Abstract
Viral infections represent a major threat for mankind. The adaptive immune system plays a key role in both viral clearance and disease pathogenesis, and, accordingly, understanding how lymphocytes interact with different viruses is critical to design more effective vaccination and therapeutic strategies. The recent advent of intravital microscopy has enabled the real-time visualization of the complex interplay between viruses and the ensuing adaptive immune response in living organisms. Here, we will review the most significant recent insights on antiviral adaptive immune responses obtained through intravital imaging. We will also discuss what challenges lie ahead and what we think are the most promising areas for future research.
Introduction
Adaptive immune responses play a crucial role in anti-viral immunity, as they mediate viral clearance or containment, they contribute to tissue damage, and they are essential for preventing reinfection and for the protection conferred by the available vaccines. In order to mount a successful adaptive immune response against a virus, lymphocytes need to move within distinct organs and tissue niches, where they interact with many different cell types that influence their behavior and function. Since no in vitro methodology can fully capture the myriad of physical and chemical cues that influence cell behavior in tissues, several groups have started to utilize intravital microscopy to visualize the dynamics of anti-viral immunity as it happens in real time in vivo in an organism [1,2]. The idea is relatively simple: an experimental animal is anesthetized and positioned on a suitable stage so that a particular organ can be microsurgically prepared for microscopic viewing [1,2]. Because of greater resolving power, increased depth penetration and reduced phototoxicity, multiphoton lasers are now the preferred option for most intravital imaging studies; however, due to generally higher temporal resolution, single photon-based epifluorescent techniques are still used for studying events that occur rapidly, such as the intravascular adhesion of leukocytes in superficial sinusoids of the liver [3••].
We will review here how intravital imaging has improved our understanding of adaptive immune responses to viruses. Specifically, we will discuss recent studies pertaining to the priming of antiviral B cells and T cells within secondary lymphoid organs; moreover, using the liver as a paradigmatic example, we will discuss how effector T cells exert their antiviral function in the periphery. For a thorough discussion of advances obtained through the use of intravital imaging pertaining to viral spread, innate antiviral immunity and adaptive immune responses to viruses that infect peripheral organs other than the liver (e.g. skin, lung, central nervous system, gut, pancreas), we refer readers to recently published reviews [4–6].
Priming of antiviral B cells
Using intravital imaging, several pioneering studies have begun to elucidate how B cells encounter antigen and become activated in lymph nodes [7–10]. Regardless of whether these studies were conducted with intact viral particles or with inert particulate antigens, they all identified the lymph node subcapsular sinus (SCS) as an important area for early B cell-antigen encounter [11,12,13••,14••]. The SCS collects unfiltered lymph from afferent lymph vessels and hosts macrophages (SCS macrophages) that are attached to a layer of extracellular matrix [15,16]. The SCS is permeable to small soluble antigens (i.e. molecules with a dynamic radius of less than ∼5.5 nm, corresponding to a molecular mass of less than ∼70 kDa) [17], but not to larger particulate antigens (like intact viruses). Indeed, it has been shown that ∼200 nm-sized structures such as synthetic microspheres or viral particles are captured and retained by SCS macrophages, which secondarily translocate these particulate antigens across the SCS floor and display them to underlying follicular B cells [11,12,13••]. Unlike non-cognate B cells, antigen-specific follicular B cells make prolonged contacts with SCS macrophages, acquire antigen, up-regulate CC-chemokine receptor 7 (CCR7) and the G protein-coupled receptor EBI2, and migrate to the B cell-T cell boundary where they recruit CD4+ T cell help necessary for maximal activation [9,14••,18–23]. The importance of SCS macrophage-mediated antigen retention for follicular B cell activation is highlighted by the observation that disruption of the SCS macrophage lining occurring during infection or inflammation impairs B cell responses to subsequent viral infection [24••]. Following activation at the B cell-T cell boundary, B cells undergo vigorous proliferation in the outer B cell follicle. Within a few days after antigen encounter, while some B cells differentiate into plasmablasts and accumulate at extrafollicular sites, others re-enter B cell follicles and give rise to germinal centers [25]. An interesting research question pertains to the spatiotemporal dynamics of B cell activation in response to viruses that fail to induce early neutralizing antibody responses (e.g. lymphocytic choriomeningitis virus [LCMV] in mice or hepatitis B virus [HBV], hepatitis C virus [HCV] or human immunodeficiency virus [HIV] in humans). It was recently shown that, upon LCMV infection, virus-specific B cells move to the inter-follicular and T cell area of the draining lymph node, where they engage in prolonged interactions with a population of CD11b+Ly6Chi inflammatory monocytes that is recruited in a type I interferon (IFN-I)-dependent and CCR2-dependent fashion [14••]. Inflammatory monocytes induce B cell apoptosis via nitric oxide and, indeed, experimental ablation of inflammatory monocytes or inhibition of their lymph node recruitment leads to increased survival of antiviral B cells and recovery of protective antibodies [14••]. Future studies should assess the relative capacity of different viruses to induce the lymph node recruitment of inflammatory monocytes and the role these cells might play in suppressing antibody responses to those infections. Recent studies have suggested that virus-induced type I interferon is not only required for the lymph node recruitment of inflammatory monocytes, but it also promotes the expansion and differentiation of virus-specific effector CD8+ T cells that kill antiviral B cells [26••] and it indirectly stimulates the differentiation of antiviral B cells into short-lived plasma cells [27••]. The detailed characterization of the molecular mechanisms whereby type I interferon hinders antiviral B cell responses might lead to novel therapy for chronic infectious diseases as well as more rational vaccination strategies.
