Abstract
Background and Objectives
Little is known about the outcomes of outpatient clinic-based elective external cardioversion (OPC-ECV) for persistent atrial fibrillation (PeAF). We investigated the acute, short-term, and long-term elective external cardioversion (ECV) outcomes.
Methods
We included 1,718 patients who underwent OPC-ECV (74% male, 61.1±11.0 years old, 90.9% long-standing PeAF, 9.1% after atrial fibrillation [AF] ablation) after excluding patients with atrial tachycardia or inappropriate antiarrhythmic drug medication, and in-patient ECV. Biphasic shocks were delivered sequentially until successful cardioversion was achieved (70-100-150-200-250 J). If ECV failed at 150 J, we administered intravenous amiodarone 150 mg and delivered 200 J.
Results
ECV failed in 11.4%, and the complication rate was 0.47%. Within 3 months, AF recurred in 55.5% (44.7% as sustaining AF, 10.8% as paroxysmal AF), and the AF duration was independently associated (odds ratio [OR], 1.01 [1.00–1.02]; p=0.006), but amiodarone was independently protective (OR, 0.46 [0.27–0.76]; p=0.002, Log rank p<0.001) against an early recurrence. Regarding the long-term recurrence, pre-ECV heart failure was protective against an AF recurrence (hazard ratio, 0.63 [0.41–0.96], p=0.033) over 32 (9–66) months of follow-up. ECV energy (p<0.001) and early recurrence rate within 3 months (p=0.007, Log rank p=0.006) were significantly lower in post-ablation patients than in those with long-standing persistent AF.
Conclusions
The success rate of OPC-ECV was 88.6%, and the complication rate was low. However, AF recurred in 55.5% within 3 months. Amiodarone was protective against short-term AF recurrences, and long-term AF recurrences were less in patients with baseline heart failure.
Keywords: Atrial fibrillation, Cardioversion, Recurrence
INTRODUCTION
Atrial fibrillation (AF) is a common rhythm disorder with a prevalence of 1–2% in the total population, with an increasing prevalence with age.1) Although rhythm control of AF may reduce heart failure mortality,2) cardiovascular mortality and hospitalization,3) and incidence of stroke,4) and improve cognitive5) and renal functions,6) most studies have been evaluated after aggressive rhythm control of AF by catheter ablation. As a traditional intervention for rhythm control, elective external cardioversion (ECV) is commonly used to treat persistent AF (PeAF). Although ECV has a high acute success rate for restoring sinus rhythm, its rate of sinus rhythm maintenance is low when using antiarrhythmic drugs (AADs).7) The outcome of ECV can vary greatly with the duration of AF,8) the type of AAD used,7) the ECV shock energy form and energy level,9) the location of the paddles,9) and the rhythm follow-up protocol after ECV.10) In previous studies on ECV, acute and short-term success rates were commonly evaluated within 1 month by combining the 2.8) Only a few previous studies have evaluated the effects of ECV in highly selective patient groups, such as long-standing PeAF or post-AF catheter ablation (AFCA) PeAF.11) Moreover, only a few studies have been conducted on elective ECV performed in an outpatient clinic rather than ECV performed in an emergency room or intensive care unit.12),13)
The purpose of this study was to evaluate the efficacy and safety of outpatient clinic-based ECV in a protocol-based single center prospective registry. The aims of this study were to evaluate the acute success rate after outpatient clinic-based ECV, short-term rhythm outcomes within 3 months, and long-term outcomes after 3 months and to identify factors associated with these rhythm outcomes. We also compared ECV outcomes between patients with long-standing PeAF and those in whom AFCA.
METHODS
Study population
The study protocol adhered to the Declaration of Helsinki. All patients provided written informed consent. From March 2009 to November 2018, a total of 1,893 long-standing PeAF patients who underwent ECV at Severance Hospital were enrolled. After excluding 175 patients with atrial tachycardia (AT), inappropriate AAD medication, or in-patient ECV, 1,718 patients were included (74% males, 61.1± 11.0 years). Among all included individuals, 1,561 patients (90.9%) had long-standing PeAF, and 157 (9.1%) patients had PeAF after AFCA. According to the American College of Cardiology (ACC)/American Heart Association (AHA) Task Force on Practice Guidelines and the Heart Rhythm Society (HRS) Expert Consensus Statement guidelines,14) long-standing PeAF was defined as AF lasting for longer than 1 year. Anti-coagulation therapy was maintained at least 3 weeks before ECV.
Echocardiogram and medical therapy
All patients underwent trans-thoracic echocardiography (Sonos 5500, Philips Medical System, Andover, MA, USA; or Vivid 7, GE Vingmed Ultrasound, Horten, Norway) prior to ECV. Left atrial (LA) chamber size (LA dimension and LA volume index), ejection fraction, the ratio of early diastolic mitral inflow velocity (E) to early diastolic mitral annular velocity (Em), and other data were acquired according to the American Society of Echocardiography guidelines.15) Transesophageal echocardiography was performed in the majority of the patients with a high risk of a stroke or high CHA2DS2-VASc score (≥2) based on the physician's discretion (358 patients, Supplementary Table 1).
We prescribed AADs in 96.5% of the patients at least 1 month before the ECV. In the remaining 3.5% of patients, the ECV was conducted without AADs because of significant sinus node dysfunction or adverse effects of the drugs. Physicians chose AADs based on current guidelines after evaluating the comorbidities of patients.14) The general dosages of each AAD were amiodarone 200–400 mg, dronedarone 400–800 mg, sotalol 80–160 mg, flecainide 150–200 mg, and propafenone 300–450 mg per day. AADs were maintained after successful ECV, but stopped in recurred patients. We reduced doses of amiodarone to 100 mg per day when the patient maintained sinus rhythm on the post-ECV Holter in the third month to minimize long-term adverse effects.
