Abstract
Background/Aims
Studies on long-term outcomes of adalimumab therapy in non-Caucasian patients with ulcerative colitis (UC) are lacking.
Methods
We analyzed long-term outcomes of Korean UC patients treated with adalimumab at the Asan Medical Center, Seoul, Korea.
Results
Between July 2013 and October 2018, adalimumab therapy was started in a total of 100 patients with UC (65 males [65.0%]; median age, 39.5 years [interquartile range, 23.3 to 49.8 years]; and median disease duration, 3.0 years [interquartile range, 1.0 to 7.0 years]). The median duration of adalimumab therapy was 13.5 months (interquartile range, 4.0 to 32.0 months). Eight of 100 patients (8.0%) received induction therapy only, four (4.0%) of whom ultimately underwent colectomy. Of 92 patients who received adalimumab maintenance therapy, 30 (30.0%) stopped adalimumab therapy due to loss of response, and one patient (1.0%) was lost to follow-up. Among the 92 patients who received adalimumab maintenance therapy, the cumulative proportions of patients remaining on adalimumab maintenance therapy were 70.0% at 1 year and 48.9% at 5 years. High partial Mayo score after 8 weeks of adalimumab therapy (hazard ratio [HR], 1.217; 95% confidence interval [CI], 1.040 to 1.425; p=0.014) and a history of exposure to two biologic agents before adalimumab therapy (HR, 4.722; CI, 1.033 to 21.586; p=0.045) were predictors of adalimumab discontinuation.
Conclusions
Long-term outcomes of adalimumab therapy in Korean UC patients appear to be comparable to those in previously published Western studies. Furthermore, previous exposure to multiple biologic agents before adalimumab therapy and disease activity after 8 weeks of adalimumab therapy were predictors of adalimumab discontinuation.
Keywords: Colitis, ulcerative; Adalimumab; Tumor necrosis factor-alpha; Prognosis
INTRODUCTION
The introduction of biologic agents, such as anti-tumor necrosis factor (anti-TNF) agents, has greatly changed treatment paradigm of patients with inflammatory bowel disease (IBD).1 According to a large-scaled retrospective cohort study on Korean ulcerative colitis (UC) patients, immunomodulators and anti-TNF agents have become used more frequently, and from earlier time points during the disease course than before.2 At the same time, cumulative rates of colectomy have decreased over the last three decades.2
In the ulcerative colitis long-term remission and maintenance with adalimumab-1, 2 (ULTRA-1,2) trials, adalimumab, a human monoclonal antibody against TNF-α, has been proven to be efficacious in inducing remission at week 8 and maintaining remission at week 54 in patients with moderate to severe UC.3-5 It was also reported to reduce risks of all-cause, UC-related, and UC- or drug-related hospitalizations as well.6 Furthermore, adalimumab is self-injectable by patients and it was also reported to be safe and efficacious in patients who lost response or are intolerant to infliximab.7
Although efficacies of adalimumab in Asian UC patients were evaluated in a previous placebo-controlled trial from Japan,8,9 studies on real-life long-term outcomes of adalimumab therapy in Asian UC patients are still lacking. Therefore, the aim of this study was to assess long-term clinical outcomes of adalimumab therapy in a real-life cohort of Korean UC patients and to investigate the predictors affecting the outcomes.
MATERIALS AND METHODS
1. Study population
This was a retrospective observational study of a single-center cohort at the IBD center of Asan Medical Center, a tertiary referral center in Seoul, Republic of Korea. Characteristics of the UC patient cohort of Asan Medical Center and patients registry were previously described.2 Patients were included if they were definitely diagnosed with UC and started to receive adalimumab therapy at the Asan Medical Center from July 2013 to October 2018. A definite diagnosis with UC was based on patients’ clinical features, laboratory, endoscopic, radiologic and histologic findings.10 Patients were excluded if they were <18 years old or adalimumab therapy was indicated for other diseases, such as ankylosing spondylitis. All study subjects were previously exposed to immunomodulators or systemic corticosteroids, but failed to at least one out of those two medications before starting adalimumab therapy.
2. Data collection
Medical data of all UC patients were prospectively collected in our IBD patient registry and reviewed retrospectively at the time of the study.2 Data on demographic characteristics (sex, age, smoking status at diagnosis and family history of IBD in the first-degree relatives), disease characteristics (UC duration and extent at starting adalimumab therapy), therapies (previous exposure to other biologic agents at starting adalimumab therapy, concomitant immunomodulators use for more than 3 months during initial 6 months of adalimumab therapy), a previous history of appendectomy before starting adalimumab therapy, a history of cytomegalovirus colitis within 3 months before, or during adalimumab therapy, and laboratory findings at starting adalimumab therapy were collected. Cytomegalovirus colitis was defined as one or more inclusion bodies detected by hematoxylin and eosin staining and/or immunohistochemistry in accordance with the current European consensus.11-15
Primary outcome was set as adalimumab discontinuation due to loss of response to adalimumab and/or a colectomy. The rate of dose intensification (DI) was also evaluated. The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2017-0402).
