Abstract
Chaga mushrooms are widely used in folk remedies and in alternative medicine. Contrary to many beneficial effects, its adverse effect is rarely reported. We here report a case of end-stage renal disease after long-term taking Chaga mushroom. A 49-year-old Korean man with end stage renal disease (ESRD) was transferred to our hospital. Review of kidney biopsy finding was consistent with chronic tubulointerstitial nephritis with oxalate crystal deposits and drug history revealed long-term exposure to Chaga mushroom powder due to intractable atopic dermatitis. We suspected the association between Chaga mushroom and oxalate nephropathy, and measured the oxalate content of remained Chaga mushroom. The Chaga mushroom had extremely high oxalate content (14.2/100 g). Estimated daily oxalate intake of our case was 2 times for four years and 5 times for one year higher than that of usual diet. Chaga mushroom is a potential risk factor of chronic kidney disease considering high oxalate content. Nephrologist should consider oxalate nephropathy in ESRD patients exposed to Chaga mushrooms.
Keywords: Chaga Mushroom, End Stage Renal Disease, Oxalate Nephropathy
Graphical Abstract
INTRODUCTION
Chaga mushroom is widely used in folk remedies and have been used in alternative medicine. The growing popularity of Chaga mushroom-based products is one of the major trends seen in the global healthcare supplement market.
Oxalate nephropathy is caused by deposition of oxalate crystals that induce renal epithelial cell injury and inflammation in the kidney.1 Oxalate nephropathy can be seen in genetic causes known as primary hyperoxaluria or in clinical settings such as enteric hyperoxaluria, ethylene glycol poisoning and excessive dietary intake of oxalate or ascorbic acid.2,3
To the contrary to well-known effects, Chaga mushroom-related side effect is rarely reported. Until now only one case of Chaga mushroom-induced oxalate nephropathy was reported in Japan.1 We here report the second case worldwide and discussed Chaga mushroom-induced oxalate nephropathy as a risk factor of chronic kidney disease.
CASE DESCRIPTION
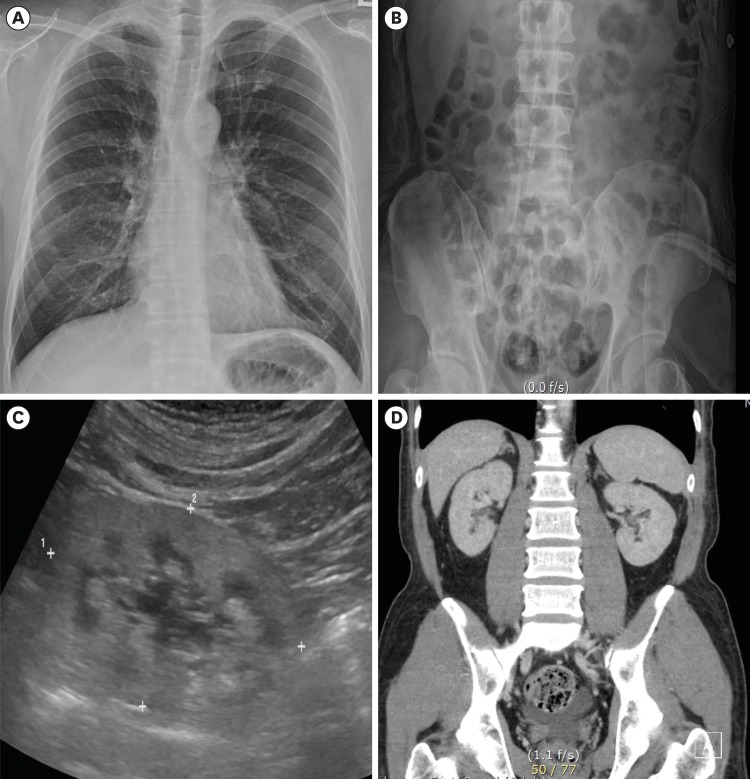
On April 6, 2018, a 49-year-old Korean man with end stage renal disease (ESRD) was transferred to our hospital for kidney transplantation. He visited emergency room at local hospital due to nausea and vomiting one month ago. Laboratory findings revealed renal failure (blood urea nitrogen 137 mg/dL, serum creatinine 20.5 mg/dL) and anemia (hemoglobin 9.1 g/dL) and metabolic acidosis on arterial blood gas analysis (pH 7.3, bicarbonate 16.4 mEq/L). Routine urinalysis revealed no proteinuria or hematuria. Complement levels were normal range and autoantibodies (Antinuclear antibody and antineutrophil cytoplasmic antibody) were negative. Chest PA showed no cardiomegaly or pulmonary edema, and Kidney-Ureter-Bladder radiography (KUB) was non-specific. Kidney ultrasonography revealed diffusely increased echogenicity and kidney size was smaller than normal (8.2 × 5.0 cm). The abdominal computed tomography showed no evidence of stone in both kidneys (Fig. 1).
