Abstract
Type I interferons (IFNs) released upon viral infections play different and opposing roles in disease outcome. This pleiotropic effect is mainly influenced by the cellular sources, timing and target cells for these molecules. The effect of type I IFN signaling on the activation and differentiation of antiviral CD4+ T cells remains ill defined, with studies reporting either a beneficial or a detrimental role, depending on the context of infection. This review will highlight several recent studies that have investigated the role of type I IFNs in the priming and polarization of CD4+ T cells and discuss areas of uncertainty that require further investigation.
Keywords: Type I interferons, CD4+ T cell, T follicular helper cells, Th1, Viral infection, T cell differentiation
1. Introduction
Type I interferons (IFNs) are innate molecules secreted by different cells upon infection [1]. They comprise several isoforms of IFN-α, one isoform of IFN-β, and the less characterized IFN-ε, IFN-κ, IFN-ω, IFN-δ, and IFN-τ. All type I IFNs act both in autocrine and paracrine fashions by binding to heterodimeric receptors composed of IFNAR1 and IFNAR2 subunits [2]. The name “interferon” was coined in concomitance with their discovery, and it was inspired by what is historically considered to be the main role for these molecules, i.e. interfering with pathogen replication [3,4]. Indeed, release of type I IFNs is one of the most potent mechanisms of innate immunity against viruses and other intracellular pathogens. However, growing evidence is pointing to a multifaceted role for type I IFNs, with either beneficial or detrimental effects in the fight against infection. This pleiotropic role is mainly due to the different cellular sources, timing and target cells for type I IFNs [5]. For instance, we and others have recently described how type I IFNs released upon lymphocytic choriomeningitis virus (LCMV) infection can have a negative impact on antiviral humoral responses by inducing apoptosis of virus-specific B cells activated early on [6–9]. The conflicting roles of type I IFNs in infection and cancer have been extensively reviewed elsewhere [5,10–12]. Here, we will discuss recent studies that investigated the role of type I IFNs in CD4+ T cell activation and polarization.
2. CD4+ T cell differentiation upon viral infections
CD4+ T cells are key players of adaptive immunity. Naïve CD4+ T cells are primed in secondary lymphoid organs (SLOs) upon binding of MHCII-peptide complexes and costimulatory molecules. Both these signals are provided by professional antigen-presenting cells (APC), mostly dendritic cells (DC), which have encountered and processed antigens either in the periphery or in SLOs [13–15]. Following the priming phase, antigen-specific naïve CD4+ T cells undergo clonal expansion and effector differentiation. During these processes, CD4+ T cells are exposed to a milieu enriched in cytokines produced by many cells including infected cells, DCs, and stromal cells. CD4+ T cells sense these cytokines and activate differentiation programs that result in their polarization towards specialized T helper cell subsets [16,17]. Infection by viruses or intracellular bacteria mainly leads to the generation of Th1 and T follicular helper (Tfh) cells. Th1 cells express the master transcription factor T-bet and produce high levels of IFN-γ, which promotes macrophage activation and enhances CD8+ T cell responses [18,19]. By contrast, Tfh cells migrate to B cell follicles where they specifically interact with cognate antigen-specific B cells and participate in germinal center (GC) reactions to ensure the generation of high-affinity, class-switched antibodies [20,21]. Both CD8-mediated cellular and B cell-mediated humoral responses are essential for proper infection control, and usually co-exist in a fine-tuned equilibrium in order to optimize anti-pathogen immunity [22–24]. The molecular and cellular determinants of CD4+ T cell differentiation into Th1 or Tfh cells in vivo, and hence the relative balance between cellular and humoral immunity, are incompletely understood [25–27]. Research in the field is complicated by the plasticity of T helper cell subsets [27] and the pleiotropic nature of many cytokines. Nonetheless, type IFNs are emerging as critical factors regulating antiviral CD4+ T cell fate.