Priming of antiviral T cells
Although subcapsular sinus macrophage play a key role in early B cell activation, they do not seem to be involved in direct priming of antiviral T cells [4]. Local infection with vaccinia virus, however, induces inflammasome activation in subcapsular sinus macrophages, which is immediately followed by cell death and release of extra-cellular apoptosis-associated speck-like protein with a CARD domain (ASC) specks [28]. This short-lived inflammasome signaling causes a vigorous lymph node recruitment of T cells and inflammatory cells from the circulation, ultimately increasing the magnitude of the T cell response [28].
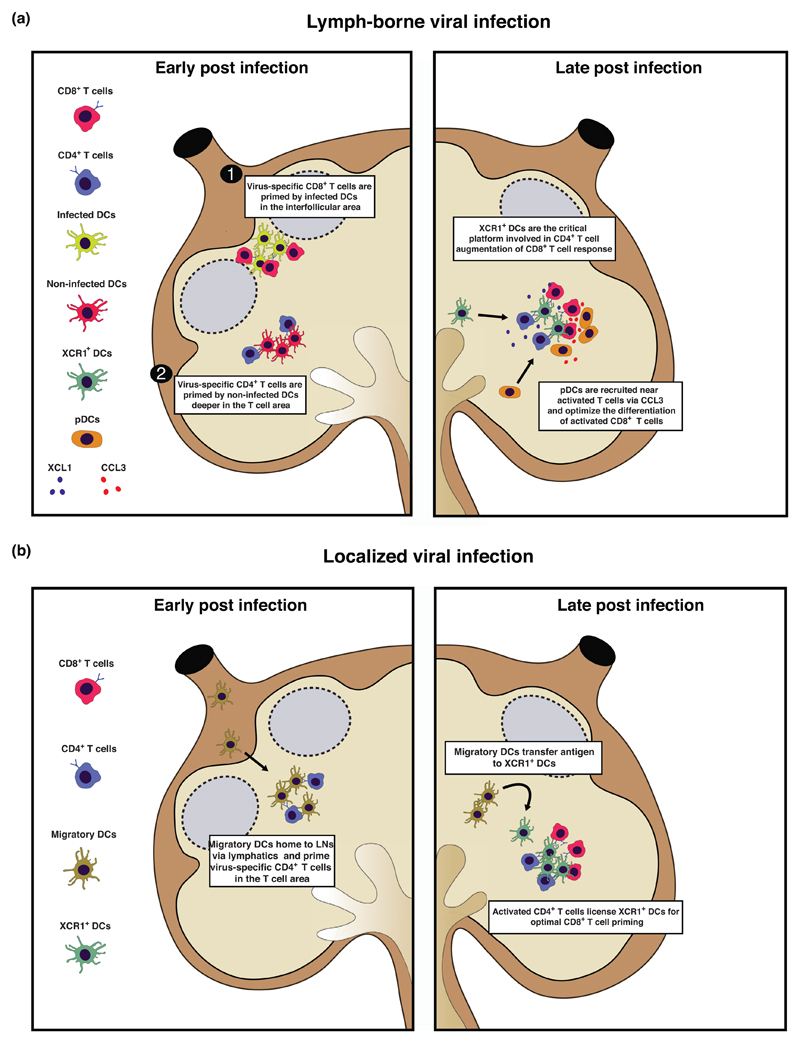
Intravital microscopy studies have revealed distinct spatiotemporal dynamics of CD4+ and CD8+ T cell activation upon viral infections [29••,30,31,32••] (Figure 1). The initial activation of CD4+ and CD8+ T cells was shown to be spatially and temporally separated, involving different dendritic cell subsets and varying between viruses and infection routes [31,32••] (Figure 1). For instance, upon subcutaneous vaccinia virus infection, naïve CD8+ T cells are initially primed by infected DCs near the SCS [29••,30,31], whereas naïve CD4+ T cells get activated deeper in the T cell zone by different non-infected DCs [31] (Figure 1). Using a cutaneous herpes simplex virus infection model (where no infected cells are found within secondary lymphoid organs), instead, it was shown that early priming of CD4+ T cells involved clustering with migratory skin DCs in the T cell area (Figure 1). By contrast, priming of naïve CD8+ T cells was delayed and did not involve migratory DCs but rather lymph-node resident XCR1+ DCs [32••] (Figure 1). At later stages of infection in both experimental systems, XCR1+ DCs present antigen to both CD4+ and CD8+ T cells [31,32••] (Figure 1). These XCR1+ DCs are the critical platform involved in CD4+ T cell augmentation of CD8+ T cell responses [31] (Figure 1). Moreover, plasmacytoid dendritic cells (pDCs) were shown to support XCR1+ DC maturation and cross-presentation, therefore indirectly optimizing CD8+ T cell priming [33] (Figure 1). Indeed, it was found that initial activation of CD8+ T cells by conventional DCs leads to production of CCL3 and CCL4 as well as XCL1 chemokines which attract pDCs and XCR1+ DCs, respectively [33] (Figure 1). The simultaneous presence of pDCs, XCR1+ DCs and CD8+ T cells within the same tissue niche allows for pDC-derived type I interferon to optimize the maturation and cross-presentation of XCR1+ DCs, thus enhancing the developing CD8+ T cell response [33] (Figure 1).
Figure 1. Antiviral CD4+ and CD8+ T cell priming is spatiotemporally separated and different upon lymph-borne or localized viral infection.
