Tuberculosis is estimated to affect 10 million people each year, but this statistic is remarkably uncertain: 30% are countries' estimates of undiagnosed, untreated, or unreported cases. Furthermore, about half of tuberculosis diagnoses globally are uncertain because they rely entirely on the non-specific clinical features of tuberculosis, without any laboratory confirmation.1
Laboratory tests for tuberculosis generally perform poorly and are scarcely available in resource-constrained settings where most cases of tuberculosis occur.1 Consequently, when assessing a patient who is unwell with symptoms such as prolonged productive cough for whom rapid laboratory tests for tuberculosis are unavailable or negative, it is often difficult to discern whether the patient has tuberculosis or a more common disease such as a bacterial pneumonia. In this situation, some guidelines recommend a trial of antibiotics, a widely practiced approach entailing a few days of broad-spectrum antibiotics that are inactive against tuberculosis. This approach assumes that symptom persistence despite a trial of antibiotics implies that tuberculosis is probable, and that improvement on a trial of antibiotics rules out tuberculosis.2 However, these assumptions have been the subject of remarkably little research and are undermined by issues such as antibiotic resistance and the placebo effect.
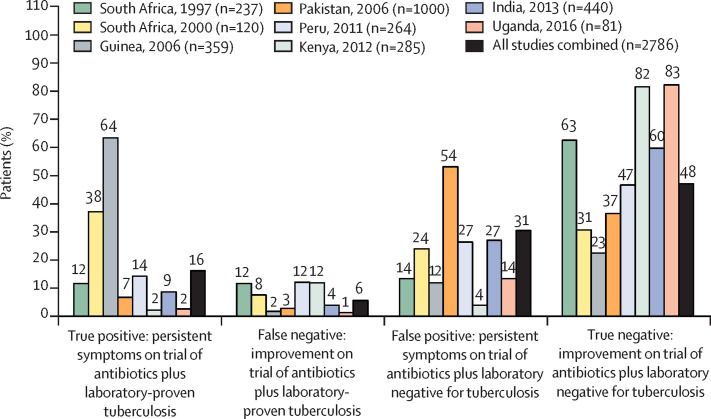
In a systematic review and meta-analysis in The Lancet Infectious Diseases, Titus Divala and colleagues3 evaluated a trial of antibiotics as if it were a diagnostic test for tuberculosis. After screening 9410 publications, they identified eight studies in which patients with initially negative rapid tuberculosis tests were treated with a trial of antibiotics, the response to which was compared with the subsequent results of more sensitive laboratory testing for tuberculosis. Of the 2786 patients who had a trial of antibiotics, overall 608 patients (22% [range 4–65]) were later found to have laboratory-proven tuberculosis. The crude results of these eight studies are summarised in the figure , which shows that whether patients' symptoms persisted on a trial of antibiotics did not usefully predict laboratory-proven tuberculosis. For example, overall, 1307 (47% [range 6–76]) of 2786 patients had symptoms that persisted despite a trial of antibiotics, implying that they had tuberculosis, but remarkably only 454 (35% [range 11–84]) of 1307 patients with persistent symptoms were later found to have laboratory-confirmed tuberculosis. Furthermore, 154 patients (25% [range 3–85]) of 608 patients later found to have laboratory-confirmed tuberculosis paradoxically improved on a trial of antibiotics, an improvement that should have ruled out this tuberculosis diagnosis. These crude percentages are complementary to the rigorous random effects meta-analysis reported by Divala and colleagues, which suggested that a trial of antibiotics had pooled sensitivity of 67% (95% CI 42–85) and specificity of 73% (58–85) versus mycobacteriology tests. This poor performance of a trial of antibiotics has important implications for policy and practice.3
Figure.
Crude data from the eight studies analysed by Divala and colleagues,3 adapted from their figure 2
If the response to a trial of antibiotics reliably indicated whether a patient had tuberculosis, then almost all of the patients would have true-positive or true-negative responses, and few patients would have false-positive or false-negative responses. Labels above each bar are the crude percentages reported in each study. Thus, the bars for all studies combined indicate the crude sum of the data from each of the eight studies. n=number of patients receiving a trial of antibiotics for suspected tuberculosis.
The methods and results of these eight studies varied so greatly that the combined statistics and pooled meta-analyses have questionable precision.4 Despite this variability, the trial of antibiotics performed poorly for ruling tuberculosis in or out in each of the eight studies. These findings are approximations because tuberculosis laboratory testing might be false positive,5 and because false-negative laboratory tests in patients who have tuberculosis are frequent.6 Furthermore, molecular testing was only included in the initial rapid testing in one of these eight studies,7 but it has since become widely used for diagnosing tuberculosis.1 None of these issues are likely to challenge Divala and colleagues' conclusion that a trial of antibiotics is unreliable for informing tuberculosis diagnosis.