Fig. 1. Radiologic evaluation. (A) Chest posterioanterior view and (B) Kidney-Ureter-Bladder radiography show no evidence of vascular calcification or kidney stone. (C) Abdominal sonography revealed increased echogenicity and decreased kidney size. (D) The abdominal computed tomography shows no evidence of kidney stone or vascular calcification. The images are published under agreement of the patient.
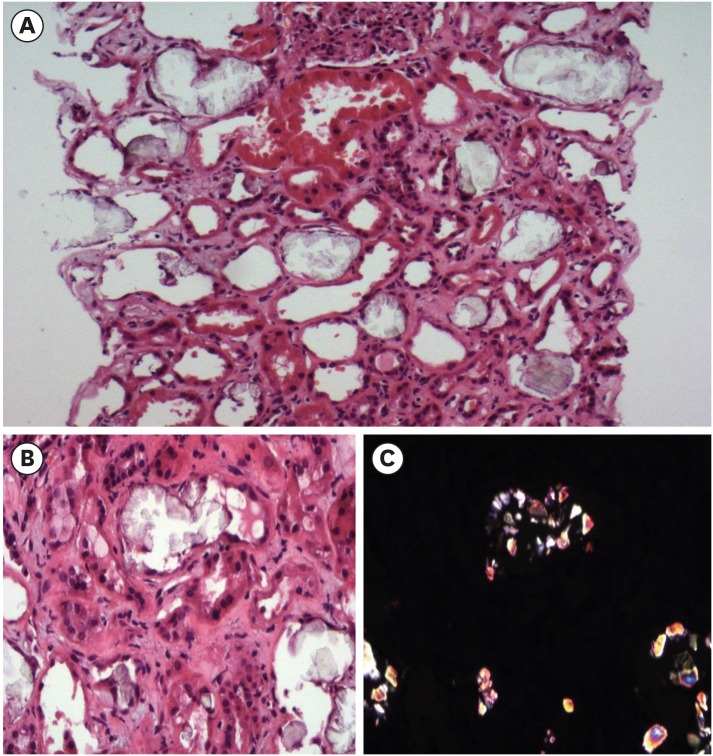
Due to uremic symptoms, emergency hemodialysis was started and the kidney biopsy was performed to identify the cause of ESRD. The kidney biopsy showed foci of tubular damage with deposition of translucent crystals of different shapes which are predominantly intraluminal, and confirmed these crystals as calcium oxalate crystals using polarized light (Fig. 2). Final report of pathology was chronic tubulointerstitial nephritis with oxalate crystal deposits and a change of ESRD.
Fig. 2. Histopathologic evaluation. (A) Tubules reveal focal severe necrosis with crystal deposits and denudation of tubular epithelial cells. H&E stain (×20). (B) Aggregates of crystals attached to tubular basement membrane are shown with denudation of tubular epithelial cells. H&E stain (× 40). (C) The same image as Fig. 2B observed under the polarizing microscope.
H&E = haemotoxylin and eosin.
He was an office worker. He received regular national health examination including renal function and urinalysis, and both results were normal until two years before admission. His height and body weight were 167 cm and 70 kg. Body mass index was 25 kg/m2. Review of medical history revealed no history of kidney stone, diabetes, hypertension and operation. He had no medical family history. From the drug history, he had taken Chaga mushroom powder for 5 years due to atopic dermatitis. Initially, he had taken 3 g daily for 4 years following company's recommendation and 9 g of powder for another year because his symptom did not improve.
Based on pathologic finding (oxalate nephropathy) and drug history (long-term exposure to Chaga mushroom), we suspected the association between Chaga mushroom and oxalate nephropathy in our case. Thus, we requested oxalate content of remained Chaga mushroom to researcher/clinical pharmacology division of Catholic Medical center clinical research coordinating center of Korea. Measurement of oxalate was performed by high performance liquid chromatography (HPLC), and analysis revealed extremely high content of oxalate in Chaga mushroom powder (0.14 g of oxalate per 1 g of Chaga mushroom powder).
We finally diagnosed this case as Chaga mushroom-induced oxalate nephropathy with kidney biopsy findings, drug history and high content of oxalate. Clinical course of our case was unfavorable. Renal function has not recovered and he is on maintenance hemodialysis for 18 months.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Catholic University of Korea (IRB No. 2019-3713-0001) and an informed consent was received from the patient and a caregiver. The images are published under agreement of the patient.