3. Adalimumab administration
All patients received subcutaneous injections of adalimumab at a dose of 160 mg at week 0 and 80 mg at week 2 for induction therapy. Subsequently, depending on their clinical response, patients administered adalimumab at a dose of 40 mg every 2 weeks as maintenance therapy from week 4. Based on the reimbursement policy of the Health Insurance Review and Assessment Service of Korea, clinical response to induction therapy was assessed after adalimumab dosing at week 8. In cases of decreased response during maintenance therapy, DI of adalimumab up to weekly administration was permitted.
4. Statistical analysis
Demographic and baseline characteristics were summarized using descriptive statistics. Categorical or nominal variables were described as numbers (%) and continuous variables as medians (interquartile range [IQR]). The Kaplan-Meier analysis was used to evaluate cumulative proportions of patients remaining on adalimumab maintenance therapy. The Cox proportional hazard regression analysis was performed to find predictors associated with adalimumab discontinuation. Variables with a p<0.1 by univariate analysis were included in the multivariate model. Statistical significance was defined as a p-value <0.05. All statistical analyses were performed using SPSS statistics version 23.0 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
1. Baseline characteristics
Between July 2013 and October 2018, adalimumab therapy was commenced on in a total of 100 UC patients at our center. Sixty-five patients (65.0%) were males and median age at diagnosis of UC was 39.5 years (IQR, 23.3 to 49.8). Median disease duration at starting adalimumab therapy was 3.0 years (IQR, 1.0 to 7.0). Forty-two patients (42.0%) were previously treated with one anti-TNF agent (37 [37.0%] with infliximab/infliximab biosimilar and 5 [5.0%] with golimumab) and two patients (2.0%) were previously treated with two biologic agents (one with infliximab and golimumab; and the other with infliximab and vedolizumab). Sixty-eight patients (68.0%) were treated with concomitant immunomodulators for more than 3 months during initial 6 months of adalimumab therapy. At starting adalimumab, extensive colitis was observed in 76% (Table 1). Median duration of adalimumab therapy for 100 patients was 13.5 months (IQR, 4.0 to 32.0).
Table 1.
Baseline Characteristics of the 100 Korean Patients with UC Who Received Adalimumab Therapy
| Variable | Value |
|---|---|
| Male sex | 65 (65.0) |
| Age at diagnosis, yr | 39.5 (23.3–49.8) |
| Duration of disease before starting adalimumab therapy, yr | 3.0 (1.0–7.0) |
| Smoking status at diagnosis of UC | |
| Never smoker | 54 (54.0) |
| Ex-smoker | 29 (29.0) |
| Current smoker | 17 (17.0) |
| Family history of inflammatory bowel disease | 7 (7.0) |
| History of appendectomy before starting adalimumab therapy | 3 (3.0) |
| p-ANCA | |
| Negative | 46 (46.0) |
| Positive | 54 (54.0) |
| ASCA IgA and IgG | |
| Both negative | 55 (55.0) |
| Any one positive | 24 (24.0) |
| Any one equivocal | 11 (11.0) |
| Not checked | 10 (10.0) |
| No. of biologic agents exposed before adalimumab therapy | |
| Biologic-naïve | 56 (56.0) |
| Exposed to 1 biologic agent | 42 (42.0) |
| Infliximab | 33 (33.0) |
| Infliximab biosimilar | 4 (4.0) |
| Golimumab | 5 (5.0) |
| Exposed to 2 biologic agents* | 2 (2.0) |
| Immunomodulator therapy for more than 3 mo during initial 6 mo of adalimumab therapy | 68 (68.0) |
| Thiopurines | 60 (60.0) |
| Methotrexate | 8 (8.0) |
| Disease extent at starting adalimumab therapy | |
| Proctitis | 0 |
| Left-sided colitis | 24 (24.0) |
| Extensive colitis | 76 (76.0) |
| Mayo endoscopic subscore at starting adalimumab therapy | |
| 0 | 0 |
| 1 | 0 |
| 2 | 22 (22.0) |
| 3 | 78 (78.0) |
| Partial Mayo score at starting adalimumab therapy | 6.0 (5.0–7.0) |
| Leukocyte count at starting adalimumab therapy, 103/μL | 7.8 (5.6–9.9) |
| Hemoglobin at starting adalimumab therapy, g/dL | |
| Male | 13.0 (10.5–14.4) |
| Female | 11.2 (9.6–12.2) |
| Platelet count at starting adalimumab therapy, 103/μL | 318.0 (269.5–402.8) |
| ESR at starting adalimumab therapy, mm/hr | |
| Male | 26.0 (13.0–43.0) |
| Female | 30.0 (14.0–43.0) |
| Serum CRP at starting adalimumab therapy, mg/dL | 0.7 (0.2–1.8) |
| Serum albumin at starting adalimumab therapy, g/dL | 3.6 (2.9–3.8) |
Data are presented as number (%) or median (interquartile range).
