NA | NA |
| Suicidality | 1 | 2 | 102 | 2·0% (0·0–5·8) | 1 | 0 | 102 | 0 (0·0–1·7) |
| Fatigue | NA | NA | NA | NA | 4 | 61 | 316 | 19·3% (15·1–23·9) |
| Frequent recall of traumatic memories | NA | NA | NA | NA | 1 | 55 | 181 | 30·4% (23·9–37·3) |
| Sleep disorder | NA | NA | NA | NA | 1 | 14 | 14 | 100% (88·0–100·0) |
| Psychotic symptoms (unspecified) | NA | NA | NA | NA | 1 | 4 | 90 | 4·4% (1·0–9·9) |
| Self-harm | NA | NA | NA | NA | 1 | 1 | 102 | 1·0% (0·0–4·2) |
NA=not available.
40 studies investigated psychiatric features after the initial infection had resolved (table 3). 35 studies describe 1192 SARS survivors and five studies describe 140 MERS survivors. They include six qualitative studies, one case report, one case series, six cross-sectional studies, and 26 cohort studies. Follow-up duration varied from 60 days to 12 years. In the post-illness stage, depressed mood (35 [10·5%; 95% CI 7·5–14·1] of 332 patients), euphoria (11 [10·8%; 5·4–17·6] of 102; in one study), pressured speech (12 [11·8%; 6·1–18·8] of 102; in one study), insomnia (34 [12·1%; 8·6–16·3] of 280), anxiety (21 [12·3%; 7·7–17·7] of 171), irritability (28 [12·8%; 8·7–17·6] of 218), memory impairment (44 [18·9%; 14·1–24·2] of 233), fatigue (61 [19·3%; 15·1–23·9] of 316), emotional lability (24 [23·5%; 5·8–32·3] of 102; in one study), traumatic memories (55 [30·4%; 23·9–37·3] of 181; in one study), and sleep disorder (14 [100·0%; 88·0–100·0] of 14; in one study) were frequently reported (table 2). Four studies assessed factors associated with psychiatric outcomes following SARS and are summarised in table 4.41, 42, 44, 46 Six qualitative papers discussed the longer-term outcomes for patients with SARS.21, 34, 37, 38, 47, 48 A major theme was the stigma that patients experienced, including from health-care professionals who did not believe their more chronic symptoms, institutions, the general public, or even their own families, friends, and colleagues.21, 34, 37, 47, 48 However, two studies discussed positive psychological outcomes, with patients gaining a better perspective on life and valuing their relationships, health, and everyday existence more.37, 38
Table 3.
Studies reporting post-illness psychiatric and neuropsychiatric outcomes of SARS-CoV and MERS-CoV infections
| Setting | Virus subtype | Study design | Follow-up timepoint | Special population | Sample size | Mean (SD)*age, years | Male cases (%) | Female cases (%) | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|
| Schneider et al (2004)67 | USA | SARS-CoV | Case report | 3 months | Pregnancy | 1 case | NR | 0 | 1 | Symptoms: anxiety |
| Cheng et al (2006)45 | Hong Kong | SARS-CoV | Case series | 2, 3, 4, 5, and 6 months after discharge | .. | 57 cases | 38·1 (10·4) | 19 (33%) | 38 (67%) | Scales: BDI, BAI (SARS Appraisal Inventory), and Thriving Scale |
| Hui et al (2005)82 | Hong Kong | SARS-CoV | Cohort | 3 months, 6 months, and 12 months | .. | 97 cases; 1939 controls | 36·9 (9·5) | 39 (40%) | 58 (60%) | Scales SF-36 (and subscales) |
| Mak et al (2009)40 | Hong Kong | SARS-CoV | Cohort | 18 months | .. | 143 cases | 38·4 (12·4) | 53 (37%) | 90 (63%) | Scale: SF-36 |
| Wing et al (2012)46 | Hong Kong | SARS-CoV | Cohort | Mean 39 months (SD NR) | .. | 181 cases | No psychiatric disorder: 44·9 (15·6); lifetime or current: 44·5 (12·0); current psychiatric condition: 45·6 (12·0) | 57 (31%) | 124 (69%) | Symptoms: fatigue, frequent recall of SARS memories; diagnoses: chronic fatigue syndrome, major depressive disorder, post-traumatic stress disorder, somatoform pain disorder, panic disorder; symptoms: fatigue, and intrusive memories; scales: HADS, IES, GAF, WHOQOL (and subscales), and WSAS (implied) |
| Han et al (2003)83 | Guandong, China | SARS-CoV | Cohort | Mean 59·7 days (SD 22·8) | .. | 69 cases | NR | 29 (42%) | 40 (58%) | Symptoms: insomnia, vexation, low spirit, fear, poor concentration, poor memory, and feelings of guilt |
| Lam et al (2006)55 | Hong Kong | SARS-CoV | Cohort | Mean 60·0 days (SD 23·9) | .. | 116 cases | 45·6 (15·1) | 51 (44%) | 65 (56%) | Scale: SF-36 |
| Guo et al (2019)58 | Guandong, China | SARS-CoV | Cohort | 12 years | .. | 67 cases | Data from original cohort only | Data from original cohort only | − | Scale: SF-36 |
| Lee et al (2019)54 | South Korea | MERS-CoV | Cohort | 12 months and 18 months | .. | 52 cases | 49·7 (12·0) | 32 (62%) | 20 (38%) | Scales: PHQ-9, FSS, and IES-R |
| Mak et al (2010)41 | Hong Kong | SARS-CoV | Cohort | 30 months | .. | 90 cases | No post-traumatic stress disorder: 40·5 (11·6); post-traumatic stress disorder: 42·8 (13·4) | 34 (38%) | 56 (62%) | Diagnoses: post-traumatic stress disorder; scales: FIC, CWCQ, and MHLC |
| Yoon et al (2016)84 | South Korea | MERS-CoV | Cohort | NR | .. | 62 cases | NR | NR | NR | Other: referral for outpatient psychiatric treatment |
| Moldofsky et al (2011)85 | Toronto, ON, Canada | SARS-CoV | Cohort | Mean 19·8 months (range 13–36) | Individuals unable to return to former occupation; mainly health-care workers | 22 cases; 21 fibromyalgia controls, 7 healthy controls | 46·3 (11·0) | 3 (14%) | 19 (86%) | Scales: BDI, PCL-C, SAQ, and WPSI |
| Lam et al (2009)49 | Hong Kong | SARS-CoV | Cohort | Mean 41·3 months (range 31–51) | .. | 233 cases | 43·3 (13·7) | 69 (30%) | 164 (70%) | Diagnoses: any psychiatric illness, post-traumatic stress disorder, depression, somatoform pain disorder, panic disorder, obsessive compulsive disorder, and chronic fatigue syndrome. Scales: HADS, IES-R, and CFQ |
