The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) challenges clinicians with a variety of presentations of COVID-19.1, 2, 3 Infection with SARS-CoV-2 is confirmed by real-time RT-PCR, typically done on nasopharyngeal (NP) swabs or, less commonly, samples from the lower respiratory tract, including bronchoalveolar lavage (BAL).4 Some data suggest that RT-PCR might be more sensitive with BAL than with NP swabs.5 Here, we present a man who developed rapidly progressive pulmonary disease and, following two negative NP tests, was diagnosed with COVID-19 on the basis of bronchoscopic biopsy and BAL after 9 days of illness.
14 days before admission to Mount Sinai Hospital (New York, NY, USA), a 34-year-old man developed fever, cough, and dyspnoea due to influenza A, confirmed by an NP swab 2 days later at a walk-in urgent care facility of the Mount Sinai Health System. No radiograph was done. The patient was previously healthy with no past hospitalisations, and works as an anaesthesiologist in a large medical centre in New York City. His only medication was emtricitabine and tenofovir alafenamide for HIV pre-exposure prophylaxis, which he had been taking once a day for 5 months (and prior to that, tenofovir disoproxil fumarate and emtricitabine once a day for 3 years). He was treated for influenza with oseltamivir for 5 days; symptoms resolved after 5 days of illness. He felt fully recovered from influenza, and returned to work in the hospital 11 days after his symptoms had resolved.
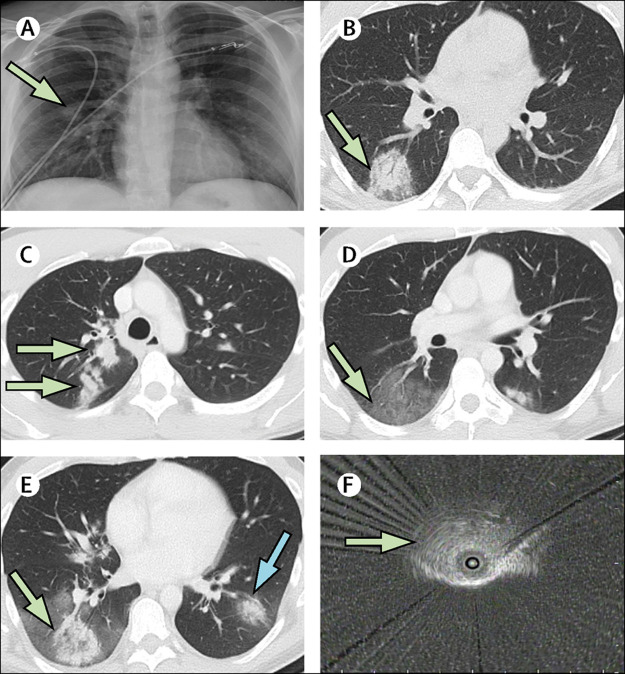
On the afternoon of his first day back at work, the patient had rapid onset of fever, chills, rigors, dry cough, and shortness of breath. On arrival at the emergency department of Mount Sinai Hospital, on the same afternoon as symptom onset (hospital day 1), his temperature was 39·4°C, blood pressure 142/80 mm Hg, heart rate 130 beats per min, and respiratory rate20 breaths per min. Oxygen saturation ranged from 89% to 99% at ambient air. On examination, he appeared acutely ill and in mild distress with normal breath sounds. Plasmalyte (2 L), vancomycin (1 g once every 12 h), and ceftriaxone (2 g once a day) were administered intravenously; all medications are detailed in the appendix (pp 1–2). The patient's white blood cell count was 13 900 cells per μL (reference range 4500–11 000), with a lymphocyte count of 9·2% (15–50). Chest radiograph revealed an ill-defined nodule in the right mid-lung (figure 1A ). The patient was admitted to the hospital. On hospital day 1, a respiratory viral panel test of an NP swab was negative, including for influenza. On day 2, RT-PCR (Roche Cobas 6800 System, Roche, Basel, Switzerland) of an NP swab was negative for SARS-CoV-2. This assay targets open-reading frame 1, a region that is unique to SARS-CoV-2, and has a sensitivity of 1–5 copies per μL.6 An HIV test was negative, and intravenous azithromycin (500 mg once a day) was started. The remainder of the laboratory tests are summarised in the appendix (pp 1–2).
Figure 1.
Imaging with chest radiograph, chest CT, and radial EBUS
(A) Admission chest radiograph (hospital day 1) with right mid-lung nodule (arrow). (B) First chest CT (day 2) with right lower lobe rounded opacity with possible halo sign (arrow). A second chest CT (day 6) showed new right upper lobe nodular opacities (C; arrows), a new large ground glass opacity in the right lower lobe (D; arrow); and enlargement of the right lower lobe rounded opacity with possible reverse halo sign (E; green arrow) and a new left lower lobe rounded opacity (E; blue arrow). (F) Radial EBUS image of right lower lobe rounded opacity (arrow) used to target the transbronchial lung biopsy on day 9. EBUS=endobronchial ultrasound.