3. Role of type I IFNs in antiviral CD4+ T cell priming during acute infections
Several reports have shown an anti-proliferative effect of type I IFNs on CD4+ T cells activated in vitro either upon anti-CD3 antibody treatment [28,29] or upon cognate antigen stimulation [29]. However, the in vivo setting is a lot more complex, since the final outcome is the net effect of type I IFNs being released by different cell types, with different kinetics and acting on different targets. For instance, it was shown that type I IFN signaling in CD4+ T cells is important for clonal expansion upon acute LCMV infection [29]. When LCMV-specific CD4+ T cells lacking the ability to sense type I IFN were transferred into LCMV-infected mice, CD4+ T cells underwent the initial activation steps (upregulation of the activation markers CD25 and CD69 and early proliferation) but accumulated at a much lower level than their WT counterparts at the peak of the immune response. Interestingly, this was observed upon LCMV but not upon Listeria Monocytogenes (LM) infection [29]; whether this is due to differences in type I IFN production upon the two infections remains to be determined. Another study went further to show that LCMV-specific CD4+ T cells lacking type I IFN receptor could accumulate at levels comparable to their WT counter-parts if NK cells were depleted during LCMV infection, suggesting that type I IFN signaling can protect antiviral CD4+ T cells from NK cell-mediated killing, similarly to what has been reported for CD8+ T cells [30,31]. The mechanisms behind this observation seem to involve a type I IFN-mediated upregulation of selected inhibitory NK cell receptor ligands and/or downregulation of natural cytotoxicity triggering receptor 1 (NCR1) ligands on CD4+ (and CD8+) T cells [30,31]. Overall, the abovementioned studies strongly suggest that CD4+ T cell-intrinsic type I IFN signaling is required for survival of primed antiviral CD4+ T cells, especially in the context of acute LCMV infection (Fig. 1). Interestingly, however, when type I IFN signaling was inhibited on all cell types by virtue of an IFNAR-blocking antibody, LCMV-specific CD4+ T cell frequencies during acute LCMV infection were not affected [32]. This unexpected result could be explained either by an incomplete IFNAR blocking by the antibody, or by the pleiotropic and sometimes opposite effects that type I IFNs play on different cellular targets during infection. For example, type I IFNs promote activation and effector function of NK cells upon viral infection [33,34], support DC maturation [35,36] and inhibit T regulatory cell-mediated suppression of antigen-specific CD4+ T cells [37,38] (Fig. 1).
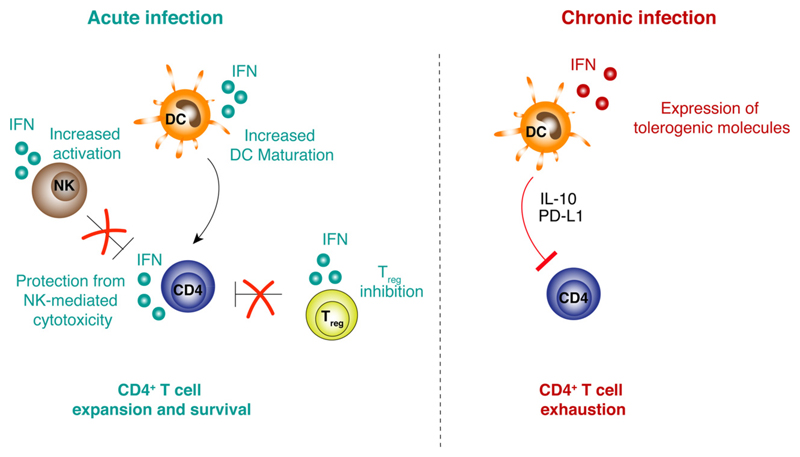
Fig. 1. Role of type I IFNs in antiviral CD4+ T cell expansion.