| Hong et al (2009)56 | Beijing, China | SARS-CoV | Cohort | Mean 53 days (SD 31), 7 months, 10 months, 20 months, and 46 months | .. | 70 cases | 38·5 (12·3) | 23 (33%) | 47 (67%) | Diagnoses: post-traumatic stress disorder; scales: IES, SAS, SCL-90, SDS, SDSS, and SF-36 |
| Mak et al (2009)86 | Hong Kong | SARS-CoV | Cohort | 30 months | .. | 90 cases;1394 controls (Hong Kong normative data) | 41·1 (12·1) | 56 (62%) | 34 (38%) | Symptoms: psychotic symptoms; diagnoses: any psychiatric disorder, post-traumatic stress disorder, anxiety disorders (and subtypes), depression (and subtypes), and substance misuse; scale: IES-R, HADS, and SF-36 |
| Bonanno et al (2008)42 | Hong Kong | SARS-CoV | Cohort | 6 months, 12, months, and 18 months | .. | 997 cases, 2410 controls (Hong Kong normative data) | 42·0 (14·0) | 389 (39%) | 608 (61%) | Scale: SF-12 |
| Lee et al (2007)53 | Hong Kong | SARS-CoV | Cohort | 12 months | .. | Two overlapping samples of 79 and 96 cases, and 145 and 112 controls | Stratified across group, year, and age range | 62 (35%) | 113 (65%) | Scales: PSS-10 (reported in 1386 participants), DASS-21, GHQ-12, and IES-R |
| Tansey et al (2007)59 | Toronto, ON, Canada | SARS-CoV | Cohort | 3 months, 6, months, and 12 months | .. | 117 cases | Median 42·0 (range 33·0–51·0) | 39 (33%) | 78 (67%) | Scale: SF-36 |
| Lau et al (2005)87 | Hong Kong | SARS-CoV | Cohort | About 2 months from onset of illness | .. | 15 cases | 35·0 (10·9) | 8 (53%) | 7 (47%) | Diagnoses: anxiety depression, and steroid psychosis; scale: WHOQOL |
| Leow et al (2005)88 | Singapore | SARS-CoV | Cohort | 3 months after recovery | .. | 61 cases | Median 36·5 (range 25·5-47·5) | 14 (23%) | 47 (77%) | Symptoms: fatigue |
| Wu et al (2005)52 | Hong Kong | SARS-CoV | Cohort | 1 month and 3 months after discharge | .. | 131 cases | 41·8 (14·0) | 57 (44%) | 74 (56%) | Scales: IES and HADS |
| Sheng et al (2005)43 | Hong Kong | SARS-CoV | Cohort | Mean 42 days (range 26–86) after discharge | .. | 102 cases | 37·6 (12·4) | 35 (34%) | 67 (66%) | Symptoms: numerous neuropsychiatric symptoms from NPSC; scales: NPSC and GHQ-28 |
| Cheng et al (2004)69 | Hong Kong | SARS-CoV | Cohort | 1 month after recovery | .. | 100 cases; 184 controls | 37·1 (12·1) | 34 (34%) | 66 (66%) | Scales: RSES, GHQ-28, and WHOQOL-BREF |
| Cheng et al (2004)44 | Hong Kong | SARS-CoV | Cohort | At least 4 weeks after discharge; mean 43·8 days (SD 13·6) | .. | 180 cases;649 healthy controls and 189 psychiatric outpatient controls | 36·9 (11·1) | 60 (33%) | 120 (67%) | Scales: BAI, BDI, and SIS |
| Ngai et al (2010)60 | Hong Kong | SARS-CoV | Cohort | 3 months, 6 months, 12 months, 18 months, and 24 months | .. | 55 cases and 538 controls (Hong Kong normative data) | 44·4 (13·2) | 19 (35%) | 36 (65%) | Scale: SF-36 |
| Hui et al (2005)89 | Hong Kong | SARS-CoV | Cohort | 3 months and 6 months | .. | 110 cases | 35·6 (9·8) | 44 (40%) | 66 (60%) | Scale: SF-36 |
| Lau et al (2005)90 | Hong Kong | SARS-CoV | Cohort | 2 weeks after discharge | .. | 171 cases and 2410 controls (Hong Kong normative data) | 37·4 (12·7) | 60 (35%) | 111 (65%) | Scale: SF-36 |
| Li et al (2006)91 | Hong Kong | SARS-CoV | Cohort | 3 months, 6 months, and 12 months | ICU admission with acute respiratory distress syndrome | 59 cases | 47·0 (16·0) | 34 (58%) | 25 (42%) | Scale: SF-36 |
| Lau et al (2005)95 | Hong-Kong | SARS-CoV | Randomised controlled trial treated as a cohort study | At least 8 weeks after discharge | Subnormal exercise tolerance | 133 cases | 37·0 (10·2) | 45 (34%) | 88 (66%) | Scale: SF-36 |
| Tso et al (2004)92 | Hong Kong | SARS-CoV | Cross-sectional | Median 6·6 weeks (SD 1·1) after onset | .. | 62 cases | 37·1 (13·0) | 28 (45%) | 34 (55%) | Symptoms: forgetfulness, depression, and insomnia |
| Lo et al (2005)93 | Singapore | SARS-CoV | Cross-sectional | 6 months | .. | 14 cases; 30 controls | Range 20–48 | 2 (14%) | 12 (86%) | Symptoms: fatigue and sleep disturbance |
| Wu et al (2005)50 | Hong Kong | SARS-CoV | Cross-sectional | 1 month | .. | 195 cases | 41·5 (14·0) | 84 (43%) | 111 (57%) | Scales: IES-R and HADS |
| Kwek et al (2006)51 | Singapore | SARS-CoV | Cross-sectional | 6 weeks and 12 weeks | .. | 63 cases; Singapore normative data as control | 34·8 (10·5) | 13 (21%) | 50 (79%) | Scales: IES, HADS, and SF-36 |
| Batawi et al (2019)57 | Saudi Arabia | MERS-CoV | Cross-sectional | Mean 13·8 months (SD 3·4) | .. | 78 cases; 57 controls (non-MERS-CoV severe acute respiratory infection) | 45·0 (13·0) | 56 (72%) | 22 (28%) | Scale: SF-36 |
| Jeong et al (2016)80 | Seoul, Gyeonggi, Chungcheong, and Gangwon, South Korea | MERS-CoV | Cross-sectional | 4, 5, and 6 months after isolation | .. | 36 cases;1656 controls without MERS who had also been isolated | 52·3 (15·0) | 18 (50%) | 18 (50%) | Scales: STAXI and GAD-7 |
| Almutairi et al (2018)34 | Saudi Arabia | MERS-CoV | Qualitative | NR | Health-care workers | 7 cases | 42·0 (16·2) | 3 (43%) | 4 (57%) | Qualitative: stigma and underestimation of illness severity |
| Siu (2016)47 | Hong Kong | SARS-CoV | Qualitative | NR | Individuals practising tai chi | 35 cases | Range 38–69 | 13 (37%) | 22 (63%) | Qualitative: emotional suffering, stigma, and passivity |
| Siu (2008)21 | Hong Kong | SARS-CoV | Qualitative | NR | .. | 30 cases | NR | NR | NR | Qualitative: stigma |
| Mok et al (2005)37 | Hong Kong | SARS-CoV | Qualitative | NR | Nurses | 10 cases | Range 20–47 | 2 (20%) | 8 (80%) | Themes: anger, guilt, unpreparedness, fear, isolation, physical symptoms, support, and changing perspective |