UC, ulcerative colitis, p-ANCA, perinuclear antineutrophil cytoplasmic antibody; ASCA, anti-Saccharomyces cerevisiae antibody; Ig, immunoglubulin; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
One patient was treated with infliximab and golimumab, and the other was treated with infliximab and vedolizumab before adalimumab therapy.
2. Disease activity at week 0 and week 8
At starting adalimumab therapy, most of the patients (n=78, 78.0%) were having severe endoscopic activity (Mayo endoscopic subscore 3) and the rest of the patients were having moderate endoscopic activity (Mayo endoscopic subscore 2). Median partial Mayo score at starting adalimumab therapy was 6.0 (IQR, 5.0 to 7.0) (Table 1). Out of 92 patients in whom results of endoscopic evaluation after 8 weeks of adalimumab therapy were available, 27 (29.4%) and 21 (22.8%) showed severe (Mayo endoscopic subscore 3) and moderate (Mayo endoscopic subscore 2) endoscopic activities, respectively, while 44 (47.8%) showed remission or mild endoscopic activity (Mayo endoscopic subscore 0–1). After 8 weeks of adalimumab therapy, median partial Mayo score decreased by 3.0 (median value at week 8, 3.0; IQR, 1.0 to 4.0).
3. Outcomes in patients who received adalimumab therapy for less than 8 weeks
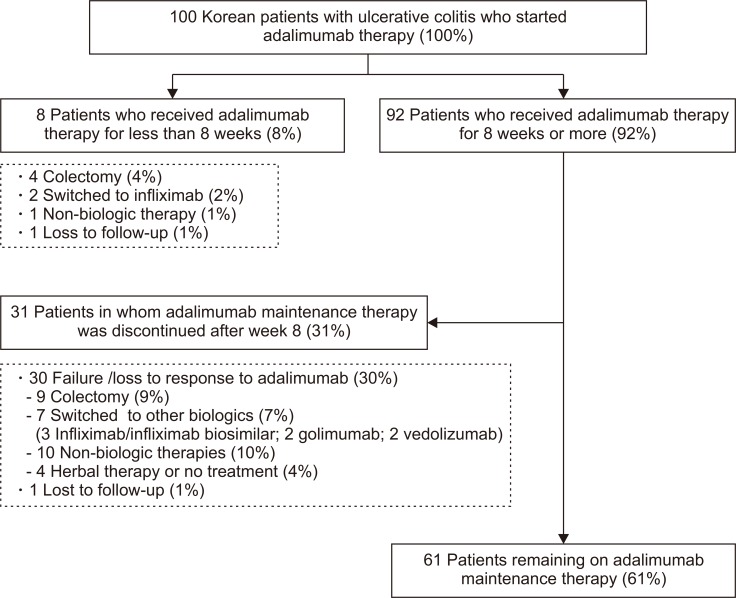
Eight out of 100 (8.0%) patients received adalimumab therapy for less than 8 weeks. Out of eight patients, four (4.0%) underwent colectomy and two (2.0%) switched to infliximab. One patient (1.0%) discontinued adalimumab after the first administration due to development of immune thrombocytopenic purpura and remained on non-biologic therapeutics (5-aminosalicylates and thiopurines) with quiescent disease. The last one (1.0%) was lost to follow-up with active disease (Fig. 1).
Fig. 1.
Flow diagram of 100 Korean patients with ulcerative colitis who received adalimumab therapy.
4. Outcomes in patients who received adalimumab therapy for 8 weeks or more
Ninety-two out of 100 patients (92.0%) received adalimumab therapy for 8 weeks or more, and median duration of adalimumab therapy among 92 patients was 17.0 months (IQR, 6.0 to 36.0). Thirty-one patients (33.7%) stopped adalimumab maintenance therapy and 61 patients (66.3%) were still remained on adalimumab therapy till the last follow-up (Table 2, Fig. 1).
Table 2.