DISCUSSION
Online search with key words “Chaga mushroom and oxalate” on PubMed shows only one report which describes a case of ESRD after ingestion of Chaga mushroom in 2014.1 Five years later, we report the second case of Chaga mushroom-induced oxalate nephropathy worldwide. Two cases are Asian (Japan and Korea) with intractable diseases (hepatocellular carcinoma or atopic dermatitis). Thus, one may think that its occurrence is limited to the Asian countries or specific diseases. Indeed, the incidence of oxalate nephropathy can vary from race to race. Previous reports have suggested that there is a racial difference between Black and Caucasian in the handling of dietary oxalate in body4,5 and this may be responsible for the difference in incidence of nephrolithiasis and oxalate nephropathy between two populations. However, there are few studies which compared oxalate metabolism between Asian and Caucasian. Chaga mushroom is so popular in healthy people and their sales are growing rapidly in global health food markets (America [49%] > Europe > Asia pacific]. Thus, the occurrence of Chaga mushroom-induced oxalate nephropathy may be underestimated, and more cases may be reported if clinicians pay attention to the association between Chaga mushroom and oxalate nephropathy.
Review of this case raised several important issues in clinical practice. First, in general, renal biopsy is not recommended to ESRD patient with contracted kidney. Thus, our case may be diagnosed as ESRD with unknown etiology if renal biopsy was not performed. Second, history taking about health foods is often overlooked. We carefully reviewed drug history and found that a patient took Chaga mushroom for long time. Third, measurement of oxalate content in Chaga mushroom powder is not available in clinical practice because of technical difficulties and high costs for measuring the content of oxalate. Indeed, in our case, measurement of oxalate was performed at public pharmacological laboratory. Taken together, we diagnosed this case as Chaga mushroom-induced oxalate nephropathy, with renal biopsy, health food history, and measurement of oxalate content.
It has been reported that the lethal dose of soluble oxalate for humans is from 2 to 30 g daily,6 and the blood levels of oxalate are further increased if oxalate-containing food is ingested without boiling or brewing. Generally, typical diets contain 200 to 300 mg of oxalate daily.7 For stone prevention, a reasonable goal is below 100 mg of oxalate daily and an ideal would be about 50 mg daily. In the case presented here, he was recommended to take 3 g Chaga mushroom per day and increased to 9 g per day because his symptom did not improve, and estimated oxalate amount was from 420 mg to 1,260 mg of oxalate per day. As a result, he was exposed to concentrations two to five times higher than the oxalate dose normally consumed by typical diets. Based on the Chaga mushroom-induced oxalate nephropathy case reports reported to date, strict guidelines for Chaga mushroom dosage based on oxalate contents are need.
It is worth noting oxalate content of the manufactured Chaga mushroom powder. The oxalate content in our case was 14.2 g per 100 g, and oxalate content of other two companies was 2.8 g or 11.2 g per 100 g, respectively. This finding demonstrates that commercial Chaga mushrooms powder has different oxalate content according to company (2.8 to 14.2 g per 100g and its content was higher than that of typical diets). According to several studies reporting on the chemical composition of Chaga mushroom, Glamoclija et al.8 identified that oxalate was the only organic acid detected in the extracts from Chaga mushroom (6.72–97.59 mg/g extract), and Shashkina et al.9 reported that oxalate was one of organic acids in the extracts from Chaga mushroom. Therefore, Chaga mushroom is expected to contain high concentrations of oxalate. Thus, any product of Chaga mushroom powder has a potential risk of oxalate nephropathy, and it is strongly recommended to establish the oxalate content of chemical composition in Chaga mushroom powder formulations.
Oxalate nephropathy is observed at various stages from acute renal failure to ESRD, and the clinical phenotype of oxalate nephropathy may be depending on ingested oxalate amount and exposure duration. The review of literature revealed that acute renal failure were developed within hours after ingestion of star fruit juice (oxalate dose, 9.2 g and 13.1 g per day)10 and chronic kidney disease (serum creatinine 1.7 mg/dL) was developed after daily excessive peanut intake (oxalate dose, 116-232 mg per day) for 2 or 3 months.3 The oxalate amount for determining “acute or chronic” is still undetermined, but our case was exposed to moderate high oxalate (420-1,260 mg per day) for long-term period (five years), and this may explain development of ESRD.