Anticoagulation was maintained according to guidelines at least 4 weeks after outpatient-based ECV.14) After that period, we maintained anticoagulation based on CHA2DS2-VASc score regardless of rhythm status.
Electrical cardioversion
We performed ECV for rhythm control in AAD-resistant AF patients based on current guidelines.14) All ECV procedures were scheduled and performed at an outpatient clinic under sedation (intravenous pentothal [1.5–2.0 mg/kg]). ECV was applied uniformly using 2 oval adhesive pre-gelled pads (each pad, 78 cm2 area) placed in an anterior-posterior position between the right sternal body at the third intercostal space and the area of the left scapular angle within the 3rd to 5th intercostal space by a physician.9) ECV was performed as a QRS-synchronized biphasic direct current shock (biphasic, Medtronic LIFEPACKVR 20; Physio-Control, Inc., Redmond, WA, USA) that began with 70 J and was serially increased to 100 J and then 150 J until sinus rhythm was achieved. If sinus rhythm was not achieved after ECV with 150 J, or if immediate recurrence of AF developed, 150 mg amiodarone intravenous infusion over 20 minutes, and ECV was serially increased to 200 J and then 250 J.16) We defined successful cardioversion as restoration and maintenance of sinus rhythm by ECV until the patient had left the clinic. Anticoagulation was strictly maintained within the therapeutic range before and after ECV.14)
Follow-up and terminology
After successful cardioversion, we checked outpatient ECG at 2 weeks after ECV and 24-hour Holter 3 months later and every 6 months thereafter, unless AF recurred. Whenever the patient reported symptoms of palpitations suggestive of arrhythmia recurrence, Holter ECG or event monitor recordings were obtained. We defined recurrence of AF as any episode of AF or AT of at least 30 seconds in duration.14) If any ECG documented an AF/AT episode within the 3-month blanking period during follow-up, the patient was diagnosed with early recurrence. Any AF/AT recurrence thereafter was diagnosed as long-term recurrence.14)
We cited the 2014 ACC/AHA/HRS Expert Consensus Statement guidelines to define paroxysmal AF14) and also cited the 2016 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure to define heart failure.17)
Data analysis
We compared clinical characteristics, echocardiographic findings, cardioversion parameters, and medications in terms of successful electrical cardioversion. Continuous variables were described as means±standard deviations or median (25–75 percentile) and were compared by analysis of variance. A χ2 test was used for the categorical variables. The independent t-test was used to compare the continuous variables between 2 groups in Tables 1 and 2. The analysis of variance test was performed for the continuous variables among the groups in Table 3, and paired t-test was used to investigate changes in echocardiographic parameters before and after cardioversion. We analyzed factors associated with clinical recurrence after cardioversion of AF by Cox proportional hazard model analysis and factors associated with early recurrence by logistic regression analysis. If any variable had a statistically significant difference (p value <0.05) in the univariate analysis, we entered it into the multivariate analysis. The age and gender were the 2 default variables included in the multivariate analysis, regardless of the p value. A Kaplan-Meier survival analysis was conducted to determine the probability of freedom from arrhythmia recurrence after cardioversion. The p values <0.05 were considered indicative of statistical significance. All analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA).
Table 1. Baseline characteristics of acute outcome of outpatient clinic-based ECV.
| Overall (n=1,718) | Success (n=1,523) | Fail (n=195) | p value | ||
|---|---|---|---|---|---|
| Age (years) | 61.1±11.0 | 61.2±11.1 | 60.1±10.2 | 0.177 | |
| Male | 1,272 (74.0) | 1,133 (74.4) | 139 (71.3) | 0.351 | |
| Body weight (kg) | 72.3±18.3 | 72.4±18.8 | 72.1±13.5 | 0.844 | |
| BMI (kg/m2) | 25.5±10.6 | 25.5±11.1 | 25.8±6.3 | 0.681 | |
| AF duration (months) | 43.4±58.2 | 43.6±58.6 | 38.7±43.3 | 0.804 | |
| Post-AFCA | 157 (9.1) | 143 (9.4) | 14 (7.2) | 0.313 | |
| CHA2DS2-VASc | 1.9±1.6 | 1.9±1.6 | 1.8±1.5 | 0.219 | |
| Heart failure | 223 (13.0) | 197 (12.9) | 26 (13.3) | 0.876 | |
| Hypertension | 901 (52.4) | 801 (52.6) | 100 (51.3) | 0.730 | |
| Age >75 years | 178 (10.4) | 163 (10.7) | 15 (7.7) | 0.194 | |
| Age 65–74 years | 701 (40.8) | 634 (41.6) | 67 (34.4) | 0.052 | |
| Diabetes | 303 (17.6) | 259 (17.0) | 44 (22.6) | 0.055 | |
| Stroke/TIA | 197 (11.5) | 182 (12.0) | 15 (7.7) | 0.079 | |
| Vascular disease | 83 (4.8) | 74 (4.9) | 9 (4.6) | 0.881 | |
| eGFR (mL/min/1.73 m2) | 76.1±15.6 | 75.9±15.9 | 77.6±13.0 | 0.117 | |
| Hemoglobin (g/dL) | 14.8±2.6 | 14.8±2.7 | 14.7±2.0 | 0.756 | |
| Echocardiogram data | |||||
| LA dimension (mm) | 45.6±6.4 | 45.6±6.5 | 46.2±5.5 | 0.180 | |
| LAVI (mL/m2) | 48.3±19.0 | 48.1±18.3 | 50.3±23.7 | 0.227 | |
| Ejection fraction (%) | 60.2±10.1 | 60.1±10.1 | 60.5±9.6 | 0.633 | |
| E/Em | 11.5±5.3 | 11.5±5.4 | 11.4±5.1 | 0.702 | |
| Antiarrhythmic drugs | 1,658 (96.5) | 1,472 (96.7) | 186 (95.4) | 0.364 | |
| Amiodarone | 767 (46.3) | 679 (46.1) | 88 (47.3) | 0.760 | |
| Dronedarone | 131 (7.9) | 117 (7.9) | 14 (7.5) | 0.841 | |
| Sotalol | 74 (4.5) | 64 (4.3) | 10 (5.4) | 0.522 | |
| Flecainide | 547 (33.0) | 492 (33.4) | 55 (29.6) | 0.292 | |
| Propafenone | 98 (5.9) | 84 (5.7) | 14 (7.5) | 0.321 | |
| Pilsicainide | 41 (2.5) | 36 (2.4) | 5 (2.7) | 0.841 | |
| ACEi/ARB | 301 (17.5) | 275 (18.1) | 26 (13.3) | 0.102 | |
| b-blocker | 484 (28.2) | 432 (28.4) | 52 (26.7) | 0.620 | |
| Statin | 310 (18.0) | 276 (18.1) | 34 (17.4) | 0.815 | |
Values are presented as number (%) or mean±standard deviation.
ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ARB = angiotensin receptor blocker; BMI = body mass index; E = early diastolic mitral inflow velocity; ECV = elective external cardioversion; eGFR = estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; Em = early diastolic mitral annular velocity; LA = left atrial; LAVI = left atrial volume index; TIA = transient ischemic attack.
Table 2. Comparison of the outpatient clinic-based ECV outcomes between the L-PeAF and post-AFCA patients.
| Overall (n=1,718) | L-PeAF (n=1,561) | Post-AFCA (n=157) | p value | ||
|---|---|---|---|---|---|
| ECV success | 1,523 (88.6) | 1,380 (88.4) | 143 (91.1) | 0.313 | |
| Successful ECV energy (J) | 144.0±69.5 | 146.2±69.1 | 122.2±70.0 | <0.001 | |
| Major complication | 8 (0.47) | 8 (0.51) | 0 (0.0) | 0.369 | |
| Stroke | 2 (0.12) | 2 (0.13) | 0 (0.0) | ||
| TIA | 1 (0.06) | 1 (0.07) | 0 (0.0) | ||
| SND requiring admission | 5 (0.29) | 5 (0.31) | 0 (0.0) | ||
| AF recurrence within 3 months | 845 (55.5) | 781 (56.6) | 64 (44.8) | 0.007 | |
| AF recurrence after 3 months | 323 (47.6) | 281 (46.9) | 42 (53.2) | 0.296 | |
| Follow-up months (25–75 percentile) | 32 (9–66) | 31 (9–65) | 41 (17–79) | 0.001 | |
Values are presented as number (%) or mean±standard deviation.
AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ECV = electrical cardioversion; L-PeAF = long-standing persistent atrial fibrillation; TIA = transient ischemic attack; SND = sinus node dysfunction.
Table 3. Characteristics based on outpatient clinic-based ECV outcome within 3 months.
| Overall (n=1,523) | Remain in NSR (n=678) | Recur as PAF (n=164) | Recur as sustaining AF (n=681) | p value | ||
|---|---|---|---|---|---|---|
| Age (years) | 61.2±11.1 | 61.9±11.4 | 62.0±11.6 | 60.4±10.7 | 0.037 | |
| Male | 1,133 (74.4) | 503 (74.2) | 114 (69.5) | 516 (75.8) | 0.254 | |
| BMI (kg/m2) | 25.5±11.1 | 25.3±7.6 | 25.7±6.7 | 25.6±14.3 | 0.870 | |
| AF duration (months) | 43.6±58.6 | 15.0±18.4 | 30.5±41.1 | 50.7±65.1 | 0.003 | |
| Post-AFCA | 143 (9.4) | 79 (55.2) | 16 (11.2)) | 48 (33.6) | 0.014 | |
| CHA2DS2-VASc | 1.9±1.6 | 2.0±1.6 | 2.0±1.5 | 1.9±1.6 | 0.552 | |
| Heart failure | 197 (12.9) | 92 (13.6) | 26 (15.9) | 79 (11.6) | 0.278 | |
| Hypertension | 801 (52.6) | 366 (54.0) | 84 (51.2) | 351 (51.5) | 0.622 | |
| Age >75 years | 163 (10.7) | 82 (12.1) | 19 (11.6) | 62 (9.1) | 0.189 | |
| Age 65–74 | 634 (41.6) | 300 (44.2) | 78 (47.6) | 256 (37.6) | 0.012 | |
| Diabetes | 259 (17.0) | 112 (16.5) | 34 (20.7) | 113 (16.6) | 0.405 | |
| Stroke/TIA | 182 (12.0) | 75 (11.1) | 14 (8.5) | 93 (13.7) | 0.122 | |
| Vascular disease | 74 (4.9) | 38 (5.6) | 5 (3.0) | 31 (4.6) | 0.347 | |
| eGFR (mL/min/1.73 m2) | 75.9±15.9 | 75.6±16.0 | 74.8±16.7 | 76.6±15.7 | 0.321 | |
| Hemoglobin (g/dL) | 14.8±2.7 | 14.8±3.5 | 14.5±2.0 | 14.8±1.7 | 0.086 | |
| Echocardiogram data | ||||||
| LA dimension (mm) | 45.6±6.5 | 45.3±6.5 | 46.7±7.4 | 45.5±6.2 | 0.067 | |
| Ejection fraction (%) | 60.1±10.1 | 60.3±10.2 | 57.8±11.5 | 60.5±9.7 | 0.023 | |
| E/Em | 11.5±5.4 | 11.8±5.7 | 12.6±6.5 | 11.0±4.6 | 0.003 | |
| Successful ECV energy (J) | 123.9±43.9 | 118.0±42.6 | 118.9±45.0 | 131.1±43.8 | <0.001 | |
| Antiarrhythmic drugs | 1,472 (96.7) | 651 (96.0) | 162 (98.8) | 659 (96.8) | 0.205 | |
| Amiodarone | 679 (46.1) | 346 (53.1) | 75 (46.3) | 258 (39.2) | <0.001 | |
| Dronedarone | 117 (7.9) | 45 (6.9) | 8 (4.9) | 64 (9.7) | 0.056 | |
| Sotalol | 64 (4.3) | 24 (3.7) | 6 (3.7) | 34 (5.2) | 0.389 | |
| Flecainide | 492 (33.4) | 193 (29.6) | 65 (40.1) | 234 (35.5) | 0.013 | |
| Propafenone | 84 (5.7) | 37 (5.7) | 5 (3.1) | 42 (6.4) | 0.271 | |
| Pilsicainide | 36 (2.4) | 6 (0.9) | 3 (1.9) | 27 (4.1) | 0.001 | |
| ACEi/ARB | 275 (18.1) | 153 (22.6) | 27 (16.5) | 95 (14.0) | <0.001 | |
| b-blocker | 432 (28.4) | 208 (30.7) | 46 (28.0) | 178 (26.1) | 0.178 | |
| Statin | 276 (18.1) | 145 (21.4) | 22 (13.4) | 109 (16.0) | 0.009 | |
Values are presented as number (%) or mean±standard deviation.
ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ARB = angiotensin receptor blocker; BMI = body mass index; E = early diastolic mitral inflow velocity; ECV = electrical cardioversion; eGFR, estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; Em = early diastolic mitral annular velocity; LA = left atrial; NSR = normal sinus rhythm; PAF = persistent atrial fibrillation; TIA = transient ischemic attack.
RESULTS
Acute outcomes of outpatient clinic-based ECV
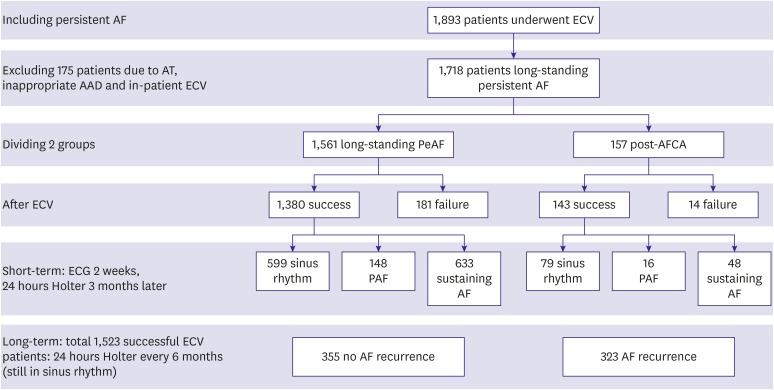
Among the 1,718 patients included in this study, outpatient clinic-based ECV successfully restored sinus rhythm in 1,523 (88.6%) and failed in 195 (11.4%, Figure 1). The required energy for successful ECV was 144.0±69.5 J. There were no significant differences in baseline characteristics between the successful ECV and failed ECV groups (Table 1). No parameters were independently associated with failed ECV in multivariate logistic regression analysis (Supplementary Table 2). The complication rate after outpatient clinic-based ECV was 0.47%: 3 patients experienced minor stroke or transient ischemic attack (TIA; 0.18%), and 5 patients had significant sinus node dysfunction requiring admission (0.29%, Table 2).
Figure 1. Flowchart of including, excluding and dividing PeAF patients into 2 groups: L-PeAF and post-AFCA.
AAD = antiarrhythmic drug; AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; AT = atrial tachycardia; ECG = electrocardiogram; ECV = elective external cardioversion; L-PeAF = long-standing persistent atrial fibrillation; PAF = paroxysmal atrial fibrillation; PeAF = persistent atrial fibrillation.
Short-term outcomes after successful ECV
Among 1,523 patients in whom outpatient clinic-based ECV was successful, 681 patients (44.7%) recurred with sustaining AF, 164 patients (10.8%) recurred with paroxysmal AF, and 678 (44.5%) remained in sinus rhythm within 3 months under AAD (Table 3). Among the patients who remained in sinus rhythm within 3 months of ECV, AF duration was shorter (p=0.003) and the proportions of post-AFCA state (p=0.014), amiodarone users (p<0.001), and statin users (p=0.009) were significantly higher than those with early AF recurrence (Table 3). A multivariate logistic regression analysis showed that the AF duration (odds ratio [OR], 1.01 [1.00–1.02]; p=0.006) and amiodarone (OR, 0.46 [0.27–0.76]; p=0.002) were independently associated with a short-term recurrence within 3 months (Table 4). Maintaining amiodarone was protective against early AF recurrence within 3 months of ECV (Log rank p<0.001, Figure 2A).