Outcomes of the 92 Korean Patients with Ulcerative Colitis Who Received Adalimumab Therapy for 8 or More Weeks
| Variable | Value |
|---|---|
| Median duration of adalimumab therapy, mo | 17.0 (6.0–36.0) |
| Discontinuation of adalimumab during maintenance therapy | 30 (32.6) |
| Colectomy | 9 (9.8) |
| Switched to other biologic agents | |
| Infliximab/infliximab biosimilar | 3 (3.3) |
| Golimumab | 2 (2.2) |
| Vedolizumab | 2 (2.2) |
| Non-biologic therapies (5-aminosalicylates, immunomodulators, or corticosteroids) | 10 (10.9) |
| Herbal therapy | 2 (2.2) |
| No treatment | 2 (2.2) |
| Loss to follow-up | 1 (1.1) |
| DI of adalimumab | 37 (40.2) |
| Median interval from starting adalimumab to DI, mo | 6.5 (3.3–13.6) |
| Steroid rescue therapy during adalimumab therapy | 32 (34.8) |
| Median interval from starting adalimumab to steroid rescue therapy, mo | 3.5 (2.0–18.3) |
| Colectomy | 9 (9.8) |
| Median interval from starting adalimumab to colectomy, mo | 8.0 (5.0–11.0) |
| History of cytomegalovirus colitis within 3 mo before, or during adalimumab therapy | 26 (26.0) |
Data are presented as median (interquartile range) or number (%).
DI, dose intensification.
Among 31 patients who stopped adalimumab during maintenance therapy, nine (9.8%) underwent colectomy. Seven (7.7%) switched to other biologic agents (three [3.3%] to infliximab/infliximab biosimilar, two [2.2%] to golimumab and two [2.2%] to vedolizumab) and 10 patients (10.9%) remained on non-biologic therapeutics (5-aminosalicylates and thiopurines). Two (2.2%) decided to receive herbal therapy and two (2.2%) did not receive any other treatment. The last one (1.1%) was lost to follow-up (Table 2).
Among 92 patients with adalimumab maintenance therapy, DI was required in 37 patients (40.2%), 6.5 months (IQR, 3.3 to 13.6) after starting adalimumab therapy. The rate of DI was calculated as 32.2% per person-year. Thirty-two (34.8%) required corticosteroid rescue therapy, median 3.5 months (IQR, 2.0 to 18.3) after starting adalimumab therapy (Table 2). In 20 patients, both of DI of adalimumab and corticosteroid rescue therapy were done, and corticosteroid rescue therapy preceded DI.
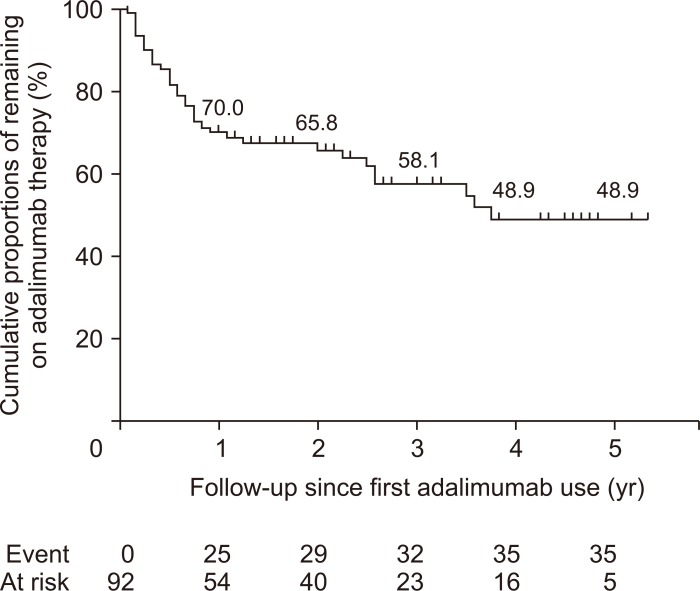
Cumulative proportions of remaining on adalimumab maintenance therapy was 70.0%, 65.8%, 58.1% and 48.9% at 1, 2, 3 and 5 years among 92 patients who received adalimumab therapy for 8 weeks or more (Fig. 2). When calculated among 100 patients who commenced adalimumab therapy, cumulative proportions of remaining on adalimumab maintenance therapy was 64.6%, 59.3%, 53.8% and 45.5% at 1, 2, 3 and 5 years, respectively.
Fig. 2.
Cumulative proportions of patients remaining on adalimumab therapy among 92 patients who received adalimumab therapy for 8 or more weeks.
5. Predictors of adalimumab discontinuation during maintenance therapy
Among all variables we assessed with the Cox proportional hazard regression analysis, disease duration before starting adalimumab therapy, previous smoking at diagnosis of UC, elevated ESR, serum albumin level at the start of adalimumab therapy, partial Mayo score after 8 weeks of adalimumab therapy, previous exposure to two biologic agents and history of cytomegalovirus colitis within 3 months of, or during adalimumab therapy were included into multivariate analysis with p<0.1 in univariate analysis. Finally, partial Mayo score after 8 weeks of adalimumab therapy (hazard ratio [HR], 1.217; 95% confidence interval [CI], 1.040 to 1.425; p=0.014) and previous exposure to two biologic agents (HR, 4.722; CI, 1.033 to 21.586; p=0.045) turned out to be associated with adalimumab discontinuation during maintenance therapy (Table 3).