Importantly, broad-spectrum antibiotics might still be necessary, not to inform tuberculosis diagnosis, but rather because the patient's illness requires antibiotic treatment, ideally guided by laboratory testing.8 However, there is compelling evidence that broad-spectrum antibiotic therapy for patients with a suspected respiratory tract infection might often be safely withheld initially, potentially reducing the risk of side-effects and antibiotic resistance.9
Divala and colleagues' findings show that a trial of antibiotics should not generally be used to decide whether to commence tuberculosis therapy in patients with negative, pending, or unavailable laboratory tests for tuberculosis. But what should health systems, clinicians, and patients do instead? When should empirical treatment be commenced rather than doing additional tests or waiting to see how the illness evolves? There is an urgent need for operational research to address this knowledge gap, which will depend on local epidemiology, the severity of the patient's illness, and the availability of repeat and more accurate laboratory tests.
More than a century after Robert Koch identified Mycobacterium tuberculosis, why do policy and practice still often include non-evidence-based algorithms for people with tuberculosis? Why are we clarifying the poor diagnostic reliability of a trial-of-antibiotics for tuberculosis only after they have been used millions of times during several decades? Both questions are answered in part by the chronic severe underfunding of tuberculosis research.1, 10 The coronavirus disease 2019 (COVID-19) emergency and resultant socioeconomic crisis will inevitably worsen the global tuberculosis pandemic by increasing tuberculosis risk factors and social determinants, and challenging health systems and access to them. It is striking that long before mortality attributed to COVID-19 approaches 1·5 million—the number of deaths caused by tuberculosis each year—there has been unprecedented investment in research that is rapidly defining how best to care for people with suspected COVID-19. This should be an inspiration to the fight for tuberculosis elimination. Similar urgency and investment are also desperately needed to inform improved care for people suspected of having the most frequent infectious cause of death, tuberculosis.
Acknowledgments
We report funding from the Wellcome Trust (awards 057434/Z/99/Z, 070005/Z/02/Z, 078340/Z/05/Z, 105788/Z/14/Z, and 201251/Z/16/Z), the UK Department for International Development (DFID) Civil Society Challenge Fund, the Joint Global Health Trials consortium (Medical Research Council [MRC], DFID, and Wellcome Trust award MR/K007467/1; MRC award MR/T040165/1), the Bill & Melinda Gates Foundation (award OPP1118545), the Sir Halley Stewart Trust, WHO, the STOP TB partnership's TB REACH initiative funded by the Government of Canada and the Bill & Melinda Gates Foundation (W5_PER_CDT1_PRISMA), and the Innovation For Health And Development charity, outside the submitted work. None of the funding organisations had any role in or placed any restrictions on the preparation or publication of this manuscript. This manuscript represents the opinion of the authors.
References
- 1.WHO . World Health Organization; Geneva: 2020. Global tuberculosis report 2019. [Google Scholar]
- 2.Global Laboratory Initiative GLI model TB diagnostic algorithms. June, 2018. http://www.stoptb.org/wg/gli/assets/documents/GLI_algorithms.pdf
- 3.Divala TH, Fielding KL, Kandulu C. Utility of broad-spectrum antibiotics for diagnosing pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30143-2. published online May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melsen WG, Bootsma MCJ, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 5.Barac A, Karimzadeh-Esfahani H, Pourostadi M. Laboratory cross-contamination of Mycobacterium tuberculosis: a systematic review and meta-analysis. Lung. 2019;197:651–661. doi: 10.1007/s00408-019-00241-4. [DOI] [PubMed] [Google Scholar]
- 6.Oberhelman RA, Soto-Castellares G, Gilman RH. A controlled study of tuberculosis diagnosis in HIV-infected and uninfected children in Peru. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walusimbi S, Semitala F, Bwanga F. Outcomes of a clinical diagnostic algorithm for management of ambulatory smear and Xpert MTB/Rif negative HIV infected patients with presumptive pulmonary TB in Uganda: a prospective study. Pan Afr Med J. 2016;23:154. doi: 10.11604/pamj.2016.23.154.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball P, Baquero F, Cars O. Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. J Antimicrob Chemother. 2002;49:31–40. doi: 10.1093/jac/49.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Spurling GKP, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev. 2017;9 doi: 10.1002/14651858.CD004417.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treatment Action Group. Stop TB Partnership . Treatment Action Group; New York: 2018. Tuberculosis research funding trends 2005–2017. [Google Scholar]