(a) Early during lymph-borne viral infections, antiviral CD8+ T cells are primed by infected lymph-node resident dendritic cells (DCs) in interfollicular areas near the subcapsular sinus (1). Subsequently, virus-specific CD4+ T cells are primed by non-infected lymph-node resident DCs deeper in the T cell area (2) (left panel). Later during infection, lymph-node resident XCR1+ DCs are recruited near activated T cells via XCL1 and represent a critical platform for CD4+ T cell augmentation of CD8+ T cell responses. In addition, pDCs are also recruited near activated T lymphocytes via CCL3 and contribute to optimize antiviral CD8+ T cell responses by producing IFN type I (right panel). (b) Early during localized viral infections, antiviral CD4+ T cells are primed by migratory DCs in the T area (left panel). Later during the infection, migratory DCs transfer viral antigens to lymph-node resident XCR1+ DCs, which are licensed by activated CD4+ T cells for optimal CD8+ T cell priming (right panel).
Whereas the spatiotemporal dynamics of antiviral CD8+ T cell priming are beginning to unravel, less is known about the dynamics of CD4+ T cells upon viral infection. For instance, we currently have a poor understanding of the spatiotemporal dynamics whereby CD4+ T cells differentiate into distinct T helper cell subsets (e.g. Th1, TFh, Th17, etc.) upon the same or different types of viral infections.
Secondary lymphoid organs also serve as sites for reactivation of central memory T cells upon secondary viral infection. Two studies have recently analyzed the lymphnode localization and dynamic behavior of central memory CD8+ T cells upon viral infection [34,35•]. Both studies reported that, upon peripheral infection, CXCL9 recruits central memory CD8+ T cells from the cortical ridge to the subcapsular sinus and IFN-γ derived from activated memory T cells activates a feed-forward circuit that enhances CXCL9 expression by both stromal cells and macrophages in the subcapsular sinus [34,35•]. This early intranodal chemokine guidance is essential for efficient antiviral memory, as CXCR3-deficient central memory CD8+ T cells were markedly compromised in their in vivo recall responses to viral infection [34]. One aspect that the two studies disagreed upon is the localization of central memory CD8+ T cells in lymph nodes under homeostatic conditions (prior to antigen re-encounter). Whereas Sung et al. showed that central memory CD8+ T cells populate the deep paracortex akin to their naïve counterparts [34], Kastenmu¨ ller et al. found central memory CD8+ T cells to be prepositioned in the peripheral paracortex [35•], near pathogen entry sites. The reason for this discrepancy might lie in the different procedures used for obtaining central memory CD8 T cells in the two studies (in vitro [34] versus in vivo [35•] generation).