Table 3.
Cox Regression Analysis: Predictors of Adalimumab Discontinuation in 92 Korean Patients with UC
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95 CI) | p-value | HR (95 CI) | p-value | |
| Male sex | 1.102 (0.540–2.253) | 0.789 | Not included | |
| Median age at diagnosis, yr | 1.017 (0.993–1.042) | 0.170 | Not included | |
| Duration of disease before starting adalimumab therapy, yr | 0.919 (0.838–1.008) | 0.075* | 0.995 (0.987–1.003) | 0.251 |
| Smoking status at diagnosis of UC | ||||
| Never smoker | Reference | |||
| Ex-smoker | 1.883 (0.925–3.836) | 0.081* | 0.775 (0.356–1.687) | 0.520 |
| Current smoker | 0.883 (0.278–2.495) | 0.745 | ||
| Family history of inflammatory bowel disease | 0.848 (0.202–3.557) | 0.821 | Not included | |
| History of appendectomy before starting adalimumab therapy | 0.047 (0.001–263.152) | 0.488 | Not included | |
| p-ANCA positivity | 0.680 (0.350–1.321) | 0.255 | Not included | |
| ASCA positivity | 1.734 (0.827–3.633) | 0.145 | Not included | |
| Immunomodulator therapy for more than 3 mo during initial 6 mo of adalimumab therapy | 0.800 (0.398–1.609) | 0.532 | Not included | |
| Disease extent at starting adalimumab therapy | Not included | |||
| Left-sided colitis | Reference | |||
| Extensive colitis | 0.826 (0.386–1.767) | 0.623 | ||
| Mayo endoscopic subscore at starting adalimumab therapy | Not included | |||
| 2 | Reference | |||
| 3 | 0.633 (0.303–1.323) | 0.224 | ||
| Partial Mayo score at starting adalimumab therapy | 1.098 (0.864–1.394) | 0.446 | Not included | |
| Leukocyte count at starting adalimumab therapy, 103/μL | 1.036 (0.968–1.108) | 0.311 | Not included | |
| Anemia at starting adalimumab therapy (<13 g/dL in male, <12 g/dL in female) | 1.207 (0.617–2.360) | 0.583 | Not included | |
| Platelet count at starting adalimumab therapy, 103/μL | 1.000 (0.997–1.003) | 0.900 | Not included | |
| Elevated ESR at starting adalimumab therapy (>9 mm/hr in male, >20 mm/hr in female) | 5.472 (1.310–22.848) | 0.020* | 4.324 (0.985–18.988) | 0.052 |
| Serum CRP at starting adalimumab therapy, mg/dL | 1.065 (0.954–1.190) | 0.261 | Not included | |
| Serum albumin at starting adalimumab therapy, g/dL | 0.637 (0.388–1.045) | 0.074* | 0.778 (0.436–1.389) | 0.396 |
| Mayo endoscopic subscore after 8 wk of adalimumab therapy | Not included | |||
| 0 | Reference | |||
| 1 | 0.708 (0.237–2.116) | 0.537 | ||
| 2 | 1.367 (0.540–3.461) | 0.509 | ||
| 3 | 1.870 (0.806–4.336) | 0.145 | ||
| Partial Mayo score after 8 wk of adalimumab therapy | 1.284 (1.118–1.474) | <0.001* | 1.217 (1.040–1.425) | 0.014† |
| No. of biologic agents exposed before adalimumab therapy | ||||
| Biologic-naïve | Reference | |||
| Exposed to 1 biologic agent | 1.753 (0.878–3.497) | 0.111 | ||
| Exposed to 2 biologic agents | 11.664 (2.418–56.268) | 0.002* | 4.722 (1.033–21.586) | 0.045† |
| Dose intensification of adalimumab | 1.483 (0.762–2.886) | 0.246 | Not included | |
| History of cytomegalovirus colitis within 3 mo before, or during adalimumab therapy | 2.920 (1.500–5.684) | 0.002* | 1.786 (0.787–4.054) | 0.165 |
UC, ulcerative colitis; HR, hazard ratio; CI, confidence interval; p-ANCA, perinuclear antineutrophil cytoplasmic antibody; ASCA, anti-Saccharomyces cerevisiae antibody; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Variables included in multivariate analysis; †Significant predictors of adalimumab discontinuation.
Patients with adalimumab maintenance therapy were classified into three groups according to partial Mayo score after 8 weeks of adalimumab therapy (group 1, partial Mayo score 0–2; group 2, partial Mayo score 3–5; group 3, partial Mayo score 6–9). The Kaplan-Meier analysis showed significant differences in cumulative proportions of patients remaining on adalimumab maintenance therapy among the three groups (group 1, 85.4% and 62.0% at 1 and 4 years; group 2, 61.6% and 38.0% at 1 and 4 years; group 3, 36.4% and 27.3% at 1 and 4 years; log-rank p=0.003) (Fig. 3).