Left panel. Type I IFNs released upon acute infections act on different cell types to support antigen-specific CD4+ T cell activation. Type I IFNs sensed by DCs increase their maturation, thus providing a more efficient antigen presentation and co-stimulation to T cells. Type I IFNs act on T regulatory cells by dampening their suppressive capabilities and allowing heightened CD4+ T activation. Type I IFNs enhance NK cell activation and effector functions. Finally, type I IFNs sensed by CD4+ T cells protect them from NK cell-mediated killing.
Right panel. Type I IFNs released upon chronic infections create an immunosuppressive environment by increasing PD-L1 and IL-10 expression by DCs. These immunoregulatory DCs induce CD4+ T exhaustion.
4. Role of type I IFNs in the antiviral CD4+ T cell response during chronic infections
The above-mentioned studies pretty much converge on the idea that type I IFN signaling (whether CD4+ T cell-intrinsic or -extrinsic) supports rather than interferes with antiviral CD4+ T cell accumulation during acute infection. The picture is quite different, however, if we look at chronic viral infections, where viral persistence induces prolonged expression of type I IFNs [39–41]. Two studies showed that blockade of type I IFN signaling during chronic LCMV infection led to a stronger CD4+ T cell response that was responsible for faster viral clearance [32,42]. Sustained type I IFN production during chronic LCMV infection induced expression of PD-L1 and IL-10 by immunoregulatory DCs, thereby contributing to an immunosuppressive environment (Fig. 1). Since in the abovementioned studies type I IFN signaling was blocked ubiquitously, however, it is impossible to definitively ascertain the target cell(s) responsible for the observed net effect. Besides DCs, there are likely additional cellular targets for type I IFN during chronic LCMV infection, potentially with opposing effects. For instance, selective deletion of type I IFN receptors in Tregs during chronic LCMV infection led to higher viral titers and increased frequency of PD-1-expressing exhausted T cells – although the effect on CD4+ T cells was less pronounced than that on CD8+ T cells [38]. It should be noted that in this latter setting, DCs could still sense type I IFNs, acquire a suppressive phenotype, and promote exhaustion of antiviral CD4+ T cells.
5. Role of type I IFNs in CD4+ T cell polarization
Since viral infections result in polarization of naïve CD4 T cells into both Th1 and Tfh subsets (although to variable degrees depending on the nature of the pathogen), a lot of effort has been devolved to the question of whether type I IFNs play a role in CD4+ T cell polarization. In vitro studies of naïve CD4+ T cell polarization have shown that type I IFNs are certainly needed for the expression of the Tfh master transcription factor Bcl-6, and to a lower extent for the induction of the Th1 transcription