| Lee et al (2005)48 | Hong Kong | SARS-CoV | Qualitative | NR | .. | 47 cases; 852 controls (neighbouring residents) | Only reported for entire cohort including non-infected | Only reported for entire cohort including non-infected | .. | Symptoms: insomnia, irritability, and low mood |
| Li et al (2004)39 | Hong Kong | SARS-CoV | Qualitative | 5 months after discharge | Children | 4 cases | Range 7–13 | 2 (50%) | 2 (50%) | Symptoms: psychological distress |
Proportions might not sum to 100% as a result of rounding. BAI=Beck Anxiety Inventory. BDI=Beck Depression Inventory. CFQ=Cognitive Failures Questionnaire. CWCQ=Chinese Ways of Coping Questionnaire. DASS-21=Depression, Anxiety and Stress Scale 21 items. FIC=Functional Impairment Checklist. FSS=Fatigue Severity Scale. GAD-7=Generalised Anxiety Disorder-7. GAF=Global Assessment of Functioning. GHQ-12=General Health Questionnaire-12. GHQ-28=General Health Questionnaire-28. HADS=Hospital Anxiety and Depression Scale. ICU=intensive care unit. IES=Impact of Event Scale. IES-R=Impact of Event Scale Revised. MERS-CoV=Middle East respiratory syndrome coronavirus. MHLC=Multidimensional Health Locus of Control. NPSC=Neuropsychiatric Symptom Checklist. NR=not reported. PCL-C=PTSD Checklist, Civilian Version. PHQ-9=Patient Health Questionnaire-9. PSS-10=Perceived Stress Scale 10. RSES=Rosenberg Self-Esteem Scale. SAQ=Sleep Assessment Questionnaire. SARS-CoV=severe acute respiratory syndrome coronavirus. SAS=Zung Self-Rating Anxiety Scale. SCL-90=Symptom Checklist 90. SDS=Zung Self-Rating Depression Scale. SDSS=Social Disability Screening Schedule. SF-12=Short Form 12 Health Survey Questionnaire. SF-36=Short Form 36 Health Survey Questionnaire. SIS=SARS Impact Scale. STAXI=State-Trait Anger Expression Inventory. WHOQOL=WHO Quality of Life. WPSI=Wahler Physical Symptom Inventory. WSAS=Work and Social Adjustment Scale.
Data are mean (SD) unless otherwise stated.
Table 4.
Factors associated with psychiatric and neuropsychiatric outcomes in SARS
| Outcome | Result | |
|---|---|---|
| Demographic | ||
| Female sex | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 3·85 (95% CI 1·18–12·54)41 |
| Female sex | Chronic illness compared with resilience (based on SF-12) | OR 2·17 (p<0·01)*42 |
| Female sex | Moderate or severe range score on the BAI or BDI | OR 1·8 (95% CI 0·9–3·6)44 |
| Female sex | Current psychiatric disorder (DSM-IV) | OR 2·0 (95% CI 1·03–3·89)46 |
| Age | Chronic illness compared to resilience (based on SF-12) | OR 1·01 (not significant)*42 |
| Health-care worker | Moderate or severe range score on the BAI or BDI | OR 3·8 (95% CI 1·8–8·2)44 |
| Health-care worker | Current psychiatric disorder (DSM-IV) | OR 2·59 (95% CI 1·38–4·87)46 |
| Health-care worker | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 2·92 (95% CI 1·08–7·88)41 |
| Married | Current psychiatric disorder (DSM-IV) | OR 1·14 (0·60–2·18)46 |
| Baseline illness | ||
| Previous chronic physical illness | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 4·38 (95% CI 1·06–18·02)41 |
| Previous chronic physical illness | Moderate or severe range score on the BAI or BDI | OR 0·8 (95% CI 0·3–2·4)44 |
| Disease-related | ||
| Presence of avascular necrosis | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 2·91 (95% CI 1·06–8·02)41 |
| Functional Impairment Checklist, disability score | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 2·44 (95% CI 1·66–3·56)41 |
| Average pain | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 1·69 (95% CI 1·31–2·19)41 |
| Distressing pain after SARS | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 36·01 (95% CI 2·10–617·59)41 |
| Psychological | ||
| SARS-related worry | Chronic illness compared to resilience (based on SF-12) | OR 1·04 (p<0·05)*42 |
| Chance locus of control (Multidimensional Health Locus of Control scale) | Post-traumatic stress disorder diagnosis (DSM-IV) | OR 1·22 (95% CI 1·09–1·37)41 |
| Frequent recall of SARS memories | Current psychiatric disorder (DSM-IV) | OR 13·5 (95% CI 6·2–29·4)46 |
| Social | ||
| Social network size | Chronic illness compared to resilience (based on SF-12) | OR 0·99 (not significant)*42 |
| Death of relative due to SARS | Moderate or severe range score on the BAI or BDI | OR 3·4 (95% CI 1·0–12·2)44 |
| Medicolegal involvement | Current psychiatric disorder (DSM-IV) | OR 7·69 (95% CI 2·15–27·6)46 |
Unadjusted ORs were reported, except for the outcomes marked. BAI=Beck Anxiety Inventory. BDI=Beck Depression Inventory. OR=odds ratio. SARS=severe acute respiratory syndrome. SF-12=Short Form 12 Health Survey Questionnaire.
Only adjusted ORs were available; 95% CIs were not available.
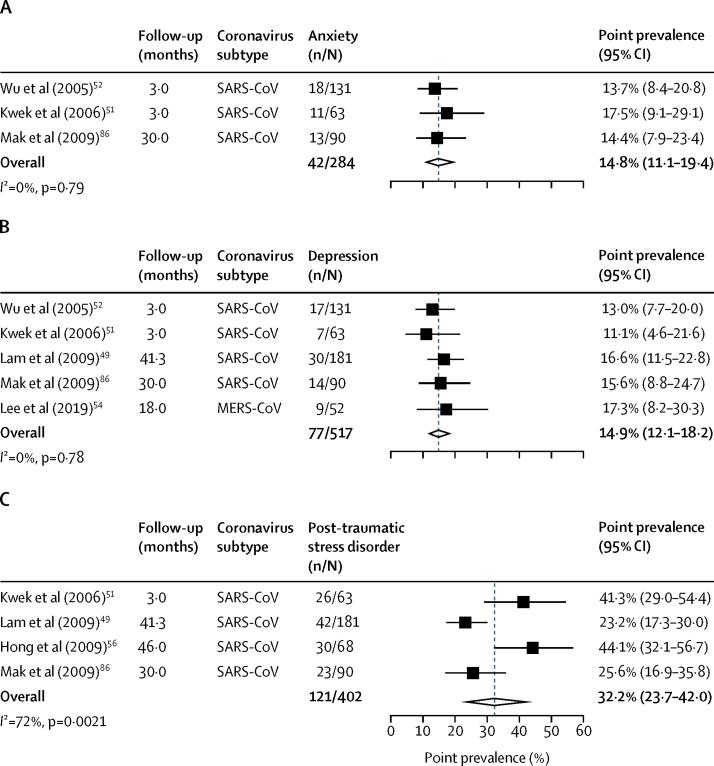
In the post-illness phase, the point prevalence of anxiety disorder diagnoses was 14·8% (95% CI 11·1–19·4; 42 of 284 cases from three studies; figure 2A) at a mean follow-up of 11·6 months (SD 12·6). The point prevalence of depression was 14·9% (95% CI 12·1–18·2; 77 of 517 cases from five studies; figure 2B) at a mean follow-up of 22·6 months (SD 16·7). The point prevalence of post-traumatic stress disorder was 32·2% (95% CI 23·7–42·0; 121 of 402 cases from four studies; figure 2C) at mean follow-up of 33·6 months (SD 14·2). Point prevalences were used in all studies except in one study,49 in which it was not clear whether the value was in fact an estimate of period prevalence.