Fig. 3.
Cumulative proportions of patients remaining on adalimumab therapy among 92 patients who received adalimumab therapy for 8 or more weeks stratified by partial Mayo score after 8 weeks of adalimumab therapy.
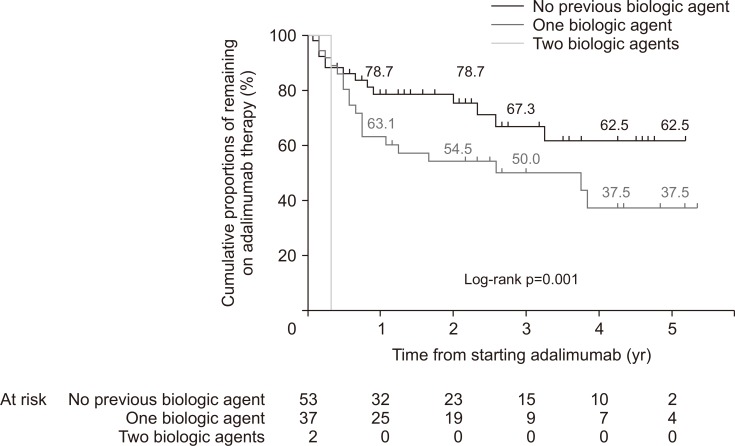
When patients were grouped according to the number of other biologic agents exposed before adalimumab therapy (group A, naïve to biologic agents; group B, exposed to one biologic agent; group C, exposed to two biologic agents), cumulative proportions of patients remaining on adalimumab maintenance therapy significantly differed as well (group A, 78.7% and 62.5% at 1 and 5 years; group B, 63.1% and 37.5% at 1 and 5 years; group C, n=2 discontinued adalimumab therapy on 125 and 148 days after starting adalimumab, respectively; log-rank test p=0.001) (Fig. 4). When comparing clinical outcomes in terms of clinical response defined by decrease of Mayo score (Mayo score decrease by 3 and 30% compared to baseline) and clinical remission (Mayo score 0–2), biologic-naïve patients (group A) showed better clinical outcomes than biologic-exposed patients (groups B and C). After 8 weeks of therapy, 46 out of 53 patients in group A (86.8%) and 25 out of 37 patients in groups B and C (67.6%) achieved clinical response, respectively (p=0.046). Also, 29 out of 53 patients in group A (54.7%) and 5 out of 37 patients in groups B and C (13.5%) achieved clinical remission (p<0.001), respectively. At 1 year of therapy (between 50 and 55 weeks after adalimumab commencement), all 46 patients in whom Mayo score was assessed achieved clinical response. However, 18 out of 27 patients (66.7%) in group A and 3 out of 19 patients (15.8%) in groups B and C achieved clinical remission (p=0.001), respectively.
Fig. 4.
Cumulative proportions of patients remaining on adalimumab therapy among 92 patients who received adalimumab therapy for 8 or more weeks stratified by the number of biologic agents exposed to before adalimumab therapy.
DISCUSSION
In this study, we analyzed long-term clinical outcomes of adalimumab therapy in non-Caucasian patients with UC. This is the first long-term study on a real-life cohort of Asian UC patients treated with adalimumab. In our study, more than 90% received adalimumab maintenance therapy and two-thirds out of patients entering maintenance therapy were still on adalimumab at the last follow-up. Cumulative proportions of patients remaining on adalimumab therapy was 70.0%, 65.8%, 58.1% and 48.9% at 1, 2, 3 and 5 years among 92 patients who received adalimumab maintenance therapy.