On hospital day 2, CT of the chest found a rounded opacity in the right lower lobe (figure 1B); the remainder of the lungs appeared normal. On day 4, the patient's peak temperature was 37·8°C, and his cough and dyspnoea had improved; intravenous azithromycin was stopped. On day 5, fever to a peak of 38·9°C occurred, and the patient developed rigors and severe coughing. Intravenous azithromycin (500 mg once a day) was restarted and oral clindamycin (300 mg once every 6 h) was administered, without clinical improvement. A repeat chest CT on day 6 showed enlargement of the right lower lobe opacity, which had become surrounded by a large new ground glass opacity. A new rounded opacity was present in the left lower lobe, and a new multilobulated opacity in the right upper lobe (figure 1C–E). The CT report suggested atypical pneumonia of fungal or viral origin. A repeat RT-PCR of an NP swab for SARS-CoV-2 was negative on day 7. Severe cough, malaise, rigors, and fever continued, and oxygen saturation was 92–94% at ambient air. Antibiotics were stopped on day 8, and prednisone 40 mg was given orally on the mornings of day 8 and day 9 for presumed cryptogenic organising pneumonia. Consulting radiologists noted CT evidence of a halo sign and reverse halo sign (figure 1B and 1E),7 suggestive of invasive fungal infection and not characteristic of previously reported CT findings for COVID-19.1, 8 On day 8, a serum galactomannan assay was ordered and pulmonary consultation was requested. The pulmonary consultant recommended bronchoscopy with transbronchial biopsy and BAL. Given the epidemic of COVID-19 in New York City at the time, the pulmonary team still considered COVID-19 a possible cause of the pulmonary disease.
On hospital day 9, bronchoscopy was done with appropriate personal and environmental protection precautions for COVID-19, including intubation. The airways appeared normal without inflammation or secretions. The original right lower lobe opacity was targeted for biopsy, and radial endobronchial ultrasound was used to select the airways leading directly to the lesion (figure 1F). Transbronchial lung biopsy and BAL were done in the right lower lobe lateral segment through the target airways. Appropriate specimens were sent for histopathological and cytological examinations and for routine bacterial, fungal, and mycobacterial cultures, and a BAL galactomannan assay. Validated testing of BAL for SARS-CoV-2 was unavailable, and thus a swab used for NP specimens was swirled in the BAL specimen and sent for RT-PCR.
On day 9, pathology reported alveolar tissue with patchy chronic inflammation, type 2 pneumocyte hyperplasia, and areas of organising intra-alveolar fibrin and fibroblastic tissue, consistent with acute lung injury. No hyaline membranes, viral cytopathic changes, giant cells, granulomas, or malignancy were present, and no microorganisms were identified on acid-fast or Grocott's methenamine silver staining (figure 2A ). Cytological examination of the BAL found marked reactive changes in pneumocytes, with lymphocytes, histiocytes, and occasional fibroblastic balls lined by pneumocytes, with negative Grocott's methenamine silver staining (figure 2B). RT-PCR for SARS-CoV-2 of the BAL specimen was positive; BAL and serum galactomannan were negative. No additional antibiotic or prednisone was given. On day 10, the patient declined treatment with hydroxychloroquine and was discharged home. Via follow-up phone calls, the patient reported that his cough and myalgias slowly resolved, and he had no fever higher than 37·8°C after discharge. No chest imaging was done after discharge.
Figure 2.
Pathology of transbronchial biopsy and BAL cytology
(A) Transbronchial biopsy showing prominent pneumocyte hyperplasia with areas of organising fibrin and fibroblastic tissue. The alveolar septa showed some mild chronic inflammatory cell infiltrates (haematoxylin-eosin stain, original magnification × 200). (B) BAL cytology specimen showing a fibroblastic ball lined by pneumocytes (Papanicolaou stain, original magnification × 400). BAL=bronchoalveolar lavage.
This patient experienced an unusual, confusing clinical course during a 10-day hospitalisation, which concluded with a diagnosis of COVID-19, confirmed only with bronchoscopic samples. An atypical presentation of SARS-CoV-2 can explain the entire clinical course during this hospitalisation, beginning with a possible mild cytokine storm and pulmonary infiltrate on hospital day 1, with initial clinical improvement followed by rapid progression within 4 days to multifocal pulmonary disease. Development of a cytokine storm has been reported in patients with COVID-19, but its occurrence within a few hours after onset of symptoms seems unusual,9 as does the initial clinical improvement. The patient's clinical course might have been affected by other factors, including the influenza infection itself or its treatment 11 days earlier, chronic antiretroviral therapy, and early initiation of treatment with azithromycin.