Figure 2.
Forest plots of pooled prevalence of anxiety (A), depression (B), and post-traumatic stress disorder (C) in individuals who recovered from coronavirus infection
MERS-CoV=Middle East respiratory syndrome coronavirus. SARS-CoV=severe acute respiratory syndrome coronavirus.
For symptom severity scores, standardised mean differences could not be generated because control groups were not used in included studies. Hence, studies using different symptom scales to assess the same symptoms (eg, Hospital Anxiety and Depression Scale [HADS] and Beck Depression Inventory [BDI]) could not be combined. The weighted mean symptom score for the HADS anxiety subscale, with a clinical cutoff of 8, was 6·5 (95% CI 3·9–9·1; assessed in 364 cases from three studies; appendix p 12).49, 50, 51, 52 The weighted mean symptom score for the HADS depression subscale, with a clinical cutoff of 8, was 6·2 (95% CI 3·7–8·6; 364 cases from three studies).49, 50, 51 The weighted mean symptom score was 10·8 (95% CI 6·9–14·7; 397 cases from three studies) for the Impact of Event Scale Revised (IES-R) Intrusion subscale,49, 50, 51, 52, 53 8·8 (5·0–12·5; 397 cases from three studies) for the IES-R Avoidance subscale,49, 50, 51, 53 8·1 (5·1–11·1; 397 cases from three studies) for the IES-R Hyperarousal subscale,49, 50, 52, 53 and 20·7 (7·8–33·5; 115 cases across two studies) for IES-R Total (clinical cutoff of 24)51, 54 at a mean follow-up time of 9·8 months (SD 10·6).
Health-related quality of life was lower in patients after SARS-CoV infection across the three mental health-related subscales of the Short Form 36 Health Survey Questionnaire (SF-36; range 0–100 points) than among the general population sample obtained using a telephone survey with an unknown response rate.103, 104 The pooled mean difference was −26·4 points (95% CI −37·0 to −15·7, p<0·0001; 187 cases from two studies) for social functioning, −15·4 (−31·2 to 0·5, p=0·057; 187 cases from two studies) for role limitation due to emotional problems, and −10·6 (−13·9 to −7·4, p<0·0001; 187 cases from two studies) for the mental health subscale at a mean follow-up time of 20·7 months (SD 9·0; appendix p 11). When combined with data from studies of SARS and MERS that did not have data from a control group,40, 51, 55, 56, 57 the weighted mean SF-36 scores were 68·1 (95% CI 60·1–76·0; assessed in 581 cases from 11 studies) for social functioning, 44·1 (43·0–45·2) for role limitation due to emotional problems, and 52·0 (51·2–52·8) for the mental health subscale (appendix p 13). With regard to employment, 446 (76·9%; 95% CI 68·1–84·6) of 580 patients from six studies had returned to work at a mean follow-up time of 35·3 months (SD 40·1; appendix p 15).46, 49, 58, 59, 60 The proportion of patients who were admitted to an ICU or ventilated are presented in the appendix (p 16). Results of heterogeneity and sensitivity analyses can be seen in the appendix (p 17).
12 studies (including seven preprints) described the features of 976 patients with acute SARS-CoV-2 infection (table 5). Seven studies (including four preprints) were from Wuhan and reported data from at least 575 unique cases. Another three preprints described 343 cases from Chongqing and Zhejiang in China, and Hong Kong. Two preprints used rating scales to systematically assess depressive and anxiety symptoms.96, 97 In one study,96 50 (35%) of 144 patients had symptoms of anxiety and 41 (28%) had symptoms of depression, although these assessments were not diagnostic. In the other study,97 26 patients with SARS-CoV-2 infection were compared with patients with other forms of pneumonia and age-matched and sex-matched healthy controls; scores on both the Hamilton Depression Scale and the Hamilton Anxiety Scale were higher for the SARS-CoV-2 group than for either of the other groups, but these scores improved significantly after the first week of their hospital stay.
Table 5.
Studies reporting acute psychiatric and neuropsychiatric outcomes of SARS-CoV-2 infections
| Preprint | Setting | Virus subtype | Study design | Special population | Sample size | Mean (SD)*age, years | Male cases (%) | Females cases (%) | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|
| Moriguchi et al (2020)64 | No | Japan | SARS-CoV-2 | Case report | .. | 1 case | 24 (NR) | 1 (100%) | 0 | Symptom: impaired consciousness; diagnosis: meningitis-encephalitis |
| Helms et al (2020)61 | No | France | SARS-CoV-2 | Case series | ICU admissions | 58 cases | NR | NR | NR | Symptoms: agitation, confusion, inattention, disorientation, and poorly organised movements in response to command; diagnoses: dysexcutive syndrome and encephalopathy; investigations: MRI brain, EEG, and CSF analysis |
| Chen et al (2020)63 | No | Wuhan, China | SARS-CoV-2 | Cohort | .. | 99 cases | 55·5 (13·1) | 67 (68%) | 32 (32%) | Symptom: confusion |
| Chen et al (2020)62 | No | Wuhan, China | SARS-CoV-2 | Cohort | .. | 21 cases | Median 56·0 (IQR 50·0–65·0) | 17 (81%) | 4 (19%) | Symptom: coma; diagnosis: hypoxic encephalopathy |
| Zhang et al (2020)98 | Yes | Wuhan, China | SARS-CoV-2 | Cohort | Deaths | 82 cases | Median 72·5 (IQR 65·0–80·0) | 54 (66%) | 28 (34%) | Symptom: consciousness problem |
| Qi et al (2020)100 | Yes | Chongqing, China | SARS-CoV-2 | Cohort | .. | 267 cases | Median 48·0 (IQR 35·0–65·0) | 149 (56%) | 118 (44%) | Symptom: confusion |
| Huang et al (2020)99 | Yes | Wuhan, China | SARS-CoV-2 | Cohort | Deaths | 36 cases | 69·2 (9·6) | 25 (69%) | 11 (31%) | Symptom: disturbance of consciousness |
| Mao et al (2020)23 | No | Wuhan, China | SARS-CoV-2 | Cohort | .. | 214 cases | 52·7 (15·5) | 87 (40%) | 127 (60%) | Symptom: impaired consciousness |
| Leung et al (2020)101 | Yes | Hong Kong | SARS-CoV-2 | Cohort | .. | 50 cases | 55·2 (19·5) | 23 (46%) | 27 (54%) | Symptom: confusion |
| Fu et al (2020)102 | Yes | Wuhan, China | SARS-CoV-2 | Cohort | .. | 50 cases | Median 64·0 (IQR 37·0–87·0) | 27 (54%) | 23 (46%) | Symptom: insomnia |
| Yang et al (2020)97 | Yes | Zhejiang, China | SARS-CoV-2 | Cohort | .. | 26 cases, 87 pneumonia controls, 30 healthy controls | Mean 56·0 (range 27·0–86·0) | 9 (35%) | 17 (65%) | Scales: HAMD and HAMA |
| Kong et al (2020)96 | Yes | Wuhan, China | SARS-CoV-2 | Cross-sectional | .. | 144 cases | 50·0 (13·7) | 70 (49%) | 74 (51%) | Scales: HADS and PSSS |
CSF=cerebrospinal fluid. EEG=electroencephalogram. HADS=Hospital Anxiety and Depression Scale. HAMA=Hamilton Anxiety Scale. HAMD=Hamilton Depression Scale. ICU=intensive care unit. NR=not reported. PSSS=Perceived Social Support Scale. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Data are mean (SD) unless otherwise stated.