factor T-bet [43]. Interestingly, culture of CD4+ T cells with type I IFNs resulted in the expression of Tfh surface markers CXCR5 and PD-1, but not in IL-21 production. IL-21 is a cytokine produced by fully differentiated Tfh, so what can be inferred from these data is that type I IFNs initiate early Tfh differentiation but other cytokines (e.g. IL-6) are needed to complete the Tfh polarization program. Type I IFNs seem to be required for Tfh differentiation upon immunization in vivo as well; however the target cell(s) responsible for this effect seems to vary according to the experimental setting, ranging from CD4+ T cell themselves to DCs and monocytes [44–46]. The differences in these settings might be explained by the complex cytokine milieu found in vivo, where synergies of type I IFNs with other cytokines such as IL-6 [43,45] and IL-1 [46] might have profoundly different effects on CD4+ T cell polarization (Fig. 2). The notion that type I IFNs drive Tfh differentiation in vivo is not universally accepted, with some studies reporting increased Tfh cell number upon IFNAR blockade during acute LCMV infection [47]. This was also shown in a more stringent setting where Tfh were poised to express Th1-related genes due to impaired STAT3-derived signals. STAT3-deficient CD4+ T cells showed higher sensitivity to type I IFNs, and treatment with IFNAR-blocking antibodies reverted the phenotype from Th1 to Tfh [47]. Interestingly, in a setting of LM infection, type I IFN signaling in CD4+ T cells was shown to promote Th1 differentiation, by synergizing with IL-12-derived signals [48]. These data potentially suggest that IFNAR signaling might suppress Tfh and favor Th1 differentiation upon certain infections (Fig. 2). It is worth mentioning, however, that type I IFN signaling during LCMV infection leads to LCMV-specific B cell apoptosis [6–9]. Thus, the detrimental effect of type I IFN on Tfh development upon LCMV infection might be due to an ineffective B cell response, which is required to reach full Tfh differentiation (Fig. 2). Moreover, it was shown that type I IFN signaling results in modest but significant suppression of Tfh cells generated upon infection with a LCMV chronic strain [49]. Whereas blocking type I IFN signaling at the onset of acute or chronic LCMV infection led to increased Tfh cells [47,49], a strikingly opposite result was obtained with CD4+ T cells primed during an established persistent infection [49]. Indeed, transfer of LCMV-specific CD4+ T cells into mice previously infected with a chronic LCMV strain led to suppressed Th1 and heightened Tfh differentiation, and this phenotype was readily reverted when IFNAR was blocked [49]. This effect was not due to signaling of type I IFNs in CD4+ T cells but to the effect of type I IFNs on other yet to be identified cells.
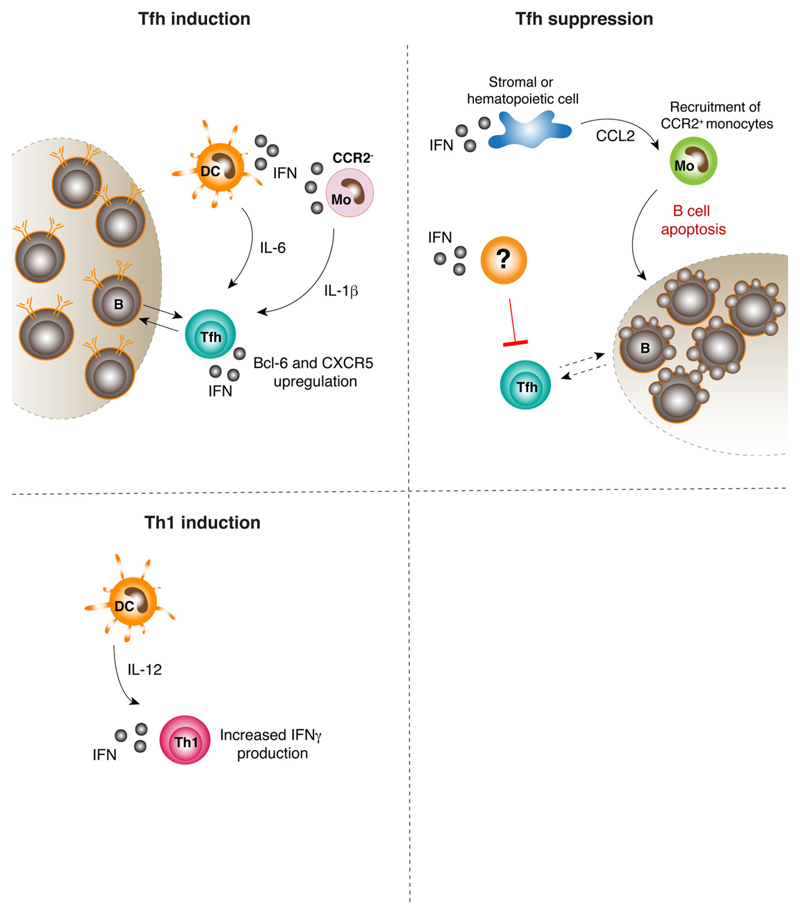
Fig. 2. Role of type I IFNs in CD4+ T cell polarization.