If SARS-CoV-2 was the sole cause of this presentation, starting on the day of admission, then the two negative NP tests were false negatives. In a study by Wang and colleagues,5 BAL was positive in 14 (93%) of 15 positive cases, whereas nasal swabs were positive in 5 (63%) of 8 positive cases, and pharyngeal swabs in 126 (32%) of 398 positive cases. The pathology from transbronchial lung biopsy, which was targeted specifically at the right lower lobe rounded opacity present on the initial chest CT, showed a pattern consistent with acute lung injury. The few peer-reviewed publications reporting pulmonary pathology in COVID-19 to date have all described acute lung injury or diffuse alveolar damage.10, 11, 12, 13, 14, 15, 16 One report of BAL in a patient with COVID-19 described a large number of aggregates of plasma cells;17 this was not seen in the specimen from our patient. Both the finding of acute lung injury in the area of lung affected at the onset of symptoms, and the positive RT-PCR test for SARS-CoV-2 in the BAL, support the diagnosis of COVID-19 to explain the entire hospital course.
Alternatively, a different infection might have caused the early sepsis presentation, with clinical improvement up to day 4 related to appropriate treatment with antibiotics, after which COVID-19 manifested on day 5, causing clinical worsening. However, no infection other than SARS-CoV-2 was identified by culture, pathology, or respiratory viral panel, procalcitonin concentration was normal in the first 48 h (appendix p 1), serum and BAL galactomannan were negative, and the pathological finding of acute lung injury in the lesion was already present on day 2, which argue against this explanation.
A third possible explanation is that cryptogenic organising pneumonia or similar inflammatory illness spontaneously worsened and improved over the hospitalisation, and the positive RT-PCR represents a hospital-acquired infection incidental to the hospital course. However, the sepsis-like presentation and the pathology and cytology findings showing an acute lung injury pattern with organising fibrin do not support the diagnosis of cryptogenic organising pneumonia.
This case represents a presentation of COVID-19 with atypical features, including sudden onset with a mild cytokine storm profile, apparent early response to antibiotics followed by rapid clinical worsening, and two negative RT-PCR tests of NP swabs, which delayed the diagnosis of COVID-19. However, for a disease that was unknown only 5 months ago, it might also be too early for clinicians to be certain of which manifestations are typical. In most patients requiring hospitalisation, COVID-19 is diagnosed by positive RT-PCR,1 and bronchoscopy is rarely required to establish the diagnosis. As noted by the American Association of Bronchology and Interventional Pulmonology, “bronchoscopy should have an extremely limited role in diagnosis of COVID-19 and only be considered in intubated patients if upper respiratory samples are negative and other diagnosis is considered that would significantly change clinical management.”18 We agree with this statement, and feel that the presentation in our patient necessitated use of bronchoscopy.
Contributors
TJH conceived the Case Report and wrote the first draft. TJH and KMR collected pathology, cytology, and viral specimens, and designed the appendix table. KMR and JM collected and collated data. TJH and CE contributed radiology images, and CE interpreted radiology images. AHS interpreted and photographed cytology specimens. MBB reviewed and photographed pathology specimens. All authors participated in the construction and editing of the manuscript and revisions. Written consent for publication was obtained from the patient.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Ai T, Yang Z, Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. published online Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song F, Shi N, Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju PK, Ghassemieh BJ, Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance. March 19, 2020. file:///C:/Users/GashD/Downloads/WHO-COVID-19-laboratory-2020.5-eng.pdf
- 5.Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche Molecular Systems cobas® SARS-CoV-2. Qualitative assay for use on the cobas® 6800/8800 systems. 2020. https://www.fda.gov/media/136049/download
- 7.Raju S, Ghosh S, Mehta AC. Chest CT signs in pulmonary disease: a pictorial review. Chest. 2017;151:1356–1374. doi: 10.1016/j.chest.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Chung M, Bernheim A, Mei X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Wu D, Guo W. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copin M-C, Parmentier E, Duburcq T, Poissy J, Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06057-8. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wichmann D, Sperhake JP, Lütgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-2003. published online May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter T, Haslbauer JD, Nienhold R. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. published online May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konopka KE, Wilson A, Myers JL. Postmortem lung findings in an asthmatic with coronavirus disease 2019 (COVID-19) Chest. 2020 doi: 10.1016/j.chest.2020.04.032. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marco Giani M, Davide Seminati M, Alberto Lucchini M, Giuseppe Foti M, Fabio Pagni M. Exuberant plasmocytosis in bronchoalveolar lavage of the first patient requiring extracorporeal membrane oxygenation for SARS-CoV-2 in Europe. J Thorac Oncol. 2020;15:e65–e66. doi: 10.1016/j.jtho.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahidi MM, Lamb C, Murgu S. American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchology Interv Pulmonol. 2020 doi: 10.1097/LBR.0000000000000681. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.