A recently published study61 of 58 patients with COVID-19 who had been admitted to two ICUs in France described agitation in 40 (69%) patients after withdrawal of sedation and neuromuscular blockade. It also reported confusion in 26 (65%) of 40 patients who were assessed using the Confusion Assessment Method for the ICU. Some patients had neuropsychiatric investigations including brain MRI (13 [22%] of 58 patients), electroencephalogram (EEG; eight [14%] patients), and lumbar puncture (seven [12%] patients). MRI demonstrated larger leptomeningeal spaces in eight (62%) of 13 patients as well as two recent asymptomatic ischaemic strokes. EEG changes were non-specific, with diffuse bifrontal slowing consistent with encephalopathy described in one of eight patients. Of seven patients who had lumbar puncture, cerebrospinal fluid analysis identified oligoclonal bands in two patients and elevated protein and IgG in another. At discharge, 15 (33%) of 45 patients who were assessed had a dysexecutive syndrome with symptoms such as inattention, disorientation, or poorly organised movements in response to command.61
The only other systematic assessment of neuropsychiatric presentations was from a preprint98 that found altered consciousness to be present in 17 (21%) of 82 patients with COVID-19 who subsequently died. Overall, altered consciousness or encephalopathy was reported in five studies.23, 62, 98, 99 Four other studies (two preprints) reported cases of confusion or disturbance of consciousness, although not systematically, with prevalence ranging between 2·0% (95% CI 0·4–10·5) and 22·2% (11·7–38·1).63, 99, 100, 101 In terms of neuropsychiatric features of specific neurological consequences of SARS-CoV-2 infection, there was one report of meningitis-encephalitis and two cases where hypoxic encephalopathy was specified in peer-reviewed studies.62, 64
Overall, for the 65 peer-reviewed studies, 32 were deemed to be of low quality, 30 were deemed to be of moderate quality, and three were deemed to be of high quality. Two preprints were of low quality, four moderate quality, and one was high quality. Across studies, the main weaknesses were due to limited assessment of pre-infection psychiatric symptoms and the lack of adequate comparison groups. Results of the study quality assessment are described in the appendix (pp 18–20).
Discussion
To our knowledge, this is the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection. We identified 72 independent studies that provided data on both the acute and post-illness psychiatric and neuropsychiatric features of coronavirus infection, including seven medRxiv preprints. The scientific literature predominantly consists of data on patients with SARS and MERS treated in hospital, so there should be caution in generalising any findings to COVID-19, particularly for patients who have mild symptoms. Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19.
In SARS and MERS in the acute stage, using data from two studies, the most important finding was that confusion occurred in 27·9% of patients, suggesting that delirium was common. Other common psychiatric findings were depression, anxiety, and insomnia. Diagnoses of mania and psychosis did occur in a small minority (0·7%), but in a small sample this diagnosis appeared to be almost entirely related to use of exogenous corticosteroids, which are rarely prescribed to treat SARS-CoV-2 infection. Notably, insomnia, emotional lability, irritability, pressured speech, and euphoria were relatively common, suggesting that although a full syndrome of mania was uncommon, subthreshold symptoms might be present.
In SARS and MERS, after recovery from the infection, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients at a follow-up period ranging between 6 weeks and 39 months. Emotional lability, pressured speech, and euphoria were only reported by patients and relatives after a short follow-up (mean 42 days [range 26–86]) in one study43 in which corticosteroids had frequently been prescribed at high doses and symptoms; therefore, it might be of limited relevance to the COVID-19 pandemic. The point prevalences of anxiety disorders, depression, and post-traumatic stress disorder were high, although the lack of adequate comparison groups or assessment of previous psychiatric disorder means that it is hard to separate the effects of the infection from the impact of an epidemic on the population as a whole or the possibility that selection bias led to the high prevalence figures. In terms of severity, mean scores for depression and anxiety on standard scales were below clinical cutoffs. Measures of health-related quality of life were considerably lower in patients with SARS than in control groups. However, the impairment in social functioning was greater than the effects on mental health (appendix p 11), suggesting that the effect of coronaviruses is broad and not specific to mental health. Some positive effects in terms of personal growth during adversity were noted.
In terms of applicability to COVID-19, conclusions must be cautious because data on the acute effects of the illness are limited and no data exist on the post-illness phase, and the higher mortality of SARS and MERS might be correlated with poorer psychiatric outcomes.11, 105 The information available suggests that in the acute stage—as in SARS and MERS—confusion is a common feature, so delirium is probably a significant clinical problem. In the longer term, the data from SARS and MERS suggest that the prevalence of depression, anxiety, post-traumatic stress disorder, and fatigue might be high, but as yet data on these diagnoses in patients with COVID-19 are preliminary or unpublished. In patients with severe illness requiring ICU admission, neurocognitive impairment might be a feature. We found only three cases of SARS-CoV-2-related psychiatric symptoms that were explicitly linked to hypoxic or encephalitic brain injury; this finding is consistent with the rarity of case reports that have associated detection of coronaviruses in the CNS with acute encephalitis or encephalomyelitis (mainly in immunocompromised or immunodeficient children).106, 107, 108
The aetiology of the psychiatric consequences of infection with coronavirus is likely to be multifactorial and might include the direct effects of viral infection (including brain infection), cerebrovascular disease (including in the context of a procoagulant state), the degree of physiological compromise (eg, hypoxia), the immunological response, medical interventions, social isolation, the psychological impact of a novel severe and potentially fatal illness, concerns about infecting others, and stigma. The immune response in SARS-CoV-2 infection is of interest and there might be a hyperinflammatory state similar to that seen in haemophagocytic lymphohisticytosis in which there are increased concentrations of C-reactive protein, ferritin, and interleukin-6, although this state is likely to be short lived.109 The link between inflammation and depression is well described and might explain some of the psychiatric morbidity.110
Survivors of critical illness are at risk of persistent psychiatric impairment after discharge from hospital. At 1 year, the pooled prevalences of clinically relevant depressive, anxiety, and post-traumatic symptoms were 29% (23–34),4 34% (25–42),5 and 34% (22–50),6 respectively. The majority of patients with severe acute respiratory distress syndrome, a key feature of severe COVID-19 illness, show impairments of memory, attention, concentration, or mental processing speed at 1 year.111 None of the studies included in this review completed systematic neuropsychological assessments apart from one report of severe SARS-CoV-2 cases, which described a dysexecutive syndrome in a third of survivors.61 Acute respiratory distress syndrome and prolonged mechanical ventilation are also associated with greater reductions in quality of life than ICU admissions for other reasons.112
Limitations include the use of preprint articles that have not been subject to peer review, exclusion of non-English-language articles, and the inclusion of studies with very small samples. A further limitation was that most studies were of low or moderate quality. Almost all of the studies we included in this review reported outcomes from patients admitted to hospital, which improves the comparability between coronavirus infections. However, although the frequencies of ICU admission and ventilation were similar for patients admitted to hospital with SARS-CoV infection (13% ICU admission and 7% ventilation) and SARS-CoV-2 (18% and 6%), they were considerably higher in patients with MERS (60% and 51%). Systematic assessment of psychiatric symptoms was rare, use of self-report questionnaires was common, and there was variation in the definition of illness and laboratory verification of infection between studies. The lack of baseline psychiatric assessments means that accurate estimates of incidence are impossible; therefore, we relied on point prevalence where possible. Few studies included objective biological measures, such as peripheral blood markers of genetic, inflammatory, immune, and metabolic function, cerebrospinal fluid measures, EEG, or brain imaging. Furthermore, few studies included comparison groups. The apparently high prevalence of common symptoms reported (such as depression, anxiety, and fatigue) could have been unrelated to the coronavirus infection and rather a consequence of selection bias. For the post-illness studies, there was substantial variation in follow-up time that hindered comparability. These factors might have contributed to heterogeneity, but there were too few studies to explore explanations of this variance.