Clinicians can suspect several causes of oxalate nephropathy such as vitamin C, enteric hyperoxaluria, history of gastrointestinal tract operation, systemic sclerosis, cystic fibrosis or primary hyperoxaluria if oxalate crystals were incidentally observed in a kidney-biopsy specimen and the next step is evaluation for the secondary oxalate nephropathy.2 First, according to the medical history taking, the patient had a normal dietary habit and there was no dietary supplement such as vitamin C. Second, enteric hyperoxaluria (state of enhanced absorption of oxalate from the gastrointestinal tract) can be excluded by normal findings of intestines and pancreas on abdominal image examination and normal bowel habits. According to the history taking, the patient had no surgical history, including any gastrointestinal tract operations. Third, systemic sclerosis is one of the causes of oxalate nephropathy.2 But our case did not meet the diagnostic criteria of systemic sclerosis, such as skin thickening, abnormal nail fold capillaries and fingertip pitting scars or digital tip ulcers.11 Forth, cystic fibrosis is one of the causes of oxalate nephropathy.2 In our case, cystic fibrosis could be excluded due to lack of clinical manifestations (history of recurrent respiratory infection and characteristic findings on radiologic examination) and family history. Last, primary hyperoxaluria should be considered the cause of oxalate nephropathy. The autosomal recessive inherited primary hyperoxaluria types I, II, and III are caused by defects in glyoxylate metabolism.12 Recurrent urolithiasis and nephrocalcinosis are the most common findings of these diseases. Our patient had no medical history of recurrent urolithiasis and family history. Therefore, we could exclude primary hyperoxaluria as the cause of oxalate nephropathy.
In conclusion, we report the patient with development of ESRD due to Chaga mushroom-induced oxalate nephropathy, and propose Chaga mushroom as an emerging risk factor of chronic kidney disease. Nephrologist should pay attention to unknown chronic kidney disease patients exposed to Chaga mushroom.
Footnotes
Funding: This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (2018M3A9E802151).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Data curation: Lee HY.
- Investigation: Lee HY.
- Supervision: Yang CW.
- Validation: Lee S.
- Writing - original draft: Lee HY, Lee S, Yang CW.
- Writing - review & editing: Lee S, Park YH, Ko EJ, Ban TH, Chung BH, Lee HS, Yang CW.
References
- 1.Kikuchi Y, Seta K, Ogawa Y, Takayama T, Nagata M, Taguchi T, et al. Chaga mushroom-induced oxalate nephropathy. Clin Nephrol. 2014;81(6):440–444. doi: 10.5414/CN107655. [DOI] [PubMed] [Google Scholar]
- 2.Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary Oxalate Nephropathy: A Systematic Review. Kidney Int Rep. 2018;3(6):1363–1372. doi: 10.1016/j.ekir.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki M, Murakami M, Matsuo K, Matsuo Y, Tanaka S, Ono T, et al. Oxalate nephropathy with a granulomatous lesion due to excessive intake of peanuts. Clin Exp Nephrol. 2008;12(4):305–308. doi: 10.1007/s10157-008-0046-5. [DOI] [PubMed] [Google Scholar]
- 4.Lewandowski S, Rodgers A, Schloss I. The influence of a high-oxalate/low-calcium diet on calcium oxalate renal stone risk factors in non-stone-forming black and white South African subjects. BJU Int. 2001;87(4):307–311. doi: 10.1046/j.1464-410x.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 5.Lewandowski S, Rodgers AL, Laube N, von Unruh G, Zimmermann D, Hesse A. Oxalate and its handling in a low stone risk vs a stone-prone population group. World J Urol. 2005;23(5):330–333. doi: 10.1007/s00345-005-0030-6. [DOI] [PubMed] [Google Scholar]
- 6.Beier RC. Natural pesticides and bioactive components in foods. Rev Environ Contam Toxicol. 1990;113:47–137. doi: 10.1007/978-1-4612-3366-4_2. [DOI] [PubMed] [Google Scholar]
- 7.Glew RH, Sun Y, Horowitz BL, Konstantinov KN, Barry M, Fair JR, et al. Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease. World J Nephrol. 2014;3(4):122–142. doi: 10.5527/wjn.v3.i4.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glamočlija J, Ćirić A, Nikolić M, Fernandes Â, Barros L, Calhelha RC, et al. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J Ethnopharmacol. 2015;162:323–332. doi: 10.1016/j.jep.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 9.Shashkina MY, Shashkin PN, Sergeev AV. Chemical and medicobiological properties of chaga. Pharm Chem J. 2006;40(10):560–568. [review] [Google Scholar]
- 10.Chen CL, Fang HC, Chou KJ, Wang JS, Chung HM. Acute oxalate nephropathy after ingestion of star fruit. Am J Kidney Dis. 2001;37(2):418–422. doi: 10.1053/ajkd.2001.21333. [DOI] [PubMed] [Google Scholar]
- 11.Asano Y, Jinnin M, Kawaguchi Y, Kuwana M, Goto D, Sato S, et al. Diagnostic criteria, severity classification and guidelines of systemic sclerosis. J Dermatol. 2018;45(6):633–691. doi: 10.1111/1346-8138.14162. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8(8):467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]