Adalimumab is the first recombinant fully human monoclonal antibody that can be self-injected subcutaneously by patients. According to Western real-life studies, it has been proven to be effective in treating patients with UC. In a prospective multicenter study from Hungary with 73 patients with active UC who were refractory to conventional therapy (67.1% of the patients were previously exposed to infliximab), 75.3% of the patients showed a short-term clinical response at week 12.16 Regarding the long-term clinical outcomes, adalimumab therapy was maintained in 48.6% with a continuous clinical response at week 52.16 In an Italian study, the cumulative probability of continuing adalimumab therapy at 12 months was reported to be 65.9% among 88 patients with UC (78.4% of the patients were previously exposed to infliximab).17 In a retrospective Irish study, rates of clinical response and remission were 52% and 42%, respectively among 52 UC patients (13% of the patients were previously exposed to infliximab) at 12 months.18 In our study, because we focused on retention rate of adalimumab rather than clinical response or remission rate, it is difficult to directly compare our results with those from Hungarian and Irish studies.16,18 However, when we calculated cumulative adalimumab retention rate at 12 months among all 100 patients who commenced adalimumab therapy like the previous Italian study, the rates were very similar between two studies (64.6% vs 65.9%).17 Limited option after stopping adalimumab therapy and reimbursement for life-long maintenance therapy of adalimumab with a 10% co-payment may have influenced on the relatively high retention rate of adalimumab in our study. A substantial rate of dose escalation (40.2%), which was similar with 35.2% from the Italian study could explain high durability of adalimumab therapy as well.17
There have been no sufficient real-life studies on the clinical benefit of adalimumab in Asian patients with UC. A placebo-controlled trial conducted in Japan showed that 50% response rate at week 8 of adalimumab therapy among anti-TNF-naïve patients with moderate to severe UC.8,9 In another prospective study from Japan, 73.3% of UC patients responded to adalimumab therapy at week 14 in 15 anti-TNF-naïve UC patients.19 Furthermore, the drop-out rate before week 54 was 40% (6/15) and the response, remission, and mucosal healing rates at week 54 were 66.7%, 53.3%, and 58.3%, respectively.19 In our study, 92% of patients entered into adalimumab maintenance therapy and the cumulative rate of maintaining adalimumab therapy until 1 year was 70.0% among the patients who received adalimumab therapy for 8 weeks or more. These figures seem to be higher when comparing with the results presented by the previously mentioned Japanese studies.8,9,19 However, this difference might be brought about because of different outcome definitions. Furthermore, as our study was conducted in real-life setting, assessment of clinical response might have been less stringent compared to a placebo-controlled trial and a prospective study from Japan.8,9,19
Partial Mayo score after 8 weeks of adalimumab therapy was found to be associated with discontinuing maintenance therapy in our study. Likewise, a previous Spanish study reported that a lack of response at week 12 was associated with an increased probability of discontinuing adalimumab and a higher rate of colectomy.20 Another study also suggested that failure of adalimumab treatment within first 3 months is associated with colectomy,21 proposing early response to adalimumab as a factor associated with a long-term durability of adalimumab.
The other factor which was related to adalimumab discontinuation in our study was previous exposure to two biologic agents. In the ULTRA-2 study, rates of clinical remission at weeks 8 and 52 were 21.3% and 22% in anti-TNF agent-naïve patients, while 9.2% and 10.2% in previously anti-TNF-exposed patients.5 Another study from Belgium reported that prior failure to infliximab was negatively associated with short-term mucosal healing (assessed between weeks 8 and 14).22 However, in the Hungarian study, previous infliximab therapy did not influence disease outcome and a need for DI.16 In the Italian study, previous immunosuppressant use was associated with a lower probability of clinical remission at week 54, however, previous infliximab use was not associated with clinical remission at week 54.17 In the Irish study, a trend towards better outcomes with adalimumab therapy in patients previously exposed to anti-TNF than in anti-TNF-naïve patients was observed.18 However, higher proportion of DI in anti-TNF exposed group than anti-TNF-naïve group (43% vs 24%) and small number of anti-TNF-exposed patients (n=7) made it difficult to draw a firm conclusion.18 Therefore, it is still difficult to conclude that previous exposure to other biologics is associated with poor outcomes in adalimumab therapy for UC because of heterogeneity in study designs and definition of outcomes.
The major limitation of our study is its retrospective design. There might be factors affecting the patients’ and clinicians’ decisions which might have been controlled and monitored more strictly in a prospectively controlled design. However, this retrospective design could be a strength of the study at the same time, by reflecting real-life circumstances more accurately. Although information bias could be made by recall errors in retrospective study, it was minimized by using our center’s IBD registry in which patients were enrolled and their data were updated prospectively by IBD specialists.2,23 The second limitation might be referral bias as our study was conducted in a single tertiary referral center. However, most moderate-to-severe UC patients using biologic agents are referred to tertiary hospitals and continuously followed, not referred back to primary or secondary medical institutions. Therefore, the patients in this study may sufficiently represent the general UC patient population receiving adalimumab therapy in Korea. Moreover, as all the patients received adalimumab therapy from a single specialized IBD center, relatively standardized treatment and follow-up strategies were ensured, thereby minimizing variations among treating clinicians. Lastly, therapeutic drug monitoring and fecal calprotectin monitoring were not systematically applied for our study subjects, which could have assisted therapeutic decision when performed.
In conclusion, long-term outcomes of adalimumab therapy in a real-life cohort of Korean UC patients appear to be similar to those in previously published Western and Asian studies. Partial Mayo score after 8 weeks of adalimumab therapy and a previous history of exposure to two other biologic agents before adalimumab therapy were found to be predictors for adalimumab discontinuation. These factors could be used for early optimization of adalimumab therapy considering their prognostic implication.