Future studies should systematically assess the prevalence of psychiatric symptoms in patients with coronavirus infections, and we suggest that a prospective cohort of patients with SARS-CoV-2 should be established. Ideally, there should be measures of mental health before infection, as well as other possible confounding factors, potentially using existing cohorts. A comparison group of other patients undergoing acute medical admissions would be helpful. There would need to be standardised measures of psychiatric disorder.
It will be important to establish whether markers of severity of infection correlate with psychiatric presentations. Case-control studies of immunoreactivity to the SARS-CoV-2 virus in psychiatric populations using serological measures, once available, will give an indication of whether infection is a risk factor for psychiatric disorders.
Given that a very large number of individuals will be infected with SARS-CoV-2, the immediate impact on mental health could be considerable. An acute rise in cases of delirium will probably prolong hospital stay; there is also some preliminary evidence that delirium was associated with raised mortality in MERS.113 There is a risk of common mental illnesses in patients with disease that require hospital admission, which might be compounded by the effects of social isolation.16 Given this psychiatric morbidity and high frequency of persistent fatigue, some patients might have difficulty in returning to their previous employment, at least in the short term, although physical—as well as mental—recovery is intrinsic to such a broad functional outcome.
In conclusion, although there are many ways in which mental health might be adversely affected by a pandemic, this review suggests, first, that most people do not suffer from a psychiatric disorder following coronavirus infection, and second, that so far there is little to suggest that common neuropsychiatric complications beyond short-term delirium are a feature. Clinicians must be aware of the possibility of depression, anxiety, fatigue, post-traumatic stress disorder, and rarer neuropsychiatric syndromes in the aftermath. The quality of studies to date has been variable, and ongoing surveillance is essential.
Acknowledgments
Acknowledgments
JPR is supported by a Wellcome Trust Clinical Training Fellowship (102186/B/13/Z). EC is supported by a UK National Institute for Health Research (NIHR) Doctoral Research Fellowship (NIHR300273). DO is supported by the UK Medical Research Council (MRC; MR/N013700/1) and King's College London member of the MRC Doctoral Training Partnership in Biomedical Sciences. TAP is supported by an NIHR Clinical Lectureship. MSZ, GL, and ASD are supported by the UK NIHR University College London Hospitals Biomedical Research Centre.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
JPR and EC screened the text. EC and DO extracted and analysed the data. JPR wrote the first draft of the manuscript with input from EC and DO. TAP, PM, PF-P, MSZ, GL, and ASD contributed to the design of the study and the final manuscript.
Declaration of interests
MSZ reports receiving personal fees from UCB Pharma for lecturing, outside the submitted work. PF-P reports personal fees from Lundbeck, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Arciniegas DB, Anderson CA. Viral encephalitis: neuropsychiatric and neurobehavioral aspects. Curr Psychiatry Rep. 2004;6:372–379. doi: 10.1007/s11920-004-0024-x. [DOI] [PubMed] [Google Scholar]
- 2.Dubé B, Benton T, Cruess DG, Evans DL. Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci. 2005;30:237–246. [PMC free article] [PubMed] [Google Scholar]
- 3.Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54(suppl 1):S44–S52. doi: 10.1016/s0895-4356(01)00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabiee A, Nikayin S, Hashem MD. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44:1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikayin S, Rabiee A, Hashem MD. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43:1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 7.Lu R, Zhao X, Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desforges M, Le Coupanec A, Dubeau P. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO 2019 novel coronavirus (2019nCoV): strategic preparedness and response plan. Feb 3, 2020. https://www.who.int/docs/default-source/coronaviruse/srp-04022020.pdf?sfvrsn=7ff55ec0_4&download=true
- 11.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . World Health Organization; Geneva: 2018. The ICD-11 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. [Google Scholar]
- 13.WHO Mental health and psychosocial considerations during the COVID-19 outbreak. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/mental-health-considerations.pdf
- 14.The Lancet Psychiatry Send in the therapists? Lancet Psychiatry. 2020;7:291. doi: 10.1016/S2215-0366(20)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewnard JA, Lo NC. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30190-0. published online March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks SK, Webster RK, Smith LE. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asmundson GJG, Taylor S. Coronaphobia: fear and the 2019-nCoV outbreak. J Anxiety Disord. 2020;70 doi: 10.1016/j.janxdis.2020.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg N, Docherty M, Gnanapragasam S, Wessely S. Managing mental health challenges faced by healthcare workers during covid-19 pandemic. BMJ. 2020;368 doi: 10.1136/bmj.m1211. [DOI] [PubMed] [Google Scholar]
- 19.Chaves C, Castellanos T, Abrams M, Vazquez C. The impact of economic recessions on depression and individual and social well-being: the case of Spain (2006–2013) Soc Psychiatry Psychiatr Epidemiol. 2018;53:977–986. doi: 10.1007/s00127-018-1558-2. [DOI] [PubMed] [Google Scholar]
- 20.Xiang Y-T, Yang Y, Li W. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu JY. The SARS-associated stigma of SARS victims in the post-SARS era of Hong Kong. Qual Health Res. 2008;18:729–738. doi: 10.1177/1049732308318372. [DOI] [PubMed] [Google Scholar]
- 22.Jones C, Humphris GM, Griffiths RD. Psychological morbidity following critical illness—the rationale for care after intensive care. Clin Intensive Care. 1998;9:199–205. [Google Scholar]
- 23.Mao L, Jin H, Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Bai W, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. published online Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu H, Chan JF-W, Yuen TT-T. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30004-5. published online April 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pleasure SJ, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1065. published online April 10. [DOI] [PubMed] [Google Scholar]