Table 4. Logistic regression analysis for early AF recurrence within 3 months of ECV.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 0.99 (0.98–1.00) | 0.051 | 0.99 (0.96–1.01) | 0.278 |
| Male | 1.02 (0.81–1.29) | 0.870 | 1.18 (0.66–2.12) | 0.577 |
| BMI (kg/m2) | 1.00 (0.99–1.01) | 0.609 | - | - |
| AF duration (months) | 1.01 (1.00–1.01) | 0.006 | 1.01 (1.00–1.02) | 0.006 |
| Post-AFCA | 0.62 (0.44–0.88) | 0.007 | 0.42 (0.14–1.21) | 0.107 |
| CHA2DS2-VASc | 0.98 (0.92–1.05) | 0.587 | - | - |
| Heart failure | 0.90 (0.67–1.22) | 0.509 | - | - |
| Hypertension | 0.90 (0.74–1.11) | 0.331 | - | - |
| Diabetes | 1.06 (0.81–1.39) | 0.651 | - | - |
| Stroke | 1.16 (0.85–1.60) | 0.339 | - | - |
| Vascular disease | 0.75 (0.47–1.20) | 0.227 | - | - |
| LA dimension (mm) | 1.01 (0.99–1.03) | 0.207 | - | - |
| Ejection fraction (%) | 1.00 (0.99–1.01) | 0.472 | - | - |
| E/Em | 0.98 (0.96–1.00) | 0.069 | - | - |
| Successful ECV energy (J) | 1.24 (1.13–1.37) | <0.001 | 1.26 (1.00–1.60) | 0.052 |
| Antiarrhythmic drugs | 1.42 (0.81–2.48) | 0.220 | - | - |
| Amiodarone | 0.60 (0.49–0.74) | <0.001 | 0.46 (0.27–0.76) | 0.002 |
| ACEi/ARB | 0.58 (0.45–0.75) | <0.001 | 0.88 (0.48–1.63) | 0.690 |
| b-blocker | 0.82 (0.65–1.02) | 0.073 | - | - |
| Statin | 0.67 (0.52–0.88) | 0.003 | 0.92 (0.52–1.64) | 0.777 |
ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ARB, angiotensin receptor blocker; BMI = body mass index; CI = confidence interval; E = early diastolic mitral inflow velocity; ECV = elective external cardioversion; Em = early diastolic mitral annular velocity; LA = left atrial; OR = odds ratio.
Figure 2. Kaplan-Meier curves for AF recurrence-free survival rate in short-term (above) and long-term (beneath) follow-up after successful ECV between groups L-PeAF, heart failure, amiodarone (red line) and post-AFCA, non heart failure, non-amiodarone (blue line).
AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ECV = elective external cardioversion; L-PeAF = long-standing persistent atrial fibrillation; PeAF = persistent atrial fibrillation.
Long-term outcomes after ECV
Table 5 summarizes the baseline characteristics according to long-term recurrence after 3 months of outpatient clinic-based ECV. Among 678 patients who maintained sinus rhythm for longer than 3 months, 323 patients (47.6%) experienced a recurrence of AF during a median of 32 (9–66) months of follow-up. The patients who remained in sinus rhythm had more heart failure (p<0.001) with lower ejection fraction (p=0.002) before ECV and comprised more β-blocker users (p=0.003) than those with long-term recurrence. Among 92 patients who experienced pre-ECV heart failure, the ejection fraction (49.4±12.5% to 56.2±11.8%, p<0.001) and left ventricular (LV) end-systolic dimension (39.9±7.4 mm to 37.9±5.8 mm, p=0.015) were significantly improved in follow-up echocardiogram at 23.2±25.1 months after ECV (Supplementary Table 3). In Cox regression analysis, baseline heart failure was independently associated with long-term AF recurrence after outpatient clinic-based ECV (hazard ratio, 0.63 [0.41–0.96]; p=0.033, Table 6). In Kaplan-Meier analysis, underlying hear failure was protective against long-term recurrence of AF after ECV (Log rank p=0.024, Figure 2B). However, amiodarone did not affect the long-term rhythm outcomes of ECV (Figure 2A).
Table 5. Patient characteristics for long-term AF recurrence after 3 months of ECV.
| Overall (n=678) | No AF recurrence (n=355) | AF recurrence (n=323) | p value | ||
|---|---|---|---|---|---|
| Age (years) | 61.9±11.4 | 62.3±11.4 | 61.4±11.4 | 0.327 | |
| Male | 503 (74.2) | 273 (76.9) | 230 (71.2) | 0.091 | |
| BMI (kg/m2) | 25.4±7.6 | 25.1±3.0 | 25.7±10.6 | 0.294 | |
| AF duration (months) | 28.8±35.6 | 15.0±18.4 | 29.1±35.9 | 0.582 | |
| L-PeAF | 599 (88.3) | 318 (53.1) | 281 (46.9) | 0.296 | |
| Post-AFCA | 79 (11.7) | 37 (46.8) | 42 (53.2) | 0.296 | |
| CHA2DS2-VASc | 2.0±1.6 | 2.1±1.7 | 1.9±1.5 | 0.068 | |
| Heart failure | 92 (13.6) | 67 (18.9) | 25 (7.7) | <0.001 | |
| Hypertension | 366 (54.0) | 197 (55.5) | 169 (52.3) | 0.408 | |
| Age >75 years | 82 (12.1) | 47 (13.2) | 35 (10.8) | 0.338 | |
| Age 65–74 years | 300 (44.2) | 173 (48.7) | 127 (39.3) | 0.014 | |
| Diabetes | 112 (16.5) | 58 (16.3) | 54 (16.7) | 0.894 | |
| Stroke | 75 (11.1) | 40 (11.3) | 35 (10.8) | 0.858 | |
| Vascular disease | 38 (5.6) | 23 (6.5) | 15 (4.6) | 0.300 | |
| eGFR (mL/min/1.73 m2) | 75.6±16.0 | 75.2±16.1 | 76.1±15.9 | 0.465 | |
| Hemoglobin (g/dL) | 14.8±3.5 | 14.7±2.0 | 14.9±4.6 | 0.438 | |
| Echocardiogram data | |||||
| LA dimension (mm) | 45.3±6.5 | 45.3±6.5 | 45.4±6.5 | 0.934 | |
| Ejection fraction (%) | 60.3±10.2 | 59.1±11.0 | 61.7±9.1 | 0.002 | |
| E/Em | 11.8±5.7 | 11.9±5.9 | 11.8±5.5 | 0.760 | |
| Successful ECV energy (J) | 118.0±42.6 | 116.6±41.9 | 119.6±43.3 | 0.361 | |
| Antiarrhythmic drugs | 651 (96.0) | 336 (94.6) | 315 (97.5) | 0.056 | |
| Amiodarone | 346 (51.0) | 178 (50.1) | 168 (52.0) | 0.927 | |
| Dronedarone | 45 (6.6) | 29 (8.2) | 16 (5.0) | 0.074 | |
| Sotalol | 24 (3.5) | 9 (2.5) | 15 (4.6) | 0.159 | |
| Flecainide | 193 (28.5) | 93 (26.2) | 100 (31.0) | 0.256 | |
| Propafenone | 37 (5.5) | 24 (6.8) | 13 (4.0) | 0.097 | |
| Pilsicainide | 6 (0.9) | 3 (0.8) | 3 (0.9) | 0.937 | |
| ACEi/ARB | 153 (22.6) | 87 (24.5) | 66 (20.4) | 0.205 | |
| b-blocker | 208 (30.7) | 127 (35.8) | 81 (25.1) | 0.003 | |
| Statin | 145 (21.4) | 80 (22.5) | 65 (20.1) | 0.444 | |
Values are presented as number (%) or mean±standard deviation.
ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ARB = angiotensin receptor blocker; BMI = body mass index; L-PeAF = long-standing persistent atrial fibrillation; E = early diastolic mitral inflow velocity; ECV = elective external cardioversion; eGFR = estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; Em = early diastolic mitral annular velocity; LA = left atrial.
Table 6. Cox regression analysis for long-term AF recurrence after 3 months of ECV.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 0.99 (0.98–1.00) | 0.030 | 0.99 (0.98–1.00) | 0.084 |
| Male | 0.80 (0.63–1.02) | 0.075 | - | - |
| BMI (kg/m2) | 1.00 (0.99–1.01) | 0.882 | - | - |
| AF duration (months) | 1.00 (1.00–1.00) | 0.885 | - | - |
| L-PeAF | 0.97 (0.70–1.34) | 0.829 | - | - |
| Post-AFCA | 1.04 (0.75–1.43) | 0.829 | - | - |
| CHA2DS2-VASc | 0.93 (0.86–1.00) | 0.039 | 1.03 (0.93–1.14) | 0.585 |
| Heart failure | 0.63 (0.42–0.95) | 0.028 | 0.60 (0.40–0.92) | 0.019 |
| Hypertension | 0.79 (0.63–0.98) | 0.030 | 0.79 (0.61–1.03) | 0.078 |
| Diabetes | 0.92 (0.68–1.23) | 0.553 | - | - |
| Stroke | 1.03 (0.72–1.46) | 0.877 | - | - |
| Vascular disease | 0.74 (0.44–1.24) | 0.251 | - | - |
| LA dimension (mm) | 1.00 (0.98–1.02) | 0.931 | - | - |
| Ejection fraction (%) | 1.01 (1.00–1.02) | 0.231 | - | - |
| E/Em | 0.98 (0.96–1.01) | 0.141 | - | - |
| Successful ECV energy (J) | 1.05 (0.95–1.16) | 0.359 | - | - |
| Antiarrhythmic drugs | 1.66 (0.82–3.34) | 0.160 | - | - |
| Amiodarone | 1.08 (0.86–1.35) | 0.508 | - | - |
| ACEi/ARB | 1.05 (0.80–1.37) | 0.752 | - | - |
| b-blocker | 0.87 (0.68–1.12) | 0.278 | - | - |
| Statin | 1.00 (0.76–1.31) | 0.996 | - | - |
AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; ACEi = angiotensinconverting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; CI = confidence interval; E = early diastolic mitral inflow velocity; ECV = elective external cardioversion; Em = early diastolic mitral annular velocity; L-PeAF = long-standing persistent atrial fibrillation; LA = left atrial; OR = odds ratio.
Although the early recurrence of AF was significantly higher in patients with a successful ECV with ≤150 J (Log rank p=0.003), the late recurrence rate did not differ between the ECV ≤150 J group and ECV >150 J group (Supplementary Figure 1). The early and late recurrence rates of AF did not significantly differ between the patients who underwent pre-ECV transesophageal echocardiography and those who did not (Supplementary Figure 1). The early and late recurrence rates of AF did not significantly differ between the patients with associated structural heart disease and those without (Supplementary Figure 1).
Long-standing PeAF versus post-AFCA recurrent AF
Among 1,718 patients who underwent outpatient clinic-based ECV, we compared 1,561 patients with long-standing PeAF and 157 patients with recurred AF after AFCA (Table 2). Although ECV success rates (p=0.313) or major complication rates (p=0.369) did not significantly differ, the energy level of successful ECV was significantly higher in patients with long-standing PeAF (146.2±69.1 J) than in those with a post-AFCA state (122.2±70.0 J, p<0.001). Early AF recurrence rate within 3 months of ECV was significantly higher in patients with long-standing PeAF (56.6%) than in those with post-AFCA (44.8%, p=0.007; Log rank p<0.001, Figure 2C). However, there was no significant difference in long-term AF recurrence after 3 months of ECV (p=0.296, Log rank p=0.501, Figure 2C).
DISCUSSION
In this single center prospective registry study, we investigated factors associated with acute success, early AF recurrence, and long-term recurrence after outpatient clinic-based ECV. This study has several characteristics. First, we included a relatively homogeneous group of patients who underwent ECV on an elective schedule in an outpatient clinic and excluded ECV cases in the emergency room or intensive care unit. Second, we performed protocol-based ECV with serial increases in ECV energy. Third, rhythm follow-up after ECV was monitored by Holter monitoring in accordance with the fixed period, and recurrence of asymptomatic paroxysmal AF could be found. After protocol-based ECV under AAD, the acute success rate was 88.6%, and the complication rate was 0.47%. Among the patients with a successful ECV, AF recurred in 55.5% within 3 months, and another 52.4% of the patients who maintained sinus rhythm for 3 months, experienced a recurrence of AF after a median follow-up period of 32 (9–66) months. Amiodarone was protective against short-term AF recurrence, and baseline heart failure was protective against long-term AF recurrence with significant improvement of LV function after ECV. We also found that successful ECV energy and early recurrence rate within 3 months were significantly lower in post-ablation patients than in those with long-standing PeAF.