Upper left panel. In some settings, type I IFNs can promote CD4+ T cell differentiation into the Tfh subset by acting at different levels. Type I IFN signaling in DCs results in production of IL-6, a Tfh-polarizing cytokine. Type I IFNs induce CCR2− monocytes to produce IL-1β, which also results in Tfh polarization. Finally, type I IFNs sensed by CD4+ T cells play a supportive role in full Tfh differentiation.
Upper right panel. Type I IFNs can also suppress CD4+ T cell differentiation into the Tfh subset, in some settings. For example, upon both acute and chronic LCMV infection, type I IFNs acting on cell types other than CD4+ T cells suppress Tfh differentiation at the onset of the infection. Moreover, type I IFNs released upon LCMV infection result in the production of CCL2 by stromal and hematopoietic cells. CCL2 triggers recruitment of CCR2+ monocytes, which induce LCMV-specific B cell apoptosis, thus potentially impairing full Tfh differentiation.
Lower left panel. Upon Listeria monocytogenes infection, type I IFN signaling in CD4+ T cells can synergize with DC-derived IL-12 to increase IFN-γ production and Th1 differentiation.
The role of type I IFNs on CD4+ T cell responses has been investigated also in the context of malaria infection, with data suggesting that type I IFN signaling in DCs dampens CD4+ T cell activation and differentiation to both Th1 and Tfh cell subsets [50–53].
Together, the available data indicate that the mechanisms underlying the different sensitivity to type I IFNs according to the type and stage of infection remain incompletely understood.
6. Concluding remarks and discussion
The studies discussed in this review show that type I IFN signaling can have disparate effects on CD4+ T cell activation, depending on the cells sensing these cytokines, the nature of the pathogen and the stage of the infection. One interpretation might be that the role of type I IFNs changes based on whether the infection is acute or chronic [10], with interferons supporting CD4+ T cell expansion in the acute phase, but inducing their exhaustion during the chronic phase [29,32,42]. Interestingly, one study showed that type I IFNs played distinct roles in polarization of CD4+ T cells depending on whether these cells were primed at the onset or during an ongoing chronic LCMV infection [49]. Indeed, type I IFN signaling promoted Th1 differentiation early on, but inhibited de novo Th1 and favored instead Tfh differentiation during an ongoing infection [49]. This suggests that even within the context of the same infection, IFNAR signaling might lead to opposed effects on CD4+ T cell polarization, depending on the timing of action. Moreover, the role of type I IFNs on CD4+ T cell polarization seem to vary even during acute infections or immunizations [44–46]. Therefore, more than distinguishing between acute and chronic infections, one should perhaps focus on the kinetics, magnitude and targets of type I IFNs in the different immunization/infection settings. For instance, during acute malaria infection IFNAR engagement suppresses CD4+ T cell responses, whereas during acute LCMV infection it supports them [29,54]. One attractive hypothesis might be that the action of type I IFNs is tightly regulated in time and space, and the cytokine milieu generated upon different infections (or at different times during the same infection) adds up to this tight regulation to confer specificity to the IFN-mediated signaling. The molecular and cellular mechanisms regulating the spatiotemporal functions of type I IFNs on CD4+ T cell differentiation remain to be fully elucidated, and might be influenced by, among others, pathogen replication kinetics, tropism, cytokine milieu or cellular target(s) [5,45,47,51]. Combination of conditional knock-out models with timed delivery of blocking antibodies might shed additional light on the complex relationship between type I IFNs and CD4+ T helper cell priming and differentiation.
Acknowledgements
We thank M. Silva for secretarial assistance and the members of the Iannacone laboratory for helpful discussions.
Funding
M.I. is supported by European Research Council (ERC) Consolidator Grant 725038, Italian Association for Cancer Research (AIRC) Grant 19891, Italian Ministry of Health (MoH) Grant GR-2011-02347925, Lombardy Foundation for Biomedical Research (FRRB) Grant 2015-0010, the European Molecular Biology Organization (EMBO) Young Investigator Program, and a Career Development from the Giovanni Armenise-Harvard Foundation; M.K. is the recipient of the Italian Ministry of Education (MIUR) grant SIR-RBSI14BAO5.