- 28.Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020 doi: 10.1016/J.BBI.2020.04.027. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kępińska AP, Iyegbe CO, Vernon AC, Yolken R, Murray RM, Pollak TA. Schizophrenia and influenza at the centenary of the 1918–1919 Spanish influenza pandemic: mechanisms of psychosis risk. Front Psychiatry. 2020;11:72. doi: 10.3389/fpsyt.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 31.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid-Based Healthc. 2018;16:195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 32.Wells G, Shea B, O'Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 33.Lee DTS, Wing YK, Leung HCM. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case-control study. Clin Infect Dis. 2004;39:1247–1249. doi: 10.1086/424016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almutairi AF, Adlan AA, Balkhy HH, Abbas OA, Clark AM. “It feels like I'm the dirtiest person in the world.”: exploring the experiences of healthcare providers who survived MERS-CoV in Saudi Arabia. J Infect Public Health. 2018;11:187–191. doi: 10.1016/j.jiph.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koller DF, Nicholas DB, Goldie RS, Gearing R, Selkirk EK. When family-centered care is challenged by infectious disease: pediatric health care delivery during the SARS outbreaks. Qual Health Res. 2006;16:47–60. doi: 10.1177/1049732305284010. [DOI] [PubMed] [Google Scholar]
- 36.Maunder R, Hunter J, Vincent L. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003;168:1245–1251. [PMC free article] [PubMed] [Google Scholar]
- 37.Mok E, Chung BP, Chung JW, Wong TK. An exploratory study of nurses suffering from severe acute respiratory syndrome (SARS) Int J Nurs Pract. 2005;11:150–160. doi: 10.1111/j.1440-172X.2005.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiwari A, Chan S, Wong A. Severe acute respiratory syndrome (SARS) in Hong Kong: patients' experiences. Nurs Outlook. 2003;51:212–219. doi: 10.1016/j.outlook.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li AM, Chan CHY, Chan DFY. Long-term sequelae of SARS in children. Paediatr Respir Rev. 2004;5:296–299. doi: 10.1016/j.prrv.2004.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mak WWS, Law RW, Woo J, Cheung FM, Lee D. Social support and psychological adjustment to SARS: the mediating role of self-care self-efficacy. Psychol Health. 2009;24:161–174. doi: 10.1080/08870440701447649. [DOI] [PubMed] [Google Scholar]
- 41.Mak IWC, Chu CM, Pan PC, Yiu MGC, Ho SC, Chan VL. Risk factors for chronic post-traumatic stress disorder (PTSD) in SARS survivors. Gen Hosp Psychiatry. 2010;32:590–598. doi: 10.1016/j.genhosppsych.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonanno GA, Ho SMY, Chan JCK. Psychological resilience and dysfunction among hospitalized survivors of the SARS epidemic in Hong Kong: a latent class approach. Health Psychol. 2008;27:659–667. doi: 10.1037/0278-6133.27.5.659. [DOI] [PubMed] [Google Scholar]
- 43.Sheng B, Cheng SKW, Lau KK, Li HL, Chan ELY. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur Psychiatry. 2005;20:236–242. doi: 10.1016/j.eurpsy.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng SK, Wong CW, Tsang J, Wong KC. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS) Psychol Med. 2004;34:1187–1195. doi: 10.1017/s0033291704002272. [DOI] [PubMed] [Google Scholar]
- 45.Cheng SKW, Chong GHC, Chang SSY. Adjustment to severe acute respiratory syndrome (SARS): roles of appraisal and post-traumatic growth. Psychol Health. 2006;21:301–317. [Google Scholar]
- 46.Wing YK, Leung CM. Mental health impact of severe acute respiratory syndrome: a prospective study. Hong Kong Med J. 2012;18(suppl 3):24–27. [PubMed] [Google Scholar]
- 47.Siu JY. Coping with future epidemics: Tai chi practice as an overcoming strategy used by survivors of severe acute respiratory syndrome (SARS) in post-SARS Hong Kong. Health Expect. 2016;19:762–772. doi: 10.1111/hex.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S, Chan LYY, Chau AMY, Kwok KPS, Kleinman A. The experience of SARS-related stigma at Amoy Gardens. Soc Sci Med. 2005;61:2038–2046. doi: 10.1016/j.socscimed.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam MH-B, Wing Y-K, Yu MW-M. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 50.Wu KK, Chan SK, Ma TM. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J Trauma Stress. 2005;18:39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwek S-K, Chew W-M, Ong K-C. Quality of life and psychological status in survivors of severe acute respiratory syndrome at 3 months postdischarge. J Psychosom Res. 2006;60:513–519. doi: 10.1016/j.jpsychores.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu KK, Chan SK, Ma TM. Posttraumatic stress after SARS. Emerg Infect Dis. 2005;11:1297–1300. doi: 10.3201/eid1108.041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee AM, Wong JG, McAlonan GM. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry. 2007;52:233–240. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- 54.Lee SH, Shin H-S, Park HY. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam SP, Tsui E, Chan KS, Lam CL, So HP. The validity and reliability of the functional impairment checklist (FIC) in the evaluation of functional consequences of severe acute respiratory distress syndrome (SARS) Qual Life Res. 2006;15:217–231. doi: 10.1007/s11136-005-1463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong X, Currier GW, Zhao X, Jiang Y, Zhou W, Wei J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: a 4-year follow-up study. Gen Hosp Psychiatry. 2009;31:546–554. doi: 10.1016/j.genhosppsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batawi S, Tarazan N, Al-Raddadi R. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS) Health Qual Life Outcomes. 2019;17:101. doi: 10.1186/s12955-019-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo L, Han Y, Li J. Long-term outcomes in patients with severe acute respiratory syndrome treated with oseltamivir: a 12-year longitudinal study. Int J Clin Exp Med. 2019;12:12464–12471. [Google Scholar]
- 59.Tansey CM, Louie M, Loeb M. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 60.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helms J, Kremer S, Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G, Wu D, Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 doi: 10.1172/JCI137244. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moriguchi T, Harii N, Goto J. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung CW, Kwan YW, Ko PW. Severe acute respiratory syndrome among children. Pediatrics. 2004;113:e535–e543. doi: 10.1542/peds.113.6.e535. [DOI] [PubMed] [Google Scholar]
- 66.Lee JY, Kim Y-J, Chung EH. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect Dis. 2017;17:498. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider E, Duncan D, Reiken M. SARS in pregnancy. AWHONN Lifelines. 2004;8:122–128. doi: 10.1177/1091592304265557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guery B, Poissy J, el Mansouf L. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng SKW, Sheng B, Lau KK. Adjustment outcomes in Chinese patients following one-month recovery from severe acute respiratory syndrome in Hong Kong. J Nerv Ment Dis. 2004;192:868–871. doi: 10.1097/01.nmd.0000147169.03998.dc. [DOI] [PubMed] [Google Scholar]
- 70.Arabi YM, Harthi A, Hussein J. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avendano M, Derkach P, Swan S. Clinical course and management of SARS in health care workers in Toronto: a case series. CMAJ. 2003;168:1649–1660. [PMC free article] [PubMed] [Google Scholar]
- 72.Hong K-H, Choi J-P, Hong S-H. Predictors of mortality in Middle East respiratory syndrome (MERS) Thorax. 2018;73:286–289. doi: 10.1136/thoraxjnl-2016-209313. [DOI] [PubMed] [Google Scholar]