ECV has been used as an effective rhythm control method for PeAF.9)11) However, it is known that the recurrence rate of AF is relatively high after the procedure.18) AF duration,11) LA size,18) comorbidity,18) method of cardioversion,9) and drug selection7) have been shown to affect the outcomes of ECV. An acute outcome reflects an assessment of the electrical intervention of ECV itself; however, the early recurrence of AF after restoration of sinus rhythm determines AAD responsiveness. In this study, the mean successful ECV energy was 144 J, utilizing biphasic shock and defibrillation patches. Therefore, it would be reasonable to start the ECV at 150 J in similar patients with AF. Although the ECV acute success rate was similar to previous studies,10)12) we could not find any independent risk factor for ECV failure.
A major concern of outpatient clinic-based ECV might be complication risk. However, ECV has been deemed as a low risk procedure even in the presence of significant heart disease in previous studies12),13) and in this study. In the present study, there was a complication rate of 0.47%: sinus node dysfunction requiring hospitalization was 0.29% and minor stroke/TIA comprised 0.18%. There were no complications associated with anesthesia. All patients underwent anticoagulation for more than 3 weeks before ECV. One of the patients who experienced peri-procedural stroke/TIA had a CHA2DS2-VASc score of 6, and 2 of them had score of 3 and a prior history of ischemic stroke. All 3 patients recovered neurologically.
Amiodarone is a mixed ion channel blocker with additional anti-adrenergic effects and significantly reduce the rate of recurrence of paroxysmal and PeAF.19),20) Because of a high rate of long-term adverse effects, amiodarone is generally reserved for patients with congestive heart failure or as a second-line AAD.14) However, rhythm control effects after restoring sinus rhythm were significantly superior with amiodarone than with sotalol or propafenone in patients with PeAF.19),20) In the SAFE-T trial,20) 1-year sinus rhythm maintenance rate was significantly higher in the amiodarone group than in the sotalol group (52% vs. 32%), although spontaneous sinus conversion rate and ECV success rate did not differ. In this study, amiodarone was superior to other AADs in regards to short-term rhythm outcomes within 3 months after ECV; however, there was no difference in ECV success rate or long-term rhythm outcomes.
The energy requirement for successful ECV was significantly lower in post-AFCA patients than in the long-standing PeAF patients. AFCA for AF has beneficial effects via multiple mechanisms,21) including isolation or abolition of trigger foci, in both PV and extra-PV sites,22) as well as modulation of autonomic innervation.23) Hwang et al.21) reported that wide circumferential PV isolation and linear ablations also reduce cardioversion threshold by reducing atrial critical mass. Although the short-term rhythm outcomes of ECV were better in patients with post-AFCA recurrence than in those with long-standing PeAF, acute success and long-term recurrence rates did not differ between the 2 groups, consistent with a previous report.
The presence of symptomatic systolic and diastolic LV dysfunction has been shown to have a negative impact upon ECV success and 30-day outcomes of cardioversion.7) LV dysfunction can lead to increases in atrial pressure and LV hypertrophy, facilitating AF maintenance or AF recurrence after ECV. However, better long-term success of ECV has been shown in patients with associated heart failure in this study with significant improvement of LV function in follow-up echocardiogram. For this reason, the majority of heart failure patients included in this study were estimated to have tachycardiomyopathy24) or AF induced cardiomyopathy.25) AF itself is a major risk factor of new-onset heart failure,26) and irregular ventricular activation alters myocardial gene expression, modulating calcium handling, cell function, and sympathetic activation.27) Therefore, long-term maintenance of sinus rhythm after successful ECV improved heart failure and reduced LA size, vice versa. It was reported that the absence of coronary artery disease, significant structural heart disease, or late gadolinium enhancement in cardiac magnetic resonance imaging predict improvement in heart failure after rhythm control of AF in patients with associated LV dysfunction.28),29)
This study was an observational cohort study from a single center that included highly selected patients referred for AF rhythm control. This study might have a selection bias for the baseline characteristics of the patients, such that our outcomes of outpatient clinic-based ECV cannot be generalized to all AF patients. The follow-up duration had a non-standard distribution in this study (32 [9–66] months). It was due to the very high chance of an early AF recurrence after the ECV, but a minority of the patients maintained sinus rhythm over a long follow-up period. We determined AF duration based on the first ECG documentation point because asymptomatic AF is common. The AAD dose was dependent on the physician's judgment rather than the strict protocol. Because we included the post-AFCA patient group, the study population was heterogeneous and could not represent the pure anti-arrhythmic drug effects. The ECG parameters are utilized in the prediction of the rhythm outcome after the ECV, but anti-arrhythmic drugs also remarkably affect the ECG parameters depending on their kind and dosage. Although we used the anterior-posterior placement for the electrode pads, the results of a pooled study analysis failed to identify any difference between the anterior-posterior and anterio-lateral pad placement in restoring sinus rhythm by the ECV. We performed protocol-based regular rhythm follow-up by Holter, the patients still have a high chance of subclinical paroxysmal AF between Holter evaluations.
In conclusions, the success rate of outpatient clinic-based ECV was 88.6% in this study, with a low complication rate, although 55.5% experienced recurrence of AF within 3 months. Amiodarone was protective against short-term AF recurrences, and long-term AF recurrences were less in patients with baseline heart failure and potential recovery of their ventricular function.
ACKNOWLEDGMENTS
We thank Mr. John Martin for his linguistic assistance.