CD8+ T cell effector function in the liver
Once primed in secondary lymphoid organs, effector CD8+ T cells need to move to infected peripheral organs in order to exert their antiviral activity. For instance, in order to control hepatotropic viral infections (such as those caused by hepatitis B virus), antigen-experienced effector cells must home to the liver, recognize pathogen-derived antigens, and deploy effector functions [36]. A recent study used advanced imaging to reveal the unique and novel mode whereby effector CD8+ T cells perform their immune surveillance function against hepatitis B virus in the liver [3]. Circulating effector CD8+ T cells were found to arrest within liver sinusoids regardless of the location of antigen-expressing hepatocytes and independently of selectins, Gαi-coupled chemokine receptors, β2-integrins and α4-integrins, PECAM-1, vascular adhesion protein 1. Rather, effector CD8+ T cells dock onto platelets that have previously adhered to sinusoidal hyaluronan via CD44. After this initial platelet-dependent arrest, effector CD8+ T cells crawl along the liver vasculature, probing hepatocytes for the presence of antigens by extending protrusions through the fenestrated sinusoidal endothelial cells. Hepatocellular antigen recognition and effector functions occur while CD8+ T cells are still confined to the intravascular space and are inhibited by the pathologic processes that characterize liver fibrosis (i.e. sinusoidal defenestration and liver capillarization). These findings might help explain why fibrosis that occurs during chronic viral hepatitis is such a predisposing factor for the development of hepatocellular carcinoma.
Conclusions and future perspectives
Although the need for cell–cell interactions in mounting successful antiviral adapative immune responses have been appreciated for many decades, it is only with the advent of intravital microscopy that important insights have been gained with respect to the identification of important antiviral immune functions that depend on the spatial and temporal location of various tissue-resident and migratory immune cells and environmental factors. While we now have a fairly good understanding of the spatiotemporal dynamics of CD8+ T cell activation in response to viral infection, that of virus-specific CD4+ T cells and B cells have lagged behind until very recently. Advances in molecular virology are finally allowing the visualization of immune responses to viruses that are notoriously difficult to manipulate genetically. Going forward, the combination of intravital microscopy with tools to study the functional space of immune cell types and states at the single-cell level (e.g. single-cell RNA-sequencing [37]) could allow the systematic identification and characterization of the cellular and molecular composition of tissue niches [38].
Acknowledgements
We thank M. Silva for secretarial assistance and all the members of the Iannacone Lab for helpful discussions. Work in the Iannacone Laboratory discussed in this review was supported by ERC grants 281648 and 725038 (to MI), Italian Association for Cancer Research (AIRC) grants 15350 and 9965 (to MI), Italian Ministry of Health grant GR-2011-02347925 (to MI), Fondazione Regionale per la Ricerca Biomedica grant 2015-0010 (to MI), European Molecular Biology Organization (EMBO) Young Investigator Program (to MI), and a Career Development Award from the Giovanni Armenise-Harvard Foundation (to MI).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sumen C, Mempel TR, Mazo IB, Andrian von UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, Fiocchi A, Vacca M, Aiolfi R, Sammicheli S, et al. Immunosurveillance of the liver by intravascular effector CD8 (+) T cells. Cell. 2015;161:486–500. doi: 10.1016/j.cell.2015.03.005. [•• This study reveals the unique way whereby intravascular CD8+ T cells exert their immune surveillance function against hepatotropic viral infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickman HD. New insights into antiviral immunity gained through intravital imaging. Curr Opin Virol. 2017;22:59–63. doi: 10.1016/j.coviro.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Murooka TT, Mempel TR. Intravital microscopy in BLT-humanized mice to study cellular dynamics in HIV infection. J Infect Dis. 2013;208(Suppl 2):S137–S144. doi: 10.1093/infdis/jit447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller SN. Effector T-cell responses in non-lymphoid tissues: insights from in vivo imaging. Immunol Cell Biol. 2013;91:290–296. doi: 10.1038/icb.2012.75. [DOI] [PubMed] [Google Scholar]
- 7.Kuka M, Iannacone M. The role of lymph node sinus macrophages in host defense. Ann N Y Acad Sci. 2014;1319:38–46. doi: 10.1111/nyas.12387. [DOI] [PubMed] [Google Scholar]
- 8.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 9.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 10.Heesters BA, van der Poel CE, Das A, Carroll MC. Antigen presentation to B cells. Trends Immunol. 2016;37:844–854. doi: 10.1016/j.it.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 13.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [•• References [11,12,13••] reveal a role for macrophages within the lymph node subcapsular sinus in the presentation of large particulate antigens, immune complexes and inactivated viruses to follicular B cells.] [DOI] [PubMed] [Google Scholar]