ACKNOWLEDGEMENTS
This study was supported by a grant (number: 2010-0774) from the Asan Institute for Life Sciences, Seoul, Korea.
Footnotes
CONFLICT OF INTEREST
B.D.Y. has received a research grant from Celltrion; consulting fees from Abbvie Korea, Celltrion, Daewoong Pharma., Ferring Korea, Janssen Korea, Kangstem Biotech, Kuhnil Pharm., Shire Korea, Takeda Korea, IQVIA, Cornerstones Health, Robarts Clinical Trials Inc. and Takeda; speaking fees from Abbvie Korea, Celltrion, Janssen Korea, Shire Korea, Takeda Korea, and IQVIA. However, all of these are not relevant to this article.
AUTHOR CONTRIBUTIONS
Study concept and design: E.H.O., B.D.Y. Data acquisition: E.H.O., J.K., N.H., S.W.H., S.H.P., D.H.Y., J.S.B., S.J.M., S.K.Y., B.D.Y. Data analysis and interpretation: E.H.O., B.D.Y. Drafting of the manuscript: E.H.O., B.D.Y. Study supervision: B.D.Y.
REFERENCES
- 1.Poullenot F, Laharie D. First line therapy of inflammatory bowel disease. Rev Prat. 2014;64:1242–1248. [PubMed] [Google Scholar]
- 2.Lee HS, Park SH, Yang SK, et al. Long-term prognosis of ulcerative colitis and its temporal change between 1977 and 2013: a hospital-based cohort study from Korea. J Crohns Colitis. 2015;9:147–155. doi: 10.1093/ecco-jcc/jju017. [DOI] [PubMed] [Google Scholar]
- 3.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–787. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn WJ, Colombel JF, D’Haens G, et al. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther. 2013;37:204–213. doi: 10.1111/apt.12145. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Sandborn WJ, Lazar A, et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology. 2014;146:110–118. doi: 10.1053/j.gastro.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Afif W, Leighton JA, Hanauer SB, et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009;15:1302–1307. doi: 10.1002/ibd.20924. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Motoya S, Hanai H, et al. Four-year maintenance treatment with adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2017;52:1031–1040. doi: 10.1007/s00535-017-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49:283–294. doi: 10.1007/s00535-013-0922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 11.Jang EY, Park SY, Lee EJ, et al. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. Clin Infect Dis. 2009;48:e121–e124. doi: 10.1086/599116. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci. 2013;58:3592–3599. doi: 10.1007/s10620-013-2828-1. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Boo SJ, Ye BD, et al. Clinical utility of cytomegalovirus antigenemia assay and blood cytomegalovirus DNA PCR for cytomegaloviral colitis patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2014;8:693–701. doi: 10.1016/j.crohns.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Park SH, Kim SH, et al. Risk factors and clinical outcomes associated with cytomegalovirus colitis in patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2016;22:912–918. doi: 10.1097/MIB.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 16.Bálint A, Farkas K, Palatka K, et al. Efficacy and safety of adalimumab in ulcerative colitis refractory to conventional therapy in routine clinical practice. J Crohns Colitis. 2016;10:26–30. doi: 10.1093/ecco-jcc/jjv169. [DOI] [PubMed] [Google Scholar]
- 17.Armuzzi A, Biancone L, et al. Italian Group for the Study of Inflammatory Bowel Disease. Adalimumab in active ulcerative colitis: a “real-life” observational study. Dig Liver Dis. 2013;45:738–743. doi: 10.1016/j.dld.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Hussey M, Mc Garrigle R, Kennedy U, et al. Long-term assessment of clinical response to adalimumab therapy in refractory ulcerative colitis. Eur J Gastroenterol Hepatol. 2016;28:217–221. doi: 10.1097/MEG.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 19.Mizoshita T, Katano T, Tanida S, et al. Prospective comparison of preference and efficacy of adalimumab and infliximab for treating ulcerative colitis naive to antitumor necrosis factor therapy. Medicine (Baltimore) 2017;96:e7800. doi: 10.1097/MD.0000000000007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taxonera C, Estellés J, Fernández-Blanco I, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther. 2011;33:340–348. doi: 10.1111/j.1365-2036.2010.04531.x. [DOI] [PubMed] [Google Scholar]
- 21.McDermott E, Murphy S, Keegan D, O’Donoghue D, Mulcahy H, Doherty G. Efficacy of Adalimumab as a long term maintenance therapy in ulcerative colitis. J Crohns Colitis. 2013;7:150–153. doi: 10.1016/j.crohns.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Papamichael K, Baert F, Tops S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis. 2017;11:53–59. doi: 10.1093/ecco-jcc/jjw122. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Yang SK, Park SK, et al. Long-term prognosis of Crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014;20:488–494. doi: 10.1097/01.MIB.0000441203.56196.46. [DOI] [PubMed] [Google Scholar]