References
- [1].Trinchieri G. Type I interferon: friend or foe. J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- [3].Pitha PM, Kunzi MS. Type I interferon: the ever unfolding story. Curr Top Microbiol Immunol. 2007;316:41–70. doi: 10.1007/978-3-540-71329-6_4. [DOI] [PubMed] [Google Scholar]
- [4].Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- [5].Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- [6].Kuka M, Iannacone M. Viral subversion of B cell responses within secondary lymphoid organs. Nat Rev Immunol. 2018;18:255–265. doi: 10.1038/nri.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moseman EA, Wu T, de la Torre JC, Schwartzberg PL, McGavern DB. Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, Cornille K, Kreutzfeldt M, Page N, Zimmer G, Geier F, Straub T, et al. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sammicheli S, Kuka M, Di Lucia P, de Oya NJ, De Giovanni M, Fioravanti J, Cristofani C, Maganuco CG, Fallet B, Ganzer L, Sironi L, et al. Inflammatory monocytes hinder antiviral B cell responses. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017;38:542–557. doi: 10.1016/j.it.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Teijaro JR. Type I interferons in viral control and immune regulation. Curr Opin Virol. 2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murira A, Lamarre A. Type-I interferon responses: from friend to foe in the battle against chronic viral infection. Front Immunol. 2016;7:609. doi: 10.3389/fimmu.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Giovanni M, Iannacone M. In vivo imaging of adaptive immune responses to viruses. Curr Opin Virol. 2018;28:102–107. doi: 10.1016/j.coviro.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19(2):89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- [16].Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- [18].Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- [19].Sercan O, Stoycheva D, Hämmerling GJ, Arnold B, Schüler T. IFN-gamma receptor signaling regulates memory CD8+ T cell differentiation. J Immunol. 2010;184:2855–2862. doi: 10.4049/jimmunol.0902708. [DOI] [PubMed] [Google Scholar]
- [20].Crotty S. Follicular helper CD4 t cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- [21].Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- [22].Arnaout RA, Nowak MA. Competitive coexistence in antiviral immunity. J Theor Biol. 2000;204:431–441. doi: 10.1006/jtbi.2000.2027. [DOI] [PubMed] [Google Scholar]
- [23].Silverstein AM. Cellular versus humoral immunology: a century-long dispute. Nat Immunol. 2003;4:425–428. doi: 10.1038/ni0503-425. [DOI] [PubMed] [Google Scholar]
- [24].Thomsen AR, Marker O. The complementary roles of cellular and humoral immunity in resistance to re-infection with LCM virus. Immunology. 1988;65:9–15. [PMC free article] [PubMed] [Google Scholar]
- [25].Schmitt N, Liu Y, Bentebibel SE, Ueno H. Molecular mechanisms regulating T helper 1 versus T follicular helper cell differentiation in humans. Cell Rep. 2016;16:1082–1095. doi: 10.1016/j.celrep.2016.06.063. [DOI] [PubMed] [Google Scholar]
- [26].Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, Kallies A, et al. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep. 2016;14:68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- [27].O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dondi E, Rogge L, Lutfalla G, Uzé G, Pellegrini S. Down-modulation of responses to type I IFN upon T cell activation. J Immunol. 2003;170:749–756. doi: 10.4049/jimmunol.170.2.749. [DOI] [PubMed] [Google Scholar]