Footnotes
Funding: This research was supported by grants (HI18C0070 and HI19C0114) from the Ministry of Health and Welfare and a grant (NRF-2017R1A2B4003983) from the Basic Science Research Program of the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT & Future Planning.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Pak HN.
- Data curation: Kim M, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, Pak HN.
- Formal analysis: Son NKL, Park JW, Yang SY.
- Supervision: Pak HN.
- Writing - original draft: Son NKL.
- Writing - review & editing: Pak HN.
SUPPLEMENTARY MATERIALS
Baseline characteristics according to the pre-ECV transesophageal echocardiography
Logistic regression analysis for acute failure of outpatient clinic-based ECV
Changes of echocardiographic parameters mean 23.2 months after ECV among the patients with baseline heart failure
Kaplan-Meier analyses for AF recurrence after ECV depending on the successful ECV energy, pre-ECV transesophageal echocardiography, and associated structural heart disease.
References
- 1.Kim D, Yang PS, Jang E, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. doi: 10.1016/j.ahj.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 3.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour M, Heist EK, Agarwal R, et al. Stroke and cardiovascular events after ablation or antiarrhythmic drugs for treatment of patients with atrial fibrillation. Am J Cardiol. 2018;121:1192–1199. doi: 10.1016/j.amjcard.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Jin MN, Kim TH, Kang KW, et al. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol. 2019;12:e007197. doi: 10.1161/CIRCEP.119.007197. [DOI] [PubMed] [Google Scholar]
- 6.Park JW, Yu HT, Kim TH, et al. Atrial fibrillation catheter ablation increases the left atrial pressure. Circ Arrhythm Electrophysiol. 2019;12:e007073. doi: 10.1161/CIRCEP.118.007073. [DOI] [PubMed] [Google Scholar]
- 7.Ecker V, Knoery C, Rushworth G, et al. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin Cardiol. 2018;41:862–870. doi: 10.1002/clc.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellman T, Kiviniemi T, Nuotio I, et al. Optimal timing for cardioversion in patients with atrial fibrillation. Clin Cardiol. 2018;41:966–971. doi: 10.1002/clc.22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joglar JA, Kowal RC. Electrical cardioversion of atrial fibrillation. Cardiol Clin. 2004;22:101–111. doi: 10.1016/s0733-8651(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 10.Frick M, Frykman V, Jensen-Urstad M, Ostergren J. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin Cardiol. 2001;24:238–244. doi: 10.1002/clc.4960240313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakazawa H, Lythall DA, Noh J, et al. Is there a place for the late cardioversion of atrial fibrillation? A long-term follow-up study of patients with post-thyrotoxic atrial fibrillation. Eur Heart J. 2000;21:327–333. doi: 10.1053/euhj.1999.1956. [DOI] [PubMed] [Google Scholar]
- 12.Botkin SB, Dhanekula LS, Olshansky B. Outpatient cardioversion of atrial arrhythmias: efficacy, safety, and costs. Am Heart J. 2003;145:233–238. doi: 10.1067/mhj.2003.112. [DOI] [PubMed] [Google Scholar]
- 13.Grönefeld G, Ehrlich JH, Hohnloser S. Comparison of outpatient vs inpatient direct current cardioversion of atrial fibrillation: Safety, efficacy and cost savings. Eur Heart J Suppl. 2003;5:H19–24. [Google Scholar]
- 14.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 16.Kim SS, Knight BP. Electrical and pharmacologic cardioversion for atrial fibrillation. Cardiol Clin. 2009;27:95–107. doi: 10.1016/j.ccl.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Pisters R, Nieuwlaat R, Prins MH, et al. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14:666–674. doi: 10.1093/europace/eur406. [DOI] [PubMed] [Google Scholar]
- 19.Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 20.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 21.Hwang ES, Nam GB, Joung B, et al. Significant reduction of atrial defibrillation threshold and inducibility by catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:1428–1435. doi: 10.1111/j.1540-8159.2012.03517.x. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Kurotobi T, Kimura R, et al. Trigger-based mechanism of the persistence of atrial fibrillation and its impact on the efficacy of catheter ablation. Circ Arrhythm Electrophysiol. 2012;5:295–301. doi: 10.1161/CIRCEP.111.964080. [DOI] [PubMed] [Google Scholar]
- 23.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinale FG, Tomita M, Zellner JL, Cook JC, Crawford FA, Zile MR. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am J Physiol. 1991;261:H308–18. doi: 10.1152/ajpheart.1991.261.2.H308. [DOI] [PubMed] [Google Scholar]
- 25.Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13:131–147. doi: 10.1038/nrcardio.2015.191. [DOI] [PubMed] [Google Scholar]
- 26.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 27.Ling LH, Khammy O, Byrne M, et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium: implications for the interaction between heart failure and atrial fibrillation. Circ Heart Fail. 2012;5:786–793. doi: 10.1161/CIRCHEARTFAILURE.112.968321. [DOI] [PubMed] [Google Scholar]
- 28.Ling LH, Taylor AJ, Ellims AH, et al. Sinus rhythm restores ventricular function in patients with cardiomyopathy and no late gadolinium enhancement on cardiac magnetic resonance imaging who undergo catheter ablation for atrial fibrillation. Heart Rhythm. 2013;10:1334–1339. doi: 10.1016/j.hrthm.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial) Circ Arrhythm Electrophysiol. 2014;7:31–38. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics according to the pre-ECV transesophageal echocardiography
Logistic regression analysis for acute failure of outpatient clinic-based ECV
Changes of echocardiographic parameters mean 23.2 months after ECV among the patients with baseline heart failure
Kaplan-Meier analyses for AF recurrence after ECV depending on the successful ECV energy, pre-ECV transesophageal echocardiography, and associated structural heart disease.