- 14.Sammicheli S, Kuka M, Di Lucia P, de Oya NJ, De Giovanni M, Fioravanti J, Cristofani C, Maganuco CG, Fallet B, Ganzer L, et al. Inflammatory monocytes hinder antiviral B cell responses. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah6789. [•• This study utilizes multiphoton intravital microscopy to analyze the spatiotemporal dynamics of B cell activation upon viral infections. It identifies the type I interferon-dependent, CCR2-dependent lymph node recruitment of inflammatory monocytes as a critical inhibitor of antiviral B cell responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark SL. The reticulum of lymph nodes in mice studied with the electron microscope. Am J Anat. 1962;110:217–257. doi: 10.1002/aja.1001100303. [DOI] [PubMed] [Google Scholar]
- 16.Farr AG, Cho Y, de Bruyn PP. The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am J Anat. 1980;157:265–284. doi: 10.1002/aja.1001570304. [DOI] [PubMed] [Google Scholar]
- 17.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Cyster JG, Dang EV, Reboldi A, Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol. 2014;14:731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 19.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 23.Yi T, Wang X, Kelly LM, An J, Xu Y, Sailer AW, Gustafsson J-A, Russell DW, Cyster JG. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity. 2012;37:535–548. doi: 10.1016/j.immuni.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaya M, Castello A, Montaner B, Rogers N, Sousa CRE, Bruckbauer A, Batista FD. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347:667–672. doi: 10.1126/science.aaa1300. [•• This study describes how viral infections disrupt the organization of lymph node subcapsular sinus macrophages, thus thwarting antibody responses to subsequent antigenic challenge.] [DOI] [PubMed] [Google Scholar]
- 25.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moseman EA, Wu T, La Torre de JC, Schwartzberg PL, McGavern DB. Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation. Sci Immunol. 2016;1:eaah3565. doi: 10.1126/sciimmunol.aah3565. [•• This study establishes that virus-induced type I interferon promotes the expansion and differentiation of cytotoxic T lymphocytes that kill antiviral B cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, Cornille K, Kreutzfeldt M, Page N, Zimmer G, Geier F, et al. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1:eaah6817. doi: 10.1126/sciimmunol.aah6817. [•• This paper describes how virus-induced type I interferon drives the deletion of antiviral B cells by supporting their differentiation into short-lived plasma cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagoo P, Garcia Z, Breart B, Lemaître F, Michonneau D, Albert ML, Levy Y, Bousso P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat Med. 2015 doi: 10.1038/nm.4016. [DOI] [PubMed] [Google Scholar]
- 29.Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [•• This paper is the first to visualize CD8+ T cell priming upon viral infection.] [DOI] [PubMed] [Google Scholar]
- 30.Hickman HD, Li L, Reynoso GV, Rubin EJ, Skon CN, Mays JW, Gibbs J, Schwartz O, Bennink JR, Yewdell JW. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J Exp Med. 2011 doi: 10.1084/jem.20102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmuller W. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell. 2015;162:1322–1337. doi: 10.1016/j.cell.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hor JL, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity. 2015;43:554–565. doi: 10.1016/j.immuni.2015.07.020. [•• References [31,32••] reveal distinct spatiotemporal dynamics of CD4+ and CD8+ T cell activation upon viral infections.] [DOI] [PubMed] [Google Scholar]
- 33.Brewitz A, Eickhoff S, Dähling S, Quast T, Bedoui S, Kroczek RA, Kurts C, Garbi N, Barchet W, Iannacone M, et al. CD8(+) T cells orchestrate pDC-XCR1(+) dendritic cell spatial and functional cooperativity to optimize priming. Immunity. 2017;46:205–219. doi: 10.1016/j.immuni.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, La Torre de JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013 doi: 10.1016/j.immuni.2012.11.012. [• References [34,35•] analyze the lymph node localization and dynamic behavior of central memory CD8+ T cells upon viral infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inverso D, Iannacone M. Spatiotemporal dynamics of effector CD8+ T cell responses within the liver. J Leukoc Biol. 2016;99:51–55. doi: 10.1189/jlb.4MR0415-150R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giladi A, Amit I. Immunology, one cell at a time. Nature. 2017;547:27–29. doi: 10.1038/547027a. [DOI] [PubMed] [Google Scholar]
- 38.Medaglia C, Giladi A, Stoler-Barak L, De Giovanni M, Salame TM, Biram A, et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science (New York, NY) 2017:eaao4277. doi: 10.1126/science.aao4277. [DOI] [PMC free article] [PubMed] [Google Scholar]