- [29].Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting edge: the direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- [30].Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- [31].Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Köhrer K, Rahbar R, Diefenbach A, Gibbert K, et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- [32].Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Madera S, Rapp M, Firth MA, Beilke JN, Lanier LL, Sun JC. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J Exp Med. 2016;213:225–233. doi: 10.1084/jem.20150712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- [35].Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- [36].Simmons DP, Wearsch PA, Canaday DH, Meyerson HJ, Liu YC, Wang Y, Boom WH, Harding CV. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J Immunol. 2012;188:3116–3126. doi: 10.4049/jimmunol.1101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Srivastava S, Koch MA, Pepper M, Campbell DJ. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J Exp Med. 2014;211:961–974. doi: 10.1084/jem.20131556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gangaplara A, Martens C, Dahlstrom E, Metidji A, Gokhale AS, Glass DD, Lopez-Ocasio M, Baur R, Kanakabandi K, Porcella SF, Shevach EM. Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment. PLoS Pathog. 2018;14:e1006985. doi: 10.1371/journal.ppat.1006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li Y, Li S, Duan X, Liu B, Yang C, Zeng P, McGilvray I, Chen L. Activation of endogenous type I IFN signaling contributes to persistent HCV infection. Rev Med Virol. 2014;24:332–342. doi: 10.1002/rmv.1795. [DOI] [PubMed] [Google Scholar]
- [40].Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010;202(Suppl 2):S297–301. doi: 10.1086/655657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work. AIDS. 2013;27:1199–1208. doi: 10.1097/QAD.0b013e32835ecb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nakayamada S, Poholek AC, Lu KT, Takahashi H, Kato M, Iwata S, Hirahara K, Cannons JL, Schwartzberg PL, Vahedi G, Sun HW, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J Immunol. 2014;192:2156–2166. doi: 10.4049/jimmunol.1300675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Riteau N, Radtke AJ, Shenderov K, Mittereder L, Oland SD, Hieny S, Jankovic D, Sher A. Water-in-oil-only adjuvants selectively promote T follicular helper cell polarization through a type I IFN and IL-6-dependent pathway. J Immunol. 2016;197:3884–3893. doi: 10.4049/jimmunol.1600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31:491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- [46].Barbet G, Sander LE, Geswell M, Leonardi I, Cerutti A, Iliev I, Blander JM. Sensing microbial viability through bacterial RNA augments T follicular helper cell and antibody responses. Immunity. 2018;48:584–598 e5. doi: 10.1016/j.immuni.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Osokine I, Snell LM, Cunningham CR, Yamada DH, Wilson EB, Elsaesser HJ, de la Torre JC, Brooks D. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc Natl Acad Sci U S A. 2014;111:7409–7414. doi: 10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haque A, Best SE, Ammerdorffer A, Desbarrieres L, de Oca MM, Amante FH, de Labastida Rivera F, Hertzog P, Boyle GM, Hill GR, Engwerda CR. Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur J Immunol. 2011;41:2688–2698. doi: 10.1002/eji.201141539. [DOI] [PubMed] [Google Scholar]
- [51].Haque A, Best SE, Montes de Oca M, James KR, Ammerdorffer A, Edwards CL, de Labastida Rivera F, Amante FH, Bunn PT, Sheel M, Sebina I, et al. Type I IFN signaling in CD8-DCs impairs Th1-dependent malaria immunity. J Clin Invest. 2014;124:2483–2496. doi: 10.1172/JCI70698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sebina I, Haque A. Effects of type I interferons in malaria. Immunology. 2018;155:176–185. doi: 10.1111/imm.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Soon MSF, Haque A. Recent insights into CD4. J Immunol. 2018;200:1965–1975. doi: 10.4049/jimmunol.1701316. [DOI] [PubMed] [Google Scholar]
- [54].Sebina I, James KR, Soon MS, Fogg LG, Best SE, Labastida Rivera F, Montes de Oca M, Amante FH, Thomas BS, Beattie L, Souza-Fonseca-Guimaraes F, et al. IFNAR1-signalling obstructs ICOS-mediated humoral immunity during non-lethal blood-stage plasmodium infection. PLoS Pathog. 2016;12:e1005999. doi: 10.1371/journal.ppat.1005999. [DOI] [PMC free article] [PubMed] [Google Scholar]