- 73.Kim H-C, Yoo S-Y, Lee B-H, Lee SH, Shin H-S. Psychiatric findings in suspected and confirmed Middle East respiratory syndrome patients quarantined in hospital: a retrospective chart analysis. Psychiatry Investig. 2018;15:355–360. doi: 10.30773/pi.2017.10.25.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alhumaid S, Tobaiqy M, Albagshi M. MERS-CoV transmitted from animal-to-human vs MERSCoV transmitted from human-to-human: comparison of virulence and therapeutic outcomes in a Saudi hospital. Trop J Pharm Res. 2018;17 [Google Scholar]
- 75.Noorwali AA, Turkistani AM, Asiri SI. Descriptive epidemiology and characteristics of confirmed cases of Middle East respiratory syndrome coronavirus infection in the Makkah Region of Saudi Arabia, March to June 2014. Ann Saudi Med. 2015;35:203–209. doi: 10.5144/0256-4947.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saad M, Omrani AS, Baig K. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackay IF, Garrah JM, Tabah BM, Freeman L, Maher MM, Macdonald CL. Adverse drug reactions associated with the use of ribavirin in the treatment of severe acute respiratory syndrome (SARS) J Popul Ther Clin Pharmacol. 2005;12:e165–e179. [Google Scholar]
- 78.Lau AC, So LK, Miu FP. Outcome of coronavirus-associated severe acute respiratory syndrome using a standard treatment protocol. Respirology. 2004;9:173–183. doi: 10.1111/j.1440-1843.2004.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chua SE, Cheung V, McAlonan GM. Stress and psychological impact on SARS patients during the outbreak. Can J Psychiatry. 2004;49:385–390. doi: 10.1177/070674370404900607. [DOI] [PubMed] [Google Scholar]
- 80.Jeong H, Yim HW, Song Y-J. Mental health status of people isolated due to Middle East Respiratory Syndrome. Epidemiol Health. 2016;38 doi: 10.4178/epih.e2016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loutfy MR, Blatt LM, Siminovitch KA. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 82.Hui DS, Wong KT, Ko FW. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han Y, Geng H, Feng W. A follow-up study of 69 discharged SARS patients. J Tradit Chin Med. 2003;23:214–217. [PubMed] [Google Scholar]
- 84.Yoon M-K, Kim S-Y, Ko H-S, Lee M-S. System effectiveness of detection, brief intervention and refer to treatment for the people with post-traumatic emotional distress by MERS: a case report of community-based proactive intervention in South Korea. Int J Ment Health Syst. 2016;10:51. doi: 10.1186/s13033-016-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mak IWC, Chu CM, Pan PC, Yiu MGC, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lau S-T, Yu W-C, Mok N-S, Tsui P-T, Tong W-L, Cheng SW. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS) Int J Cardiol. 2005;100:167–169. doi: 10.1016/j.ijcard.2004.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leow MK-S, Kwek DS-K, Ng AW-K, Ong K-C, Kaw GJ-L, Lee LS-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol. 2005;63:197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hui DS, Joynt GM, Wong KT. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lau HM-C, Lee EW-C, Wong CN-C, Ng GY-F, Jones AY-M, Hui DS-C. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil. 2005;86:1134–1140. doi: 10.1016/j.apmr.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li TS, Gomersall CD, Joynt GM, Chan DPS, Leung P, Hui DSC. Long-term outcome of acute respiratory distress syndrome caused by severe acute respiratory syndrome (SARS): an observational study. Crit Care Resusc. 2006;8:302–308. [PubMed] [Google Scholar]
- 92.Tso EYK, Tsang OTY, Choi KW. Persistence of physical symptoms in and abnormal laboratory findings for survivors of severe acute respiratory syndrome. Clin Infect Dis. 2004;38 doi: 10.1086/383580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lo YL, Leong HN, Hsu LY. Autonomic dysfunction in recovered severe acute respiratory syndrome patients. Can J Neurol Sci. 2005;32:264. [PubMed] [Google Scholar]
- 94.Cheng SK-W, Tsang JS-K, Ku K-H, Wong C-W, Ng Y-K. Psychiatric complications in patients with severe acute respiratory syndrome (SARS) during the acute treatment phase: a series of 10 cases. Br J Psychiatry. 2004;184:359–360. doi: 10.1192/bjp.184.4.359. [DOI] [PubMed] [Google Scholar]
- 95.Lau HM-C, Ng GY-F, Jones AY-M, Lee EW-C, Siu EH-K, Hui DS-C. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust J Physiother. 2005;51:213–219. doi: 10.1016/S0004-9514(05)70002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong X, Zheng K, Tang M. Prevalence and factors associated with depression and anxiety of hospitalized patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.24.20043075. published online April 5. (preprint) [DOI] [Google Scholar]
- 97.Yang L, Wu D, Hou Y. Analysis of psychological state and clinical psychological intervention model of patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.22.20040899. published online March 24. (preprint) [DOI] [Google Scholar]
- 98.Zhang B, Zhou X, Qiu Y. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.02.26.20028191. published online Feb 27. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y, Yang R, Xu Y, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.02.27.20029009. published online March 5. (preprint) [DOI] [Google Scholar]
- 100.Qi D, Yan X, Tang X. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. medRxiv. 2020 doi: 10.1101/2020.03.01.20029397. published online March 3: (preprint). [DOI] [Google Scholar]
- 101.Leung KS-S, Ng TT-L, Wu AK-L. A territory-wide study of early COVID-19 outbreak in Hong Kong community: a clinical, epidemiological and phylogenomic investigation. medRxiv. 2020 doi: 10.1101/2020.03.30.20045740. published online April 7. (preprint) [DOI] [Google Scholar]
- 102.Fu S, Fu X, Song Y. Virologic and clinical characteristics for prognosis of severe COVID-19: a retrospective observational study in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.04.03.20051763. published online April 6. (preprint) [DOI] [Google Scholar]
- 103.Lam CLK, Gandek B, Ren XS, Chan MS. Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF-36 Health Survey. J Clin Epidemiol. 1998;51:1139–1147. doi: 10.1016/s0895-4356(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 104.Lam C, Lauder I, Lam T, Gandek B. Population based norming of the Chinese (HK) version of the SF36 health survey. Hong Kong Pract. 1999;21:460–470. [Google Scholar]
- 105.Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020 doi: 10.3201/eid2606.200320. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morfopoulou S, Brown JR, Davies EG. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 107.Nilsson A, Edner N, Albert J, Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis. 2020;52:419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- 108.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 109.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 111.Hopkins RO, Weaver LK, Pope D, Orme JF, Jr, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 112.Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38:2386–2400. doi: 10.1097/CCM.0b013e3181f3dec5. [DOI] [PubMed] [Google Scholar]
- 113.Rockwood K. Delays in the discharge of elderly patients. J Clin Epidemiol. 1990;43:971–975. doi: 10.1016/0895-4356